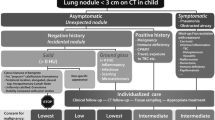
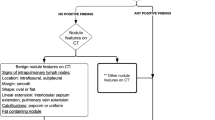
Abstract
No guidelines are in place for the follow-up and management of pulmonary nodules that are incidentally detected on CT in the pediatric population. The Fleischner guidelines, which were developed for the older adult population, do not apply to children. This review summarizes the evidence collected by the Society for Pediatric Radiology (SPR) Thoracic Imaging Committee in its attempt to develop pediatric-specific guidelines.
Small pulmonary opacities can be characterized as linear or as ground-glass or solid nodules. Linear opacities and ground-glass nodules are extremely unlikely to represent an early primary or metastatic malignancy in a child. In our review, we found a virtual absence of reported cases of a primary pulmonary malignancy presenting as an incidentally detected small lung nodule on CT in a healthy immune-competent child.
Because of the lack of definitive information on the clinical significance of small lung nodules that are incidentally detected on CT in children, the management of those that do not have the typical characteristics of an intrapulmonary lymph node should be dictated by the clinical history as to possible exposure to infectious agents, the presence of an occult immunodeficiency, the much higher likelihood that the nodule represents a metastasis than a primary lung tumor, and ultimately the individual preference of the child’s caregiver. Nodules appearing in children with a history of immune deficiency, malignancy or congenital pulmonary airway malformation should not be considered incidental, and their workup should be dictated by the natural history of these underlying conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As members of the Society for Pediatric Radiology (SPR) Thoracic Imaging Committee, we appreciate the prior comments made by Dr. Paul Thacker [1], now a committee member, in his Letter to the Editor of this journal on the need for practice guidelines on how to manage pulmonary nodules incidentally detected on CT studies in children. The Fleischner guidelines [2], which are based on a careful evaluation of a large number of studies that specifically address incidental pulmonary nodules detected in adults older than 35, were issued nearly a decade ago. However, the Fleischner recommendation to perform a single low-dose follow-up CT at 6–12 months for managing a pulmonary nodule in patients younger than 35 years does not apply to children because the only study discussing young individuals with lung nodules, on which this recommendation is based [3], did not include patients younger than 21 years. Although a few publications in the medical literature specifically address pulmonary nodules in pediatric oncology patients [4–6], we are aware of only one recently published uncontrolled retrospective study applicable to pulmonary nodules detected on abdominal CT scans in children [7].
In this vacuum of useful information fuelled by today’s risk-averse climate, we suspect that many radiologists are inappropriately recommending follow-up CT scans of these lesions [8, 9], resulting in numerous children being subjected to the risks of unnecessary radiation and potential sedation, and their caregivers to the expense and stress associated with these studies that are recommended by criteria developed for adults. In a recent opinion article, authored by Stephen Swensen [9] from the Mayo Clinic and co-authored by a variety of experts in patient safety, bioethics and consumer advocacy, the suggestion was made that follow-up with chest CT of a 3-mm lung nodule incidentally detected on a pediatric CT scan for appendicitis would be “inappropriate for this situation.” As pediatric imagers, we should not yield to the temptation to make follow-up recommendations that are not rooted in sound scientific evidence, merely to obviate potential lawsuits. Navigating between the two extremes of ignoring pulmonary nodules and issuing unfounded and overly risk-averse recommendations for imaging follow-up may require that pediatric radiologists be prepared to engage in direct personal dialogue with referring physicians, pediatric patients and their caregivers.
Lung cancer in children
In the most recent review article on pediatric lung cancer [10], there is no reported initial presentation as an incidental pulmonary nodule — these rare tumors are often aggressive and tend to be symptomatic at the time of diagnosis [11–14].
Primary bronchogenic carcinoma is extremely rare in children and adolescents, and rare reported cases of squamous cell carcinoma present with a large tumor with a secondary respiratory infection [15]. In addition, squamous cell carcinoma can occur secondary to recurrent respiratory papillomatosis in children, and this underlying condition is usually longstanding and well known [16]. There is one case report of a 2.5-cm squamous cell carcinoma, detected in Japan in 1974 with tuberculosis fluoroscopy screening in an asymptomatic 15-year-old [17].
Pleuropulmonary blastoma, the most common primary lung malignancy of childhood, usually presents before the age of 6 years, and has a varied appearance ranging from cystic to solid [18] but is typically a larger mass when solid and is not a diagnostic consideration in the setting of an incidental small solid pulmonary nodule. The cystic form of pleuropulmonary blastoma can be small [13] but should not be confused with a solid pulmonary nodule.
Neoplasms associated with the central airways (e.g., carcinoids, mucoepidermoid carcinomas) are nearly always symptomatic at the time of diagnosis and usually present as an endobronchial mass with post-obstructive atelectasis or pneumonitis [13, 19–22]; their presentation as an asymptomatic pulmonary nodule has not been reported in the pediatric population. There is an isolated case report of a carcinoid tumor with ectopic adrenocorticotropic hormone (ACTH) secretion leading to Cushing syndrome in a child, demonstrated on CT as a 2-cm peripheral solid pulmonary nodule [23].
Leiomyomas have presented as multiple pulmonary nodules on CT in a child with human immunodeficiency virus [24], and a pulmonary leiomyosarcoma has been described in a symptomatic recipient of a renal transplant following bilateral Wilms tumor resection [25]. Since the literature review in the latter publication [25] linked these smooth muscle tumors to the immunosuppression caused by chronic infection with the Epstein-Barr virus (similar to in post transplant lymphoproliferative disease), these nodular lung lesions cannot truly be regarded as “incidental”.
Nodule definition
When reporting a pulmonary nodule detected on a CT scan in a child, it is important to employ uniform terminology [6, 26]. The Fleischner Society in its glossary of terms for thoracic imaging [27] defines pulmonary nodules broadly, describing a pulmonary nodule on CT as “a rounded or irregular opacity, well or poorly defined, measuring up to 3 cm in diameter. Nodules can be solid (soft tissue attenuation), non-solid (ground-glass attenuation), or part-solid (consisting of both ground-glass and solid soft-tissue attenuation components).”
Descriptively, ground-glass opacity is defined as an area of increased lung attenuation with preserved bronchial and vascular markings. Through attenuation measurements in a slice that is sufficiently thin and that passes though the largest circumference of the nodule (so to obviate partial volume averaging errors), a ground-glass nodule is defined by obtaining Hounsfield values lower than zero throughout the nodule, as opposed to a solid nodule, which has Hounsfield values all greater than zero. It should be noted that even in the adult literature, there remains confusion as to the semantics of nodule characterization: some have objected to the use of the term “ground-glass nodule (or opacity)” in the Fleischner definition and would prefer that the descriptors “solid and sub-solid (or part-solid)” be used in reference to lung nodules [28]. Unfortunately, the broad definition of a nodule in the Fleischner glossary has resulted in the use of the term “nodule” for any small pulmonary opacity (colloquially designated as a “ditzel”) encountered on chest CT in children.
However, many of these nodules detected in children as defined by the Fleischner criteria are in fact ill-defined, irregular or linear in shape, or have ground-glass characteristics. In general, the presumption is made that the majority of these lesions represent infection/inflammation, scarring or microatelectasis [29], but this is somewhat uncertain because of the lack of systematic follow-up of these lesions. This knowledge deficit prompted us to review the existing literature on nodule characteristics with regard to their clinical significance in pediatric care.
Nodule characteristics
In a recent study of pulmonary nodules detected on screening CT in older adults [30], the following factors were associated with primary bronchogenic carcinoma: larger size, lower number, location in upper lobes, positive family history for lung cancer, and a spiculated border. The available literature on the characteristics of pediatric pulmonary nodules is largely limited to those detected in children with a known extrapulmonary malignancy [31]. In a series of 81 nodules detected in 41 such children [5], sharply defined nodules were more likely to be malignant, and nodule size was not correlated with malignancy. However, in another series of 30 children with osteosarcoma [4], and in a third series of 111 children with a variety of extrapulmonary malignant primaries [6], a nodule size over 5 mm was associated with a higher chance of the nodule being malignant, whereas nodule size and morphology criteria were found to be less helpful to correctly identify benign nodules.
Solid or lobular nodules with sharp borders are most suggestive of metastatic disease in the setting of known primary extrapulmonary malignancy with a proclivity for lung metastases, such as Wilms tumor, sarcoma, hepatoblastoma or testicular carcinoma. Metastatic disease is much more likely to be a cause of a malignant nodule in a child than is a primary lung tumor [2, 14, 29]. Calcification is common in some pediatric metastatic nodules, particularly those of osteosarcoma. Cavitation also occurs occasionally. Even when there is a known underlying malignancy, about 1/3 of biopsied pulmonary nodules are found to be benign with etiologies that include granulomatous diseases, infections, inflammatory myofibroblastic lesion, drug reaction, scarring and intrapulmonary lymph nodes [32]. The latter category of a solid pulmonary nodule in a child is usually characterized by its predominantly subcarinal location, distribution within 15 mm of a pleural surface (including a fissure), an oval or polygonal shape, and its connection with the pleura via linear extensions (pleural tags) [33, 34].
In older adults, primary adenocarcinoma of the lung (in particular its subdivision bronchioalveolar carcinoma) has been described as frequently exhibiting a growth pattern respecting the interstitial architecture of the lung (the “lepidic” growth pattern, as described by Heitzman [35]), giving rise to ground-glass attenuation in the lung parenchyma [36–38], as opposed to the “hilic” pattern of growth, where the lung parenchyma is displaced by the enlarging solid mass, which characterizes more aggressive cancers. Whereas the significance of ground-glass nodules as a precursor to adenocarcinoma in adults is debated [39–41], and because this tumor predominates in younger adults (especially in women) with lung cancer without a history of smoking [42–44], we researched the literature on adenocarcinoma, in particular bronchioalveolar carcinoma, in children.
Brody and Mark [45] reported a large bronchioalveolar carcinoma in a 15-year-old boy with the clinical presentation of a progressive lung consolidation. Dosanjh [46] reported a well-differentiated mucin-producing bronchioalveolar carcinoma in a 15-year-old immigrant girl with a paucity of symptoms and an indolent clinical course who eventually presented with multiple unresectable cavitary lung lesions demonstrated on CT. We found a more recent report of two children in their early teens who presented with findings of associated chronic infection including pulmonary tuberculosis [47].
Bronchioalveolar carcinoma has been encountered in children and adolescents treated for extrathoracic malignancies [48–51]. Okui et al. [50] reported a 14-year-old girl who presented with a large solitary osteosarcoma metastasis and who had a co-existing 3-mm ground-glass nodule on CT that demonstrated little progression over a year but was histologically proved to be a synchronous bronchioalveolar carcinoma.
A recent review article on the risk of malignancy in pulmonary cysts in early childhood [52] summarized 21 cases of bronchioalveolar carcinoma reported in patients with type 1 (large cyst type) congenital pulmonary airway malformation (CPAM) of the lung, with a median age of 18 years (range 6 to 75 years). Most of these occurred as incidental findings [53, 54], but some presented with symptoms of recurrent pneumonia [55] or metastatic disease [56, 57]. For this reason, tissue sampling of all detected lung nodules has been recommended in children with a history of extrathoracic malignancies [50] or CPAM [53].
The second of the two cases of bronchioalveolar carcinoma reported by Ohye et al. [55] occurred in an asymptomatic 15-year-old girl. In this case a 2.5-cm nodule was incidentally detected on chest radiography and was successfully resected after “persistence of the roentgenographic finding”; however, details of the imaging time interval and the appearance on CT were not reported. Perhaps with the exception of this case, none of the reported pediatric bronchioalveolar carcinoma cases had an initial presentation as an incidental pulmonary (solid or ground-glass) nodule on CT.
It appears from our review that in children, unlike in adults, a true primary neoplastic lung lesion occurs as a solid mass with a hilic pattern of growth or as a mixed solid–cystic or completely cystic mass, as may be the case in a pleuropulmonary blastoma. When growing centrifugally, it displaces rather than infiltrates the surrounding lung parenchyma, and when small it does not engulf any aerated lung tissue. In an asymptomatic child who has no history of an extrathoracic malignancy or a CPAM, we did not find any evidence that when ground-glass attenuation is demonstrated within the majority of an incidentally detected nodule, this could signify the presence of a malignancy. We therefore suggest that in such children, the category of ground-glass nodules should be dealt with separately from entirely solid nodules.
A related issue is the significance of the halo sign, i.e. the presence of ground-glass opacity surrounding a solid pulmonary nodule. This has traditionally been regarded as a specific sign for invasive pulmonary aspergillosis [58, 59] but has also been described in many other entities leading to hemorrhage or infiltration in the surrounding lung parenchyma. These include infectious/inflammatory conditions (e.g., other fungal infections, granulomatosis with polyangiitis [formerly referred to as Wegener granulomatosis], tuberculosis, bacterial and viral infections) and neoplastic conditions (through lepidic tumor infiltration in the surrounding lung parenchyma such as in lymphoma or bronchioalveolar carcinoma, or through rapid growth leading to surrounding pulmonary hemorrhage or infarction, as has been described around large osteosarcoma metastases in a child [60]). All of these conditions with a positive halo sign are unlikely to present in asymptomatic children with a negative clinical history, and require a condition-specific workup.
In summary, existing guidelines for detection and follow-up are based on the relatively high frequency of bronchogenic carcinoma in older adults. Childhood lung cancer is a different disease, and it would probably be necessary to follow many thousands of nodules to identify even one early lung cancer, which is not a practical endeavor. Unfortunately, because of the anecdotal information referenced above, we cannot definitively state that a lung cancer cannot possibly begin as an asymptomatic small nodule in a child. There is simply not enough evidence accumulated from a sufficient number of nodule CT follow-up studies to reliably predict the natural history of these lesions in an individual case.
Some institutions may have good long-term follow-up of children with asymptomatic pulmonary nodules, which could be used as a surrogate for benign nodules, if stability on follow-up is reasonably long enough. We request that if any readers have anecdotal evidence of the transition of an incidental asymptomatic pulmonary nodule into a confirmed primary malignancy, that this be reported to the SPR Thoracic Imaging Committee. Failing the accumulation of such evidence, we should discourage applying the Fleischner algorithm to children, but unfortunately we are not in a position to propose an alternative set of guidelines. In fact, any official promulgation of such guidelines by the SPR would give the impression of more certainty than exists, and this may cause more harm than good by making erroneous assumptions systemic rather than isolated. Because of the extremely small number of primary malignancies that present initially as asymptomatic pulmonary nodules, such guidelines are unlikely to be developed for our patients. What should be recognized is that there is a likelihood of harm, rather than benefit, in applying adult guidelines to nodules identified in children. Therefore, the management of these lesions comes down to using clinical judgment in an individualized setting.
Conclusion
-
(1)
Although there are very few data, the incidence of a small solid lung nodule evolving into a primary lung cancer in an otherwise healthy (immune-competent) child appears to be extremely low. The benefit of routine follow-up CT scanning in children is doubtful and potentially harmful because of the additional exposure to ionizing radiation or the detection of additional insignificant incidental findings. However, careful discussion and consideration should be given to individual cases.
-
(2)
Linear opacities are more likely related to prior infection, inflammation or atelectasis and should not be considered solid nodules.
-
(3)
Ground-glass opacities in children are likely caused by infection or inflammation and are very unlikely to represent a malignancy in an asymptomatic child without a history of malignancy. Likewise, the presence of the halo sign of ground-glass opacity surrounding a solid pulmonary nodule is often indicative of an aggressive lesion with hemorrhage or infiltration in the adjacent lung parenchyma and is very unlikely to be encountered in lung nodules that are truly detected incidentally.
-
(4)
Malignant lung nodules in children are much more likely to be caused by metastatic disease rather than primary lung tumor.
References
Thacker PG (2013) The incidental pulmonary nodule: an impetus for guidelines. Pediatr Radiol. doi:10.1007/s00247-013-2762-6
MacMahon H, Austin JH, Gamsu G et al (2005) Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 237:395–400
Gadgeel SM, Ramalingam S, Cummings G et al (1999) Lung cancer in patients <50 years of age: the experience of an academic multidisciplinary program. Chest 115:1232–1236
Brader P, Abramson SJ, Price AP et al (2011) Do characteristics of pulmonary nodules on computed tomography in children with known osteosarcoma help distinguish whether the nodules are malignant or benign? J Pediatr Surg 46:729–735
McCarville MB, Lederman HM, Santana VM et al (2006) Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology 239:514–520
Silva CT, Amaral JG, Moineddin R et al (2010) CT characteristics of lung nodules present at diagnosis of extrapulmonary malignancy in children. AJR Am J Roentgenol 194:772–778
Breen M (2014) Clinical significance of incidental pulmonary nodules detected on abdominal CT in pediatric patients. Pediatr Radiol 44:S105–S106
Feely MA, Hartman TE (2011) Inappropriate application of nodule management guidelines in radiologist reports before and after revision of exclusion criteria. AJR Am J Roentgenol 196:1115–1119
Swensen SJ, Duncan JR, Gibson R et al (2014) An appeal for safe and appropriate imaging of children. J Patient Saf 10:121–124
Dishop MK, Kuruvilla S (2008) Primary and metastatic lung tumors in the pediatric population: a review and 25-year experience at a large children’s hospital. Arch Pathol Lab Med 132:1079–1103
Lal DR, Clark I, Shalkow J et al (2005) Primary epithelial lung malignancies in the pediatric population. Pediatr Blood Cancer 45:683–686
Keita O, Lagrange JL, Michiels JF et al (1995) Primary bronchogenic squamous cell carcinoma in children: report of a case and review of the literature. Med Pediatr Oncol 24:50–52
Hartman GE, Shochat SJ (1983) Primary pulmonary neoplasms of childhood: a review. Ann Thorac Surg 36:108–119
Cohen MC, Kaschula RO (1992) Primary pulmonary tumors in childhood: a review of 31 years’ experience and the literature. Pediatr Pulmonol 14:222–232
Shelley BE, Lorenzo RL (1983) Primary squamous cell carcinoma of the lung in childhood. Pediatr Radiol 13:92–94
Cook JR, Hill DA, Humphrey PA et al (2000) Squamous cell carcinoma arising in recurrent respiratory papillomatosis with pulmonary involvement: emerging common pattern of clinical features and human papillomavirus serotype association. Mod Pathol 13:914–918
Niitu Y, Kubota H, Hasegawa S et al (1974) Lung cancer (squamous cell carcinoma) in adolescence. Am J Dis Child 127:108–111
Priest JR (2012) Pleuropulmonary blastoma. In: Schneider DT, Brecht IB (eds) Rare tumors in children and adolescents. Pediatric oncology. Springer Verlag, Berlin, pp 213–221
Curtis JM, Lacey D, Smyth R et al (1998) Endobronchial tumours in childhood. Eur J Radiol 29:11–20
Lack EE, Harris GB, Eraklis AJ et al (1983) Primary bronchial tumors in childhood. A clinicopathologic study of six cases. Cancer 51:492–497
Rizzardi G, Bartolaccini L, Terzi A (2011) Bronchial carcinoid tumors in children — a review. Eur Oncol Hematol 7:196–199
Wang LT, Wilkins EW Jr, Bode HH (1993) Bronchial carcinoid tumors in pediatric patients. Chest 103:1426–1428
De Matos LL, Trufelli DC, Das Neves-Pereira JC et al (2006) Cushing’s syndrome secondary to bronchopulmonary carcinoid tumor: report of two cases and literature review. Lung Cancer 53:381–386
Chadwick EG, Connor EJ, Hanson IC et al (1990) Tumors of smooth-muscle origin in HIV-infected children. JAMA 263:3182–3184
Suzuki K, Urushihara N, Fukumoto K et al (2011) A case of Epstein-Barr virus-associated pulmonary leiomyosarcoma arising five yr after a pediatric renal transplant. Pediatr Transplant 15:E145–148
Naidich DP (2010) Part-solid nodules: two steps forward. Radiology 255:16–18
Hansell DM, Bankier AA, MacMahon H et al (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722
Miettinen OS, Henschke CI, Smith JP et al (2014) Is ground glass descriptive of a type of pulmonary nodule? Radiology 270:311–312
Eggli KD, Newman B (1993) Nodules, masses, and pseudomasses in the pediatric lung. Radiol Clin N Am 31:651–666
McWilliams A, Tammemagi MC, Mayo JR et al (2013) Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 369:910–919
Robertson PL, Boldt DW, De Campo JF (1988) Paediatric pulmonary nodules: a comparison of computed tomography, thoracotomy findings and histology. Clin Radiol 39:607–610
Rosenfield NS, Keller MS, Markowitz RI et al (1992) CT differentiation of benign and malignant lung nodules in children. J Pediatr Surg 27:459–461
Alpert JB, Naidich DP (2011) Imaging of incidental findings on thoracic computed tomography. Radiol Clin N Am 49:267–289
Edey AJ, Hansell DM (2009) Incidentally detected small pulmonary nodules on CT. Clin Radiol 64:872–884
Heitzman ER (1984) The lung: radiologic–pathologic correlations. Mosby, Maryland Heights
Lee HY, Lee KS (2011) Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 26:106–118
Garfield DH, Cadranel JL, Wislez M et al (2006) The bronchioloalveolar carcinoma and peripheral adenocarcinoma spectrum of diseases. J Thorac Oncol 1:344–359
Lee KS, Kim Y, Han J et al (1997) Bronchioloalveolar carcinoma: clinical, histopathologic, and radiologic findings. Radiographics 17:1345–1357
MacMahon H, Bankier AA, Naidich DP (2013) Response. Radiology 268:306–307
Naidich DP, Bankier AA, MacMahon H et al (2013) Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 266:304–317
Bommart S, Kovacsik HV, Pujol JL et al (2013) Management of part-solid nodules. Radiology 268:306–307
DeCaro L, Benfield JR (1982) Lung cancer in young persons. J Thorac Cardiovasc Surg 83:372–376
Ramalingam S, Pawlish K, Gadgeel S et al (1998) Lung cancer in young patients: analysis of a surveillance, epidemiology, and end results database. J Clin Oncol 16:651–657
Liu NS, Spitz MR, Kemp BL et al (2000) Adenocarcinoma of the lung in young patients: the M. D. Anderson experience. Cancer 88:1837–1841
Brody JS, Mark EJ (1976) Case 4–1976. N Engl J Med 294:210–217
Dosanjh A (1992) Bronchioalveolar carcinoma in a 15-year-old girl. Clin Pediatr (Phila) 31:253–254
Park JA, Park HJ, Lee JS et al (2008) Adenocarcinoma of lung in never smoked children. Lung Cancer 61:266–269
Kayton ML, He M, Zakowski MF et al (2010) Primary lung adenocarcinomas in children and adolescents treated for pediatric malignancies. J Thorac Oncol 5:1764–1771
Bernardi Fdel C, Garcia JL, de Almeida MT et al (2013) Minimally invasive adenocarcinoma of the lung in a young patient treated for osteosarcoma. Pediatr Dev Pathol 16:387–390
Okui M, Goto T, Hayashi Y et al (2013) Bronchioloalveolar carcinoma as a second malignancy in a pediatric osteosarcoma survivor: case report. World J Surg Oncol 11:135
Travis WD, Linnoila RI, Horowitz M et al (1988) Pulmonary nodules resembling bronchioloalveolar carcinoma in adolescent cancer patients. Mod Pathol 1:372–377
Priest JR, Williams GM, Hill DA et al (2009) Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol 44:14–30
Granata C, Gambini C, Balducci T et al (1998) Bronchioloalveolar carcinoma arising in congenital cystic adenomatoid malformation in a child: a case report and review on malignancies originating in congenital cystic adenomatoid malformation. Pediatr Pulmonol 25:62–66
West D, Nicholson AG, Colquhoun I et al (2007) Bronchioloalveolar carcinoma in congenital cystic adenomatoid malformation of lung. Ann Thorac Surg 83:687–689
Ohye RG, Cohen DM, Caldwell S et al (1998) Pediatric bronchioloalveolar carcinoma: a favorable pediatric malignancy? J Pediatr Surg 33:730–732
Kaslovsky RA, Purdy S, Dangman BC et al (1997) Bronchioloalveolar carcinoma in a child with congenital cystic adenomatoid malformation. Chest 112:548–551
Ramos SG, Barbosa GH, Tavora FR et al (2007) Bronchioloalveolar carcinoma arising in a congenital pulmonary airway malformation in a child: case report with an update of this association. J Pediatr Surg 42:E1–4
Kim Y, Lee KS, Jung KJ et al (1999) Halo sign on high resolution CT: findings in spectrum of pulmonary diseases with pathologic correlation. J Comput Assist Tomogr 23:622–626
Pinto PS (2004) The CT halo sign. Radiology 230:109–110
Tomiyama N, Ikezoe J, Miyamoto M et al (1994) CT halo sign in metastasis of osteosarcoma. AJR Am J Roentgenol 162:468
Conflicts of interest
Dr. Podberesky discloses the following relationships: Toshiba of America Medical Systems (speaker’s bureau), GE Healthcare, Philips Healthcare and Siemens Healthcare (travel reimbursement), Guerbet (consultant), Amirsys (chapter royalties).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westra, S.J., Brody, A.S., Mahani, M.G. et al. The incidental pulmonary nodule in a child. Pediatr Radiol 45, 628–633 (2015). https://doi.org/10.1007/s00247-014-3267-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3267-7