Abstract
The incidental detection of small lung nodules in children is a vexing consequence of an increased reliance on CT. We present an algorithm for the management of lung nodules detected on CT in children, based on the presence or absence of symptoms, the presence or absence of elements in the clinical history that might explain these nodules, and the imaging characteristics of the nodules (such as attenuation measurements within the nodule). We provide suggestions on how to perform a thoughtfully directed and focused search for clinically occult extrathoracic disease processes (including malignant disease) that may present as an incidentally detected lung nodule on CT. This algorithm emphasizes that because of the lack of definitive information on the natural history of small solid nodules that are truly detected incidentally, their clinical management is highly dependent on the caregivers’ individual risk tolerance. In addition, we present strategies to reduce the prevalence of these incidental findings, by preventing unnecessary chest CT scans or inadvertent inclusion of portions of the lungs in scans of adjacent body parts. Application of these guidelines provides pediatric radiologists with an important opportunity to practice patient-centered and evidence-based medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“Medicine is a science of uncertainty and an art of probability.”— William Osler (1849–1919).
The question of how to practically manage incidentally detected pulmonary nodules fits within the broader issue of how to handle incidental findings (incidentalomas) that are increasingly identified in children as a result of a higher reliance on advanced cross-sectional imaging studies [1–4]. This issue, as it pertains to the adult population, has received attention in the lay news media [5–7] and in a recent bioethical directive by the Obama administration [8] and is only recently being formally recognized by the profession and addressed in review articles [3, 9]. Yet the detection of incidentalomas occurs with relative frequency in children as well (mainly adrenal [10] and pulmonary in origin [11]), presumably related to the increase in the use of pediatric CT in recent years [12, 13]. This, in combination with recent rapid technological advances in CT, has allowed for the routine detection of lung nodules as small as 1–2 mm on scans that cover all or part of the chest [11, 14].
Risks are inherent to both too much and too little attention to incidentally detected findings on advanced cross-sectional imaging studies. Legal authorities argue that in our era of patient-centered care, characterized by shared-informed decision-making, there is really no place for the older paternalistic approach by treating physicians to dismiss or ignore incidental findings [1, 2, 8]. Along similar lines, one could argue that it is no longer appropriate for radiologists to simply describe a pulmonary nodule that is incidentally detected without providing guidance on how to manage it. By abetting in and facilitating the increased use of CT for indications that did not exist 20 years ago, we have indirectly helped create this problem, and we believe our profession is now ethically obligated to help develop a solution [11].
Although it is usually the responsibility of the patient’s personal physician to directly communicate with the family and to determine further care, the radiologist is likely to have greater experience in the management of incidentally detected pulmonary nodules. Sharing such experience by providing suggestions for follow-up is likely to be appreciated by the referring physician, and may well lead to improved patient care.
Clinical management
With the exception of the discussion following a case report of a benign lung nodule in the French literature [15] and the above-referenced book chapter [11], there is a lack of published clinical guidelines that are truly and scientifically evidence-based for the management of an incidentally identified pulmonary nodule in a child. In a recent retrospective study of 62 children with lung nodules incidentally detected on a total of 7,912 abdominal CT scans performed over 7 years at a large children’s hospital [16], of the 31 children who had CT follow-up only two were found to harbor malignancy, and both had a history of underlying extrapulmonary malignant disease. This fact exposes a weakness of this study, since by including these patients in their study group, these authors used a broad definition of an “incidental pulmonary nodule on CT.” Tellingly, in none of the remaining 31 children, who had no history of extrapulmonary malignancy and who had no CT follow-up, was a malignancy detected on clinical follow-up. Based on this, the authors suggested that follow-up CT is not needed in incidentally detected lung nodules in children in the absence of a history of malignancy [15]. Despite the findings of this single retrospective study, the identification of a solid nodule in a child without a history of malignancy not having the characteristics of an intrapulmonary lymph node remains a vexing situation. Should the child be screened for a clinically occult malignancy? Should the child be evaluated for granulatomatous disease or other non-neoplastic process that can be associated with solid pulmonary nodules? If the nodule is incidentally identified in a lung base included on an abdominal CT, should the remainder of the lungs be imaged by a complete chest CT or a chest radiograph?
A potentially more effective way to address these questions would be to develop estimates of the likelihood that a child would have a previously undetected primary extrapulmonary malignancy that would present with a metastatic lung nodule on a CT scan performed for unrelated reasons. Reasonable risk estimates could be placed on the different outcomes of this scenario, and risk/benefit ratios may be calculated. Based on the outcome of this risk analysis, one could propose to the family an approach of “watchful waiting” (which may not include any follow-up imaging) after consideration of the following (adapted, with permission, from [11]):
-
(1)
Is the child otherwise healthy, growing and developing well, or is there any significant weight loss, hemoptysis, respiratory distress, lymphadenopathy or paraneoplastic syndrome that persists after the acute illness or trauma that precipitated the CT scan resolves?
-
(2)
Does the history and physical exam reveal anything more than what would be expected from the chief complaint that prompted the CT scan?
-
(3)
What is the pre-test probability, e.g., does the child live in an endemic area for pulmonary granulomatous disease? Does the child have a known history of aspiration, immunodeficiency, or an extrapulmonary malignancy? This may be supported by a basic clinical workup for infectious/granulomatous disease, immune disorder and screening for occult oncological disease. For the latter, if an imaging test is believed to be indicated, we might consider one that does not use ionizing radiation and that is age-appropriate (e.g., a screening abdominal US to rule out an occult Wilms tumor in a child younger than 5 years).
-
(4)
Has there been a pertinent history of travel, exposure or inhalation?
-
(5)
Can the family commit to a plan of watchful waiting, and is the family reliable to present for scheduled follow-up?
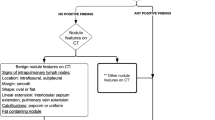
Figure 1 summarizes a conceptual framework that is helpful in the diagnostic management of a lung nodule detected on CT in a child. Please note that although this diagram has the appearance of a flow chart or algorithm, with a few exceptions all branches of this chart lead to the same recommendation to provide individualized care guided by the post-test level of concern that the nodule may be malignant, and by the degree of tolerance for risk of the child’s caregivers, the referring physician and the interpreting radiologist.
Flow chart illustrates a conceptual framework for the diagnostic management of a lung nodule measuring up to 3 cm in diameter on CT in a child. Note that this is not a classic flowchart algorithm for mutually exclusive categories. It indicates that our suggested general management strategy is identical for all categories (with the exception of a classic benign nodule), namely individualized care, as influenced by our differential post-test level of concern for malignancy (displayed as being connected by vertical lines to the appropriate category), and by the degree of tolerance for risk of the caregivers
Communication with the family
The appropriate mechanism for communicating directly with the family depends on the individual radiologist, referring physician and local care paradigm. When engaging in a discussion with a family in whom a child was diagnosed with (an) incidental pulmonary nodule (s), especially when any degree of cancer phobia is detected in that family, a balanced discussion of the merits and potential harms of imaging follow-up might include an attempted individual estimate of the cancer risk from diagnostic CT [17–19] vis-à-vis a projection of the risk that the nodule (s) may represent an early manifestation of a pulmonary (or, more likely, extrapulmonary) malignancy. The latter risk, as was discussed in part 1 of this review, is currently unknown but is projected to be extremely low. In addition to the potential radiation risk, the risk of percutaneous biopsy or thoracoscopic surgery (as well as that related to sedation or anesthesia) may be discussed, as appropriate, in case a decision is made to proceed to tissue sampling of the nodule (s) in question. The careful conduction of such a discussion and the documentation of the understanding of the risks and benefits of follow-up with CT satisfy the requirement of informed decision-making as advocated by the Image Gently campaign and other authorities [20–23]. Physicians who engage in such discussions with parents and other caregivers should be familiar with the principles of risk communication [24–27]. The anxiety caused by the communication to parents that “something abnormal” (such as an incidental lung nodule) was found while scanning their child may be magnified in families who have experienced a diagnosis of lung cancer — perhaps by one of the CT screening studies now being advocated for older adults with risks factors. They might respond differently to hearing about risk that is perceived to be immediate (i.e. “we cannot rule out that this lung nodule is an early lung cancer”) vs. one that occurs in the distant future (“repeated CT scans to follow up this nodule might cause cancer in 20 years”), irrespective of the relative magnitudes of the risks that are communicated. There will be families who cannot tolerate the anxiety associated with any degree of uncertainty and opt for immediate resolution (“when it is abnormal take it out now”); others will opt for imaging follow-up (“how often will my child need to be scanned to be sure?”); and yet others might agree with a plan for watchful waiting without follow-up imaging. No matter what the outcome of this discussion and irrespective of our own opinions on this matter as health care providers, it befits the modern paradigm of personalized medicine and informed decision-making to respect the decision of the family as an exercise of patient autonomy [28, 29]. The careful and ethical conduction of such a consultation with the family is, therefore, more an art than a science [30], and needs to be properly recognized as valuable and accountable care that radiologists may engage in within the future financial structure of the health care system [31].
Prevention
A more fundamental way to address this problem is to lower the frequency of incidentally detected lung nodules by discouraging the use of chest CT for unproven clinical indications [4, 32–35] through the rigorous justification of CT [36]. Examples of this are the substitution of chest CT by radiography [37] or fast MRI [38, 39] for the evaluation of minor chest wall deformities [40] such as pectus excavatum. In the care of the multi-trauma patient who sustained blunt injury to the torso, the omission of the routine chest CT from the initial total body scan has been shown not to influence patient management or outcomes, because (with the exception of suspected traumatic aortic injury) all clinically relevant chest injuries are adequately demonstrated on the upper slices of the abdominal scan acquisition [33, 41, 42]. Chest CT has been shown to have the highest yield in influencing patient management in certain congenital heart conditions, congenital lung malformations, febrile neutropenia, screening for pulmonary metastases, bronchiectasis and certain diffuse lung diseases [34], but it has less influence in focal parenchymal and pleural diseases in otherwise healthy children.
The value of chest CT for the characterization of diffuse lung diseases when no biopsy is indicated has been questioned [43], but some diffuse lung diseases such as bronchiolitis obliterans and neuroendocrine hyperplasia of infancy have highly specific CT findings that obviate the need for lung biopsy, and others such as the genetic disorders of surfactant metabolism can be suspected on the basis of CT findings and thereby prompt appropriate referral for genetic testing [44]. In all other situations, the omission of chest CT when not clinically indicated and the continued reliance on radiography should, apart from its cost- and radiation-saving aspects, also decrease the frequency of incidentally detected pulmonary nodules.
A second strategy to reduce the frequency of incidentally detected lung nodules is to inform parents of the risk of this possibility as part of an informed consent process prior to performing a chest CT, as has recently been proposed [8, 45]. This approach remains controversial even in adults, because the perceived risk may be much larger than the actual risk. In addition, such an approach has more impact in the adult population, where incidentalomas are far more prevalent than in children; the approach might occasionally be of value when a pediatric practitioner contemplates a scan merely for medicolegal reasons or because he or she succumbs to excessive pressure from parents or clinicians.
A more rewarding strategy to follow, when the decision has been made that a CT scan is indicated, is to purposefully avoid scanning (parts of) the lungs when adjacent regions are scanned. For instance, when abdominopelvic CT is done to evaluate for a localized disease process, such as appendicitis or renal stones, the lung bases need not routinely be included. When upper extremities are scanned, the inadvertent inclusion of the lungs within the field of view can be avoided by raising the extremities. Such strategies avoid causing potential harm by “looking in the wrong places” [46], but they require pediatric imagers to carefully adhere to a focused, problem-oriented approach that has traditionally distinguished our subspecialty from the “rule-out” and “early detection through screening” mindset that prevails in adult medicine. More to the point, this requires us to have a more active role in the protocolling of studies and the real-time reviewing of preliminary findings when our patients are on the scanner, to decide whether the clinical question has been adequately addressed, or whether there is an indication to extend the scan coverage over adjacent regions such as the chest. In this sense, pediatric imagers have a unique opportunity more than in any other field of radiology to practice personalized, patient-centered medicine, in which these decisions are rooted in principles of cost-effectiveness, beneficence and evidence-based practice rather than in a futile quest for absolute certainty in all clinical scenarios [47].
Conclusion
Because of the lack of definite information on the natural history of small lung nodules that are truly detected incidentally on a CT scan in a child, no general guidelines for the management of these nodules can be formulated. Therefore management should be individualized, dependent on the risk tolerance of the caregivers. Elements to consider include the presence or absence of symptoms and clues in the clinical history that may explain these nodules, as well their imaging characteristics. A focused search for clinically occult extrathoracic disease processes (including malignant disease), immune deficiency and granulomatous disease may also be indicated. In order to lower the frequency of incidentally detected lung nodules in children, careful thought should be paid to preventing unnecessary chest CT or inadvertent inclusion of parts of the lungs in scans of adjacent body parts.
References
Berlin L (2013) Medicolegal: Malpractice and ethical issues in radiology: the incidentaloma. AJR Am J Roentgenol 200:W91
Berlin L (2013) How do you solve a problem like incidentalomas? Applied Radiology, February 2013. http://cdn.agilitycms.com/applied-radiology/PDFs/Issues/2013-02-Articles-AR_02-13_Berlin.pdf. Accessed 14 Oct 2014
Frank L, Quint LE (2012) Chest CT incidentalomas: thyroid lesions, enlarged mediastinal lymph nodes, and lung nodules. Cancer Imaging 12:41–48
Weinstein MC, Skinner JA (2010) Comparative effectiveness and health care spending — implications for reform. New Engl J Med 362:460–465
Libby P (2010) The ‘incidentaloma’ problem with medical scans. The New York Times, 8 June 2010. http://consults.blogs.nytimes.com/2010/06/08/the-incidentaloma-problem-with-medical-scans/. Accessed 14 Oct 2014
Ofri D (2013) The fallout of a chance medical finding. The New York Times, 17 Jan 2013. http://well.blogs.nytimes.com/2013/01/17/the-fallout-of-a-chance-medical-finding/. Accessed 14 Oct 2014
Kristof N (2010) A scare, a scar, a silver lining. The New York Times, 5 June 2010. http://www.nytimes.com/2010/06/06/opinion/06kristof.html. Accessed 14 Oct 2014
Gutman A, Wagner JW, Allen AL et al (2013) Anticipate and communicate. Ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts. In: Presidential Commission for the Study of Bioethical Issues, Washington DC, Dec 2013. http://bioethics.gov/sites/default/files/FINALAnticipateCommunicate_PCSBI_0.pdf. Accessed 14 Oct 2014
Berland LL, Silverman SG, Gore RM et al (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 7:754–773
Lightner ES, Levine LS (1993) The adrenal incidentaloma. A pediatric perspective. Am J Dis Child 147:1274–1276
Cameron Coates A, Zwerdling R (2012) Pulmonary incidentalomas. In: Cleveland R (ed) Imaging in pediatric pulmonology. Springer, New York Dordrecht Heidelberg London, pp 373–375
Forghani N, Cohen RA, Abbott MB (2006) Radiation risks of CT scans. Pediatr Rev 27:79
Menoch MJ, Hirsh DA, Khan NS et al (2012) Trends in computed tomography utilization in the pediatric emergency department. Pediatrics 129:e690–697
Silva CT, Amaral JG, Moineddin R et al (2010) CT characteristics of lung nodules present at diagnosis of extrapulmonary malignancy in children. AJR Am J Roentgenol 194:772–778
Pouessel G, Thumerelle C, Santos C et al (2005) Pulmonary hamartochondroma: a rare cause of solitary pulmonary nodule in children. J Radiol 86:79–82
Breen M (2014) Clinical significance of incidental pulmonary nodules detected on abdominal CT in pediatric patients. Pediatr Radiol 44:S105–S106
Westra SJ (2014) The communication of the radiation risk from CT in relation to its clinical benefit in the era of personalized medicine: Part 1: the radiation risk from CT. Pediatr Radiol 44:S515–S518
Westra SJ (2014) The communication of the radiation risk from CT in relation to its clinical benefit in the era of personalized medicine: Part 2: benefits versus risk of CT. Pediatr Radiol 44:S525–S533
Broder JS, Frush DP (2014) Content and style of radiation risk communication for pediatric patients. J Am Coll Radiol 11:238–242
Brink JA, Goske MJ, Patti JA (2012) Informed decision making trumps informed consent for medical imaging with ionizing radiation. Radiology 262:11–14
Frush DP (2003) Responsible use of CT. Radiology 229:289–291
Goske MJ, Bulas D (2009) Improving health literacy: informed decision-making rather than informed consent for CT scans in children. Pediatr Radiol 39:901–903
Berlin L (2014) Shared decision-making: is it time to obtain informed consent before radiologic examinations utilizing ionizing radiation? Legal and ethical implications. J Am Coll Radiol 11:246–251
Calman KC (1996) Cancer: science and society and the communication of risk. BMJ 313:799–802
Edwards A, Elwyn G (2001) Understanding risk and lessons for clinical risk communication about treatment preferences. Qual Health Care 10:i9–13
Lloyd AJ (2001) The extent of patients’ understanding of the risk of treatments. Qual Health Care 101:i14–18
Redelmeier DA, Rozin P, Kahneman D (1993) Understanding patients’ decisions. Cognitive and emotional perspectives JAMA 270:72–76
Hofmann B, Lysdahl KB (2008) Moral principles and medical practice: the role of patient autonomy in the extensive use of radiological services. J Med Ethics 34:446–449
Graff J (2014) Patient perspectives on radiation dose. J Am Coll Radiol 11:243–245
Gunderman RB (2005) The medical community's changing vision of the patient: the importance of radiology. Radiology 234:339–342
Allen B Jr, Levin DC, Brant-Zawadzki M et al (2011) ACR white paper: strategies for radiologists in the era of health care reform and accountable care organizations: a report from the ACR Future Trends Committee. J Am Coll Radiol 8:309–317
Rattan AS, Laor T, Ryckman FC et al (2010) Pectus excavatum imaging: enough but not too much. Pediatr Radiol 40:168–172
Holscher CM, Faulk LW, Moore EE et al (2013) Chest computed tomography imaging for blunt pediatric trauma: not worth the radiation risk. J Surg Res 184:352–357
Schneebaum N, Blau H, Soferman R et al (2009) Use and yield of chest computed tomography in the diagnostic evaluation of pediatric lung disease. Pediatrics 124:472–479
Picano E (2004) Sustainability of medical imaging. BMJ 328:578–580
Malone J, Guleria R, Craven C et al (2012) Justification of diagnostic medical exposures: some practical issues. Report of an International Atomic Energy Agency consultation. Br J Radiol 85:523–538
Mueller C, Saint-Vil D, Bouchard S (2008) Chest X-ray as a primary modality for preoperative imaging of pectus excavatum. J Pediatr Surg 43:71–73
Marcovici PA, LoSasso BE, Kruk P et al (2011) MRI for the evaluation of pectus excavatum. Pediatr Radiol 41:757–758
Birkemeier KL, Podberesky DJ, Salisbury S et al (2012) Limited, fast magnetic resonance imaging as an alternative for preoperative evaluation of pectus excavatum: a feasibility study. J Thorac Imaging 27:393–397
Donnelly LF, Taylor CN, Emery KH et al (1997) Asymptomatic, palpable, anterior chest wall lesions in children: is cross-sectional imaging necessary? Radiology 202:829–831
Renton J, Kincaid S, Ehrlich PF (2003) Should helical CT scanning of the thoracic cavity replace the conventional chest X-ray as a primary assessment tool in pediatric trauma? An efficacy and cost analysis. J Pediatr Surg 38:793–797
Pinette W, Barrios C, Pham J et al (2012) A comparison of thoracic CT and abdominal CT for the identification of thoracic blunt trauma. Am J Surg 204:927–931
Vrielynck S, Mamou-Mani T, Emond S et al (2008) Diagnostic value of high-resolution CT in the evaluation of chronic infiltrative lung disease in children. AJR Am J Roentgenol 191:914–920
Guillerman RP, Brody AS (2011) Contemporary perspectives on pediatric diffuse lung disease. Radiol Clin North Am 49:847–868
Kole J, Fiester A (2013) Incidental findings and the need for a revised informed consent process. AJR Am J Roentgenol 201:1064–1068
Schroeder AR, Redberg RF (2013) The harm in looking. JAMA Pediatr 167:693–695
Wellbery C (2010) The value of medical uncertainty? Lancet 375:1686–1687
Acknowledgments
Although all authors are members of the Society for Pediatric Radiology (SPR) Thoracic Imaging Committee, the opinions expressed here are solely those of the authors and are not endorsed by the SPR.
Conflicts of interest
Dr. Podberesky discloses the following relationships: Toshiba of America Medical Systems (speaker’s bureau), GE Healthcare, Philips Healthcare and Siemens Healthcare (travel reimbursement), Guerbet (consultant), Amirsys (chapter royalties).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Westra, S.J., Thacker, P.G., Podberesky, D.J. et al. The incidental pulmonary nodule in a child. Pediatr Radiol 45, 634–639 (2015). https://doi.org/10.1007/s00247-014-3269-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3269-5