Abstract
Background
Gadolinium-based contrast agents (GBCAs) have been used for magnetic resonance (MR) imaging over the last three decades. Recent reports demonstrated gadolinium retention in patients’ brains following intravenous administration. Since gadolinium is a highly toxic heavy metal, there is a potential for adverse effects from prolonged retention or deposition, particularly in children. For this reason, the Society (SPR) for Pediatric Radiology Quality and Safety committee conducted a survey to evaluate the current status of GBCAs usage among pediatric radiologists.
Objective
To assess the usage of GBCAs among SPR members.
Materials and methods
An online 15-question survey was distributed to SPR members. Survey questions pertained to the type of GBCAs used, protocoling workflow, requirement of renal function or pregnancy tests, and various clinical indications for contrast-enhanced MRI examinations.
Results
A total of 163 survey responses were compiled (11.1% of survey invitations), the majority of these from academic institutions in the United States. Ninety-four percent reported that MR studies are always or usually protocoled by pediatric radiologists. The most common GBCA utilized by survey respondents were Eovist (60.7%), Ablavar (45.4%), Gadovist (38.7%), Magnevist (34.4%) and Dotarem (32.5%). For several clinical indications, survey responses regarding GBCA administration were concordant with American College of Radiology (ACR) Appropriateness Criteria, including seizures, headache and osteomyelitis. For other indications, including growth hormone deficiency and suspected vascular ring, survey responses revealed potential overutilization of GBCAs when compared to ACR recommendations.
Conclusion
Survey results demonstrate that GBCAs are administered judiciously in children, yet there is an opportunity to improve their utilization with the goal of reducing potential future adverse effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gadolinium is a highly paramagnetic heavy metal used as a base for contrast agents in magnetic resonance imaging (MRI). The free gadolinium ion (Gd3+) itself is highly toxic because it is similar in size and chemical activity to ionic calcium (Ca2+), and can therefore antagonize a variety of voltage-gated calcium channels. To overcome this potential toxicity, a gadolinium-based contrast agent (GBCA) consists of gadolinium and a chelating agent (often polyaminocarboxylic acid), which binds the metal ions to form a chemically stable compound that can be safely excreted [1].
The configuration of the chelation agent molecule may be linear or macrocyclic. Furthermore, the chelation agent molecule may be ionic or nonionic (Table 1). It has been demonstrated that macrocyclic ionic agents have the highest chemical stability, in contrast to nonionic linear agents, which are the least stable. [1].
The first GBCA to be approved by the U.S. Food and Drug Administration (FDA) in 1988 was Magnevist, a linear ionic GBCAs. For nearly two decades, GBCA boasted an exceptional safety record in the literature. However, in 2006, the association between GBCAs and nephrogenic systemic fibrosis (NSF) in patients with impaired renal function was first recognized [2]. The majority of NSF cases reported thus far (78%) have been associated with the linear nonionic agent Ominscan. Additional NSF cases have been reported with Magnevist (20%), a linear ionic agent, and Optimark (2%), a linear nonionic agent [3].
In response to this heightened awareness of NSF in the last decade, the radiology community has become more cautious with gadolinium administration in cases of impaired renal function. Many international agencies such as the European Medicines Agency, the European Society for Urological Radiology, the American College of Radiology (ACR), and the FDA have published alerts, precautions and recommendations on the use of GBCAs [4].
According to the ACR manual on contrast media (version 10.1-2015), as of September 2012, only 23 unique NSF cases have been reported in children, and all of these patients were 6 years or older. Since there is no specific consensus for pediatric guidelines, the ACR recommends following adult guidelines for administering GBCAs in children with impaired renal function. This report states that caution should be used when administering these contrast agents, particularly in preterm neonates and infants due to renal immaturity and potentially low glomerular filtration rates [5].
While it was initially thought that GBCAs are excreted by the kidneys and are safe in patients with normal renal function, studies from 2004 through 2006 demonstrated retention of gadolinium in the skeleton [6, 7]. More recent studies have demonstrated retention of gadolinium in the brain, including the dentate nuclei and globi pallidi [8,9,10]. In light of these reports, the Society for Pediatric Radiology (SPR) Quality and Safety committee sought to evaluate the current status of GBCA usage among the society’s members in an effort to share best practices and reveal potential opportunities for further investigation.
Materials and methods
A 15-question survey (Appendix A, available online) was created using SurveyMonkey (San Mateo, CA), an online survey vendor, and was distributed to all members of the SPR on November 2015. A link to the survey was contained in the email. A reminder email was sent 6 weeks following the initial invitation. The first part of the survey was designed to gather background and demographic information from the respondents, including type of institute in which the member practices (e.g., academic versus nonacademic, pediatric-focused versus general hospital) and country of residence of the participant.
Subsequent questions were intended to characterize the practice of GBCA administration to children. First, we determined whether pediatric radiologists protocol and interpret MRI examinations and whether they communicate with ordering physicians in cases of discrepancy between the exam requisition and the department protocol regarding the administration of a GBCA.
Additional questions addressed the brand(s) of GBCAs administered to the general pediatric population and specifically to neonates and infants younger than 1 year of age, and which renal function and pregnancy tests, if any, are required prior to GBCA administration.
Members were also asked whether they make any adjustments in cases of impaired renal functions, and whether they have any additional special restrictions or contraindications for GBCA administration in neonates or infants younger than 1 year of age.
Finally, we sought to clarify for which clinical indications GBCAs are administered. Soliciting all possible clinical indications for GBCA use would be beyond the capability and scope of this survey. Instead, we selected a sample of indications for MRI examinations within subspecialty disciplines, including neuroradiology, musculoskeletal and body imaging studies, with emphasis on entities that may be controversial in regard to GBCA administration, such as imaging for headaches or seizures in children.
The survey results were collected using SurveyMonkey® web-based software in February 2016, and were further analyzed using Microsoft Excel® (Microsoft, Redmond, WA).
Results
One hundred sixty-seven responses were received. This represents 11.1% out of a total of 1,498 emailed invitations to SPR members. Four responders were excluded from the analysis: three are radiologists who did not reply to any of the non-demographic questions, and one is a teleradiologist who remotely interprets studies from multiple sites but does not participate in protocoling studies. The total number of responses included in the analysis was 163. Of note, not all the responders replied to all the survey questions, and some of the questions had an option to select more than one answer.
Respondent demographics
The majority of the respondents (128/160, 80.0%) practice either in free-standing children’s hospitals or in children’s hospitals or departments contained within general hospitals: 93.1% of respondents (149/160) were from either academic (100/160, 62.5%) or hybrid (49/160, 30.6%) institutions.
Of the survey participants, 82.1% (133/162) reside in the United States; 7 (4.3%) were from Canada, and the remaining 22 (13.6%) were from multiple other countries (Table 2).
Protocoling and interpreting MRI examinations
According to the survey, in the majority of departments surveyed (149/162, 92.0%), studies are always (119/162, 73.5%) or usually (30/162, 18.5%) protocoled by pediatric radiologists. With regard to study interpretations, the majority of the participants (145/162, 89.5%) replied that the studies are either always (104/162, 64.2%) or usually (41/162, 25.3%) interpreted by pediatric radiologists.
For the question about possible discordance between the order requisition and the radiologist’s protocol regarding GBCA administration, the responses were mixed. Of the 161 responders to this question, 46.0% (74) stated they would contact the ordering physician when a noncontrast examination was requested and the protocol by the radiologist calls for administration of GBCA. Sixty (36.7%) responders would contact the ordering physician if the request calls for administration of GBCA, but the radiologist protocol does not require it, and 40 (24.8%) stated they do not contact the ordering physician at all in regard to this matter.
GBCA brand
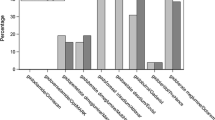
Survey respondents reported using the following agents in a decreasing order of frequency: Eovist® (99/163, 60.7%) as a specific agent for liver imaging and Ablavar (74/163, 45.4%) as a specific agent for vascular imaging, followed by Gadovist (63/163, 38.7%), Magnevist (56/163, 34.4%), Multihance (54/163, 33.1%) and Dotarem (53/163, 32.5%) (Table 3). When the results were filtered to include only participants residing in the United States, there was a slight decrease in the rate of usage of Magnevist (to 29.3%), and a slight increase in the utilization of the macrocyclic agents Gadovist, Dotarem and Prohance compared to unfiltered results (Table 4).
When filtering the results to include only respondents from dedicated pediatric facilities (i.e. free-standing pediatric hospitals and pediatric facilities within general hospitals) that are also academic institutions, there was also a mild decrease in the usage of Magnevist (to 29.8%), and a mild increase in the usage of the macrocyclic agents Dotarem and Prohance compared to unfiltered results (Table 4).
In neonates and infants younger than 1 year, Ablavar and Eovist are used less commonly (34/158, 21.5%, and 28/158, 17.7%, respectively) than in the general pediatric population. The most commonly used agents for this patient age group are Gadovist, Dotarem and Multihance (59/158, 37.3%; 45/158, 28.5%, and 41/158, 26.0%, respectively) (Table 3).
Renal function and pregnancy tests prior to GBCA administration
The majority of respondents (96/163, 58.9%) require renal function tests only on selected patients: 28.8% (48/163) commented that they require these tests when there is clinical suspicion for renal dysfunction and 29.5% (19/163) stated that they require renal function tests for most or all pediatric patients. The most commonly required tests were glomerular filtration rate (GFR) (19 affirmative responses), and creatinine and GFR (11 responders).
The majority of respondents (104/158, 65.8%) stated that they would not change the GBCA brand when renal functions are abnormal. Of the remaining 34.2% (54/158), 16 stated they would not administer GBCA, 2 would decrease the dosage, and 1 responder would not administer GBCA if the GFR was less than 30 mL/min/1.73 m2. Twenty-five respondents stated that they would administer a different agent in the case of abnormal renal function.
The majority of the respondents (109/155, 70.3%) said they would not use different criteria for determining renal function in neonates and infants less than 1 year old.
Only 39.1% (63/161) respondents require pregnancy tests for all or most teenage patients prior to GBCA administration.
Specific requirements for neonates or infants <1 year
The majority of respondents (124/158, 78.5%) stated they do not have any additional specific requirements or adjustments for neonates or infants younger than 1 year old. Of those survey responders who stated they do have additional requirements, 14 (9%) reported that they use GBCAs cautiously in this age group and try to avoid it if possible.
Indications for GBCA use
The responses for the survey question regarding the clinical indications of GBCA usage are detailed in Table 5. Indications for which the majority of the respondents administer GBCA always or usually include the following in decreasing order of frequency: osteomyelitis (143/159, 90.0%), MR enterography (133/153, 86.9%), suspected central nervous system infection (120/152, 78.9%), MR urography (122/155, 78.7%), arthritis (118/157, 75.2%), MR venography of the chest, abdomen and pelvis for suspected deep venous thrombosis (116/154, 75.1%), cystic hygroma assessment (111/155, 71.6%), MRI of the thorax for a vascular ring (101/154, 65.6%), suspected dural venous sinus thrombosis (96/153, 62.7%), neonatal infection (94/151, 62.2%), cardiac MR (89/154, 57.8%) and MRI of the brain for growth hormone deficiency (83/152, 53.6%).
Additional indications that were added in the comments section to this question included the following:
-
For follow-up of response to chemotherapy.
-
Whenever precontrast images revealed a finding for which contrast will increase the specificity.
-
For evaluation of a malignancy.
-
MRI of the brain for white matter metabolic diseases.
-
MRI of the brain for evaluation of precocious puberty.
-
MRI of the brain for developmental delay.
-
MRI of the brain and internal acoustic canals for hearing loss.
-
MRI of the spine in case of a syrinx.
-
For cystic musculoskeletal mass that does not appear to communicate with a joint.
-
Perthes disease.
-
For evaluation of vascular anomalies.
-
For evaluation of liver disease including neonatal hepatitis and biliary atresia.
Discussion
Recent reports demonstrating deposition of gadolinium in brain parenchyma following intravenous GBCA administration [8,9,10] largely generated the impetus to conduct this survey. While the overall response rate amongst all SPR members surveyed was low (11.1%), the majority of the respondents came from large academic pediatric institutes in North America. Hence, the results of the survey may be used to increase the awareness and guide practitioners in other institutes to optimize their practice of GBCA administration in children to minimize the potential long-term adverse effects of gadolinium.
At the majority of the survey respondents’ institutes, pediatric radiologists protocol and interpret MRI examinations. The authors say this is desirable as pediatric radiologists may be more informed about the possibility of long-term risks of GBCAs and may use them more judiciously, particularly in neonates and infants.
The majority of the respondents require renal function tests only on selective patients prior to administration of GBCAs. The authors say they believe this to be a reasonable standard of care as most healthy children have normal renal function. Since ACR-based guidelines for reducing the risks of NSF from gadolinium rely on GFR values for both adults and children [5], we believe this is a reasonable choice for assessing renal function when indicated.
The majority of respondents do not test routinely for pregnancy in teenage girls prior to administering gadolinium. A reasonable practice may be to initially privately question patients about sexual activity and for the possibility of pregnancy, and when in doubt to obtain a urine or blood beta-human chorionic gonadotropin (bHCG) test prior to the administration of GBCAs.
Macrocyclic GBCAs feature the highest chemical stability and are currently considered the safest, while nonionic linear agents are the least stable agents [1]. Gadovist and Dotarem are macrocyclic agents that are both commonly used by the survey participants. Eovist is a linear ionic agent and hence is considered medium risk. It is used by the majority of the survey participants (60.7%), predominantly for hepatobiliary imaging, leveraging the fact that 50% of it is excreted through the biliary system [11].
Ablavar (linear ionic) is a blood pool agent due to its ability to temporarily bind to serum albumin, which makes it ideal for MR angiograms [12]. According to our survey, Ablavar is widely used (45.4%) for imaging cardiovascular pathology. Unfortunately, its production will be discontinued at the time of this publication. A substantial number of the survey participants reported using Magnevist and Multihance® (34.4% and 33.1%, respectively). Both of these GBCAs are linear ionic and therefore do not exhibit the same chemical stability and safety profiles as do macrocyclic GBCAs. Magnevist in particular has been associated with cases of NSF [3]. Moreover, these agents are used by more than 20% of the respondents in neonates and infants younger than 1 year old. The authors speculate that there may be cost advantages or contractual agreements that may support the use of these agents over macrocyclic agents at certain institutions. Nevertheless, according to a Society of Chairs of Radiology in Children’s Hospitals (SCORCH) survey conducted in 2011 [13], the usage of these GBCAs was significantly higher - 81% of the survey participants were using Magnevist and 38% were using Multihance. Our survey demonstrates that although linear GBCAs are still widely utilized for pediatric MR imaging, there has been a substantial decline in their usage rate in recent years.
In the neonatal period, renal function may not be fully developed [5]. Furthermore, neonates and infants younger than 1 year are in a stage of rapid brain and other organ development. Consequently, this subset of patients may be at higher risk of suffering adverse effects from long-term deposition of gadolinium in their tissues. Although there is no specific evidence-based linkage between gadolinium deposition in young children and poorer health outcomes, many of the survey participants report a more cautious approach with gadolinium administration in this age group. Many survey respondents avoid gadolinium when possible, and some report never administering GBCAs in neonates.
Clinical indications for GBCA administration
The determination of whether GBCA is indicated is a multifaceted decision based on factors such as the specific indication, known underlying pathology, or unexpected or incidentally discovered findings. In our study, 19 specific clinical indications were surveyed, each receiving between 150 and 159 responses. The authors intended to provide a broad overview of current GBCAs usage in potentially controversial contexts. For many indications, the survey responses are in keeping with ACR-established guidelines for standard of care. Several examples include:
-
Seizures: ACR appropriateness criteria for seizures in children specify several circumstances where MRI is “usually appropriate,” including in the settings of partial seizures, generalized seizures (neurologically abnormal) and intractable seizures. For each of these indications, brain MRI without contrast is rated 9 (“usually appropriate”) and brain MRI with contrast is rated 6 to 7 (“may be appropriate” to “usually appropriate,” respectively). Generally, a GBCA is administered to clarify an abnormality or if there is concern for infection or inflammation [14]. This survey indicates that for seizures, 57.3% of respondents “sometimes, rarely or never” administer GBCAs, versus 24.7% who “always or usually” administer it for this indication. When specific concern for central nervous system infection was indicated, 78.9% of respondents stated they “always or usually” administer GBCAs. These results suggest that the majority of respondents were utilizing GBCAs in accordance with established ACR appropriateness criteria for seizure imaging.
-
Headache: In children with headaches and neurological deficits, it is usually appropriate to obtain a noncontrast MRI examination. MRI with gadolinium also received a score of 8 (“usually appropriate”), but was only recommended if noncontrast images warranted its use. Survey results indicated that only 7.8% “always or usually” administer GBCAs, while 74.5% “sometimes, rarely or never” administer it in the setting of headache. These findings suggest that respondents’ practices are generally congruent with ACR guidelines. Based on these observations, it would be desirable that a radiologist would evaluate precontrast images during the examination to determine the need for GBCA administration, rather than uniformly administer contrast to all patients with this indication.
-
Osteomyelitis: Following acquisition of radiographs, MRI with contrast to evaluate for soft-tissue or bone infections is usually appropriate, receiving a score of 9 (“usually appropriate”), while noncontrast MRI was rated 7 in patients in whom contrast is contraindicated. Survey respondents indicated that 90.0% “always or usually” administer GBCA when osteomyelitis is suspected. Interestingly, for children age 5 and younger who are limping and concern for infection is present, both contrast-enhanced and noncontrast MRI exams were given ACR scores of 7. These findings suggest that it may be helpful to evaluate the precontrast images for any bone marrow signal abnormalities or joint effusion that may suggest an infectious or inflammatory process and administer GBCA only when such abnormalities are present [15].
Survey results also revealed some practice patterns where GBCAs were potentially overutilized as compared to ACR recommendations, including:
-
Growth hormone deficiency: More than half (54.6%) of the respondents indicated they “always or usually” administer GBCAs for growth hormone deficiency. The neuroendocrinology variant for growth hormone deficiency and panhypopituitarism rated noncontrast MRI, including thin slices of the sella, as “usually appropriate” with a rating of 7. However, the usage of gadolinium in this context only received a rating score of 5, corresponding to “may be appropriate” [16]. These survey findings suggest that the current practice pattern potentially overutilizes GBCA administration, at least for some patients in the setting of growth hormone deficiency, and may serve as an indication that deserves greater scrutiny.
-
Suspected vascular ring: This indication serves as an example of the potential difficulties in determining consensus guidelines for gadolinium administration. No specific ACR appropriateness criteria are currently available for suspected vascular ring in children, with the closest applicable criteria being known or suspected congenital heart disease in adults. For great vessel assessment, both MR angiography without and with gadolinium receive “may be appropriate” rating scores of 6 [17]. Furthermore, cardiovascular imaging is often a highly individualized, patient-specific imaging discipline. Among our survey respondents, 41.6% report “always” and 24.0% report “usually” administering gadolinium for a suspected vascular ring. With 65.6% “always/usually” versus 25.3% “sometimes/rarely/never” responses, this indication may be an area for further clarification regarding GBCA utility.
Finally, the issue of sedation may impact radiologists’ decisions regarding contrast administration. For example, many radiologists may be more inclined to conduct additional sequences or administer GBCAs when patients are anesthetized. From a practical standpoint, it may be better “to do more than less” in the eventuality that more information is needed at future interpretation, potentially obviating another sedation event. This increased willingness to administer gadolinium must be weighed against the risks of prolonging anesthesia and potential adverse effects related to GBCAs. Survey results indicate that the majority of responders administer GBCA under anesthesia, with 57.1% using it “sometimes,” 14.9% “always” and 21.7% “usually.” With 36.6% of the respondents “always/usually” giving gadolinium in the setting of patient sedation, this may be an opportunity to consider the aforementioned risks and balance them accordingly.
This study has several limitations, of which the most significant is the low response rate of the SPR membership (11.1%). The authors attempted to reduce this limitation through an email reminder following the original survey invitation, though clearly additional methods of reaching out to membership may have been necessary to augment this rate. Another substantial limitation to our study was lack of ascertaining the number of institutions represented in the survey. This was difficult, in part, because of the logistics of such a survey question, as a drop-down menu likely would not have been inclusive. Hence, there is a strong possibility that multiple respondents from a small number of institutes participated in the survey, which may introduce bias to the results.
Additional limitations are related to the survey questions. Questions 7 and 10 in the survey (Appendix A) did not specify at what rate different brands are used in a certain institution. The results demonstrate that the most commonly used brands are Eovist® and Ablavar® (specific agents for liver and cardiovascular imaging, respectively). While these agents are used by the majority of institutions, their rate of usage at any specific site is likely low. In regard to adjustments in cases of borderline or abnormal renal function, question 11 in the survey (Appendix A) addresses only GBCA brand selection and not dosage adjustments or whether GBCAs would not be administered at all. Retrospectively, it would have been more appropriate to ask whether any adjustments are done when renal function is borderline or abnormal.
Conclusion
The majority of our 163 gadolinium usage survey respondents practice in academic pediatric institutes. While the results of the survey indicate that GBCA are generally used judiciously, there are still opportunities for improvement. The following measures are suggested by the Quality and Safety Committee to reduce unnecessary exposure of children to gadolinium. These recommendations are the consensus opinions of committee members based on the survey results exhibiting practice patterns, and how these patterns compare to ACR recommended guidelines.
-
1.
Whenever possible, pediatric studies should be protocoled by pediatric radiologists with the requisite knowledge of indications and potential risks of gadolinium usage in children.
-
2.
For many clinical indications, along with studies performed with anesthesia, pre-contrast images should be reviewed while the patient is in the magnet to assess the need of gadolinium.
-
3.
If there are protocoling questions or concerns, the radiologist should reach out to the ordering clinicians directly to clarify the need for gadolinium, as opposed to strict adherence to requests for contrast administration.
-
4.
In children who have received multiple administrations of gadolinium for follow-up MR examinations, the radiologist should assess the added value of contrast and explore the feasibility of a noncontrast study.
-
5.
Macrocyclic agents generally feature superior safety profiles to linear agents and are preferred. If cost or availability preclude their use, then linear ionic agents are preferred over linear nonionic agents.
-
6.
Exercise greatest caution in neonates and infants, as the long-term effects of gadolinium deposition in tissues have yet to be elucidated.
References
Port M, Idée JM, Medina C et al (2008) Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 21:469–490
Ramalho J, Semelka RC, Ramalho M et al (2015) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37:1192–1198
Kanal E, Maravilla K, Rowley HA (2014) Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 35:2215–2226
Khawaja AZ, Cassidy DB, Al Shakarchi J et al (2015) Revisiting the risks of MRI with gadolinium based contrast agents-review of literature and guidelines. Insights Imaging 6:553–558
ACR manual on contrast media version 10.1 (2015) http://www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed 7 Dec 2016
Gibby WA, Gibby KA, Gibby WA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Invest Radiol 39:138–142
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Invest Radiol 41:272–278
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Matsuda M, Oba H et al (2015) Gadolinium deposition after contrast-enhanced MR Imaging. Radiology 277:924–925
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Leyendecker JR (2009) Gadoxetate disodium for contrast magnetic resonance imaging of the liver. Gastroenterol Hepatol 5:698
Rigsby CK, Popescu AR, Nelson P et al (2015) Safety of blood pool contrast agent administration in children and young adults. AJR Am J Roentgenol 205:1114–1120
Trout AT, Dillman JR, Ellis JH et al (2011) Patterns of intravenous contrast material use and corticosteroid premedication in children--a survey of Society of Chairs of Radiology in Children’s Hospitals (SCORCH) member institutions. Pediatr Radiol 41:1272–1283
Hayes L, Coley B, Karmazyn B et al (2012) Headache – Child. ACR appropriateness criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Beaman F, von Herrmann P, Kransdorf M et al (2016); Suspected osteomyelitis, septic arthritis, or soft tissue infection. ACR Appropriateness Criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Seidenwurm D, Wippold F, Cornelius R et al (2012) Neuroendocrine Imaging. ACR Appropriateness Criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Ho V, Biko D, White R et al. (2011) Known or suspected congenital heart disease in the adult. ACR Appropriateness Criteria. acsearch.acr.org/list. Accessed 7 Dec 2016
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 165 kb)
Rights and permissions
About this article
Cite this article
Blumfield, E., Moore, M.M., Drake, M.K. et al. Survey of gadolinium-based contrast agent utilization among the members of the Society for Pediatric Radiology: a Quality and Safety Committee report. Pediatr Radiol 47, 665–673 (2017). https://doi.org/10.1007/s00247-017-3807-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3807-z