Abstract
Pleuropulmonary blastoma (PPB) is a malignant sarcoma of lung and pleura in children under age 6 years. PPB is in the family of dysembryonic developmental tumors of early childhood such as WT (nephroblastoma), embryonal rhabdomyosarcoma (ERMS), neuroblastoma, medulloblastoma, retinoblastoma (Rb), and others. Histopathologically, PPB recapitulates primitive pleuropulmonary mesenchyme from which it is thought to arise (Dehner 1994; Manivel et al. 1988). Because of its rarity, PPB was recognized as a diagnostic entity only in the 1980s (Dehner 1994; Manivel et al. 1987, 1988). In contrast to ∼500 Wilms tumors (WT) occurring each year in the United States, 25–50 PPBs are estimated to occur annually.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Background
Pleuropulmonary blastoma (PPB) is a malignant sarcoma of lung and pleura in children under age 6 years. PPB is in the family of dysembryonic developmental tumors of early childhood such as WT (nephroblastoma), embryonal rhabdomyosarcoma (ERMS), neuroblastoma, medulloblastoma, retinoblastoma (Rb), and others. Histopathologically, PPB recapitulates primitive pleuropulmonary mesenchyme from which it is thought to arise (Dehner 1994; Manivel et al. 1988). Because of its rarity, PPB was recognized as a diagnostic entity only in the 1980s (Dehner 1994; Manivel et al. 1987, 1988). In contrast to ∼500 Wilms tumors (WT) occurring each year in the United States, 25–50 PPBs are estimated to occur annually.
Despite its rarity, PPB is particularly important among the developmental tumors of childhood for three reasons: first, PPB has a unique, age-related spectrum of presentations and pathology from birth to age 72 months (Dehner 1994; Manivel et al. 1988; Priest et al. 1997, 2009). Second, PPB (OMIM #601200) is a strong marker for familial dysplastic and neoplastic disease in the PPB family tumor and dysplasia syndrome (PPB-FTDS) (Priest et al. 1996, 2009). Finally, the underlying genetic basis of most PPB and PPB-FTDS is mutations in the critical gene DICER1, which participates in the highly conserved RNA interference mechanism for posttranscriptional gene silencing (Hill et al. 2009; Seitz 2010). The disease spectrum of the PPB-FTDS, discussed below, appears to result from dysregulated gene silencing as a novel mechanism of childhood dysplasia and neoplasia.
2 Manifestations of PPB
PPB is classified into three interrelated clinicopathologic entities occurring from birth to age 6 years; 96% of PPBs are diagnosed by age 72 months (Fig. 25.1).
The three PPB types are based on gross pathologic morphology augmented by radiographic and microscopic evidence (Dehner 1994; Hill et al. 2008). Type I PPB is the earliest stage of tumorigenesis, may be recognized in prenatal ultrasonography (Miniati et al. 2006) and typically occurs in infancy (Fig. 25.1) (Hill et al. 2008; Priest et al. 2006). Radiographically and grossly, type I PPB is a relatively innocuous-appearing air-filled multilocular cyst in the peripheral lung parenchyma (Fig. 25.2). Until examined pathologically, type I PPB is usually considered as a congenital pulmonary airway malformation (CPAM) (Priest et al. 2009; Stocker 2002). Only rarely are type I cysts fluid filled or infected. Beneath a benign respiratory epithelium, cyst walls and septa contain a scattered, sometimes sparse, population of malignant small cells, suggesting a rhabdomyomatous lineage (Hill et al. 2008).
Type II PPB occurs generally in 1–3 year olds (Fig. 25.1) and has both type I cysts or cyst remnants and grossly visible thickened cyst walls or septa, solid mural nodules or larger tumor excrescences (Fig. 25.3). The solid tumefactions result from sarcomatous expansion of the subepithelial malignant cells of type I PPB with overgrowth of cyst walls and septa. The solid portions clearly reveal a mixed pattern sarcoma, described below.
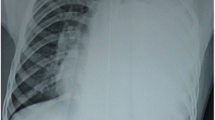
Examples of type II PPB (different patients shown). (a) Chest radiograph showing solid nodules within large air-filled cyst in left hemithorax. (b) Gross pathology showing polypoid nodules of sarcoma botryoides inside opened pulmonary cyst (Photograph courtesy of David Kelly, M.D., Children’s Health System, Birmingham, AL, USA)
Type III PPB (Fig. 25.4a) is a completely solid mixed-pattern sarcoma, described below.
Example of extensive type III PPB in a 33-month-old child. (a) Coronal CT image at carina, at diagnosis. (b) CT image 12 weeks later following four courses of IVADo chemotherapy. This tumor arose in parietal pleura, and resection did not involve lobectomy. This patient had no evidence of disease 15 months from diagnosis
Clear examples of lung cysts progressing over 6–24 months to types II and III PPB are known; such cysts must represent unrecognized type I PPB. Similarly, recurrences of type I PPB are characteristically type II or III disease (Priest et al. 2006). It is not known what proportion of types II and III PPB is preceded by purely cystic type I PPB.
In addition to progression of PPB types over the first 6 years of life, type I PPB may regress and persist without malignant potential. The residual characteristic of multiloculated cyst, termed type Ir (regressed) PPB, may be diagnosed at any age; the radiographic appearance is that of type I PPB (Fig. 25.2a). Type Ir PPB may be multiple or bilateral and is typically small (<2–3 cm diameter) but may occupy up to approximately 30% of the hemithorax. Type Ir PPB is most often recognized in relatives of PPB patients (Priest et al. 2009; Hill et al. 2008), just as retinocytoma is an occult manifestation of predisposition in hereditary Rb families (Dimaras et al. 2008). Regression of type II or III PPB has not been observed.
Type I PPB presents with respiratory difficulty from a large cyst or pneumothorax or as an incidental finding on chest radiograph or prenatal ultrasound (Priest et al. 1997, 2009; Miniati et al. 2006). Pneumothorax, including tension pneumothorax, suggests PPB and is not common in CPAM (Priest et al. 2009; Stocker 2002). Type II PPB may also present with pneumothorax, but typically types II and III PPB present with cough, mild to severe respiratory distress, and nonspecific symptoms such as fever, malaise, and anorexia. Types II and III PPB are often diagnosed as “pneumonia”; after failure to improve, investigations reveal tumor. Type III PPB, and occasionally type II PPB, often occupies an entire hemithorax (Fig. 25.4a). Types II and III PPB may extend into venous and arterial great vessels, leading to vascular symptoms and systemic embolism (Priest et al. 2011a). Type Ir PPB may present with pneumothorax but is most often occult and discovered incidentally on chest computed tomography (CT) during the work-up of another disease or when surveying PPB relatives for disease.
Types II and III PPB may metastasize (not observed in type I PPB) most frequently to cerebral parenchyma, estimated to occur in 11% and 55%, respectively, of types II and III PPB cases; the chest is free of disease in 50% of cerebral metastasis cases (Priest et al. 2007). Cerebrospinal fluid dissemination occurs virtually only after prolonged cerebral disease; cytology is rarely positive. Other metastatic sites include the pleural space, lung parenchyma, bones, and liver. Marrow disease is known in only one of 500–600 described cases. Pleural effusion is moderately common in types II and III PPB, but cytology is rarely positive. Thoracic lymph node infiltration is very rare. Bone and brain metastases may be present at diagnosis; metastases and recurrences occur from diagnosis to approximately 36 months later.
3 Pathology Overview
At gross examination, types I and Ir PPB may appear unicystic or multicystic; however, at low magnification, both demonstrate a multilocular architecture with delicate septa which are characteristic and suggest the PPB diagnosis (Hill et al. 2008). In type I PPB, a population of small primitive mesenchymal cells is found in the stroma beneath a benign respiratory epithelial lining; these cells may be a localized single focus, several foci or a diffuse proliferation, resembling the cambium layer effect of a sarcoma botryoides. The primitive small cells may display rhabdomyoblastic differentiation, and prominent eosinophilic cytoplasm may be present. The presence of rhabdomyoblasts is not necessary for the pathologic diagnosis. Small nodules of immature cartilage are often found in the septa and are not necessarily accompanied by the small primitive cells. Because the small primitive cells or nodules of cartilage may be exquisitely focal, it may be necessary to submit an entire cyst specimen for microscopic examination. In type Ir PPB, the small cell population is not seen; there may be evidence of necrotic or hyalinized cyst walls and septa and hemosiderin-laden macrophages.
Type II PPB retains evidence of type I cysts but grossly demonstrates areas of solid tumor. The evidence for residual cysts may be clinical (pneumothorax), radiographic or pathologic. Type III PPB is completely solid. Elsewhere in the lungs, types II and III PPB patients may have separate, scattered, typically small Type I or Ir PPB. Microscopically, the solid areas of types II and III PPB reveal an aggressive, primitive mixed-pattern sarcoma: blastema, anaplasia, ERMS, chondrosarcoma, undifferentiated spindle-cell proliferations, necrosis; rarely, other tissue types such as neuroblastoma are present. Anaplasia may be dramatic with frequent bizarre mitotic figures; anaplasia is rare in type I PPB (∼6% of cases) but present in 77% and 90%, respectively, of types II and III PPB (Hill et al. 2008). Tumors may be extremely friable with large areas of necrosis; pseudocysts, secondary to necrosis, do not designate type II disease. Small biopsies of large tumors may sample a monomorphic histopathology, typically ERMS; the International PPB Registry does not consider such samples diagnostic of PPB unless a mixed pattern is seen in subsequent resection specimens. Fine needle aspiration cytology is not recommended for diagnosis. Type III PPB may arise in and be entirely pleural based, including tumors of the parietal pleura (Fig. 25.4a). Malignant epithelium is not seen in PPB, differentiating it from “pulmonary blastoma”, a biphasic tumor of adulthood, which nevertheless occurs rarely in young children (Dehner 1994).
The differential diagnosis of type I PPB includes CPAM, which is radiographically indistinguishable from type I PPB, a newly recognized fetal lung interstitial tumor (FLIT) (Dishop et al. 2010) and cystic primary pleuropulmonary synovial sarcoma (CPPSS) (Cummings et al. 2010). FLIT is typically seen in newborns; although the histology may be reminiscent of type I PPB, the radiographic appearance of an airless opaque mass is typical of FLIT and unlike type I PPB. Although CPPSS may present with pneumothorax (Belcher et al. 2007), it is a disease of teen and young adult years during which type I PPB is extraordinarily unlikely (Cummings et al. 2010). A subtle spindle-cell population in cyst walls and septa suggests type I PPB; gene fusion markers for synovial sarcoma are useful (Cummings et al. 2010).
The differential diagnosis of solid PPB includes “monomorphic” pulmonary ERMS, biphasic adult-type pulmonary blastoma, and monomorphic spindle-cell proliferations, such as monomorphic pulmonary synovial sarcoma in teens.
4 PPB Treatment
No prospective treatment trials for any PPB type have been done because of the disease’s rarity.
Surgical extirpation is the primary treatment for type I PPB. Type I PPB may be exophytic and readily removed at a stalk, or it may deeply replace and distort one or more lobes, requiring lobectomy. Wedge resections suffice for intermediate disease. No case is described where unilateral pneumonectomy was necessary for type I PPB. Some type I PPBs are widely multifocal (unilateral or bilateral); surgical removal is not possible; the largest lesion(s) should be removed for pathologic examination and further treatment based on pathologic findings. A patient may have both types I and Ir cysts. Postoperative i.e. chemotherapy scan is recommended to determine whether residual cysts remain, which may have been compressed and undetected initially. For type I PPB, adjunctive chemotherapy may be useful (vincristine (V), dactinomycin (A), and cyclophosphamide (C)) (Priest et al. 2006). Among approximately 100 registry type I PPB patients, only one is known who received adjuvant chemotherapy and then had recurrence; however, many type I patients have been cured with surgery alone (Priest et al. 2006). No chemotherapy is recommended for patients with only type Ir PPB.
Types II and III PPB require multimodal therapy. These tumors often occupy >50% of a hemithorax (Fig. 25.4a); surgeons may prefer biopsy to resection. Core needle biopsies may not sample diverse histologic subtypes but will probably yield a “sarcoma” diagnosis, allowing selection of neoadjuvant chemotherapy. Multiple core needle biopsies or open excisional biopsy are recommended. Large tumors are sometimes resected and may be encapsulated or conversely extremely friable with pre- or intraoperative rupture, gross pleural spillage, and piecemeal resection.
Types II and III PPB are aggressive sarcomas, and chemotherapy recommendations are the same for both types. Adjuvant and neoadjuvant regimens typically follow aggressive sarcoma therapies. In Europe, “VAIAd” (courses of V, A, and ifosfamide (I) alternating with V, doxorubicin (Ad), and I) is used for PPB (Kirsch et al. 2005; Indolfi et al. 2007). Regimens including platinum compounds, etoposide, or other anthracyclines are generally no longer used in Europe. In the United States, VAC, VACAd, and VAC alternating with Ad-cisplatin have been used most often. Since 2007, the International PPB Registry has recommended “IVADo” (Do, doxorubicin), four courses at 3-week intervals, followed by IVA continuation therapy. IVADo employs four agents together for maximal early effect, especially for neoadjuvant use. Neoadjuvant responses range from approximately 40 to 90 + % three-dimensional volume reduction during 16 weeks of IVADo (Fig. 25.4b). PPB cannot be controlled only by chemotherapy, and surgical resection at week 12 or 16 must be considered. Aggressive resections, including extrapleural pneumonectomy for an extensively involved hemithorax, may be appropriate. Diaphragmatic resections and more rarely small chest wall resections have been done. Children appear to tolerate well lobectomy or pneumonectomy, although no long term studies of PPB survivors have been done.
Adjunctive radiation therapy may be considered for focal sites of known residual. No study has focused specifically on PPB outcome vis-à-vis radiotherapy usage. Sarcoma doses seem necessary; mediastinal disease margins may be troublesome because of anthracycline use. Whole-lung radiation at lung tolerance doses would seem not to be useful but has not been formally evaluated.
Treatments for PPB recurrences are highly varied. Thoracic recurrence therapy is likely to include surgery, relapse sarcoma regimens, or new agents, with or without focal radiation. A small proportion of children with recurrent chest disease survive. For responsive disease or “consolidation” of a disease-free condition, high-dose chemotherapy with autologous stem-cell reconstitution has been used for several PPB patients who have survived.
Cerebral metastases can be cured with excellent outcomes (Priest et al. 2007); perhaps 10–20% of children with cerebral metastases survive (unpublished International PPB Registry observation). General guidelines include neurosurgical resection followed by radiotherapy, which may include high-dose short-course focal therapy (“gamma knife”) protocols. If a cerebral metastasis occurs during chemotherapy, one could consider interdigitating an ifosphamide, carboplatinum, etoposide regimen, found useful in treating cerebral metastases of clear cell sarcoma of kidney (Radulescu et al. 2008), with the original chemotherapy regimen. Some brain metastases have been cured with surgery and radiation (Priest et al. 2007).
5 Prognosis
Figure 25.5 presents survival data by PPB type for 295 registry-confirmed patients treated heterogeneously at institutions around the world, comparing diagnoses before and after the year 2000. In general, it appears as if rates of survival are somewhat improved for more recent diagnoses; reasons for this are not known. Overall survivals are approximately 90% for type I PPB and 40–60% for types II and III PPB.
Life-table recurrence-free and overall survival by PPB type in 100 patients diagnosed prior to year 2000 and 180 patients diagnosed in year 2000 and later (based on diagnosis date for International PPB Registry enrollees consecutively accessioned when pathologic diagnosis is centrally confirmed). Log rank p values shown
6 DICER1 Mutations and the PPB Family Tumor and Dysplasia Syndrome (PPB-FTDS)
In 2009, germline mutations were described in PPB patients from families exhibiting PPB, lung cysts, CN, and ERMS (Hill et al. 2009). Subsequently, DICER1 mutations have been described in 50–70% of PPB patients and in many associated diseases in the PPB family tumor and dysplasia syndrome (Table 25.1) (Hill et al. 2010; Slade et al. 2011; Bahubeshi et al. 2010; Rio Frio et al. 2011; Priest et al. 2011b). PPB-FTDS affects 33% of families in which PPB is diagnosed, making PPB one of the strongest markers among pediatric malignancies for syndromic/familial disease (Plon and Malkin 2005; Scott et al. 2006). The PPB-FTDS phenotype is unique and has highly variable expressivity, autosomal dominant inheritance, many unaffected carriers, multifocal and bilateral disease, young age at diagnosis for certain phenotypic conditions compared to their sporadic counterparts and affects individuals predominantly under age 20 years. Both PPB and the PPB-FTDS also occur without DICER1 mutation.
7 Disease Screening and Genetic Counseling
Given the pleiotropy, varied expressivity, and relatively low disease penetrance in the PPB-FTDS, clinical screening recommendations are complex. Diseases may occur in many organs (Table 25.1) over the first two decades of life. PPB is the most frequent manifestation of the FTDS. Because type I PPB is highly curable and may evolve into advanced types II and III PPB with a much less favorable outlook (Fig. 25.5), identifying individuals genetically at risk and detecting and eradicating type I PPB by 8–12 months of age are the rational goals for any screening program.
References
Bahubeshi A, Bal N, Rio Frio T et al (2010) Germline DICER1 mutations and familial cystic nephroma. J Med Genet 47:863–866
Belcher E, Lawson MH, Nicholson AG, Davison A, Goldstraw P (2007) Congenital cystic adenomatoid malformation presenting as in-flight systemic air embolisation. Eur Respir J 30:801–804
Cummings NM, Desai S, Thway K et al (2010) Cystic primary pulmonary synovial sarcoma presenting as recurrent pneumothorax: report of 4 cases. Am J Surg Pathol 34:1176–1179
Dehner LP (1994) Pleuropulmonary blastoma is the pulmonary blastoma of childhood. Semin Diagn Pathol 11:144–151
Dimaras H, Khetan V, Halliday W et al (2008) Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet 17:1363–1372
Dishop MK, McKay EM, Kreiger PA et al (2010) Fetal Lung Interstitial Tumor (FLIT): a proposed newly recognized lung tumor of infancy to be differentiated from cystic pleuropulmonary blastoma and other developmental pulmonary lesions. Am J Surg Pathol 34:1762–1772
Hill DA, Jarzembowski JA, Priest JR, Williams G, Schoettler P, Dehner LP (2008) Type I pleuropulmonary blastoma: pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol 32:282–295
Hill DA, Ivanovich J, Priest JR et al (2009) DICER1 mutations in familial pleuropulmonary blastoma. Science 325:965
Hill DA, Wang JD, Schoettler P et al (2010) Germline DICER1 mutations are common in both hereditary and presumed sporadic pleuropulmonary blastoma [abstract]. Lab Invest 90:311
Indolfi P, Bisogno G, Casale F et al (2007) Prognostic factors in pleuro-pulmonary blastoma. Pediatr Blood Cancer 48:318–323
Kirsch S, Leuschner I, Int-Veen C et al (2005) Sixteen children with pleuropulmonary blastoma – results of the German Cooperative Soft Tissue Sarcoma Group [abstract]. Sarcoma 9:88
Manivel C, Wick M, Dehner L (1987) Thoracopulmonary blastoma – reassessment of an embryonal neoplasm. Lab Invest 56:48
Manivel JC, Priest JR, Watterson J et al (1988) Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer 62:1516–1526
Miniati DN, Chintagumpala M, Langston C et al (2006) Prenatal presentation and outcome of children with pleuropulmonary blastoma. J Pediatr Surg 41:66–71
Plon SE, Malkin D (2005) Childhood cancer and heredity. In: Pizzo PA (ed) Principles and practice of pediatric oncology, vol 5. Lippincott Williams & Wilkins, Philadelphia, pp 14–37
Priest JR, Watterson J, Strong L et al (1996) Pleuropulmonary blastoma: a marker for familial disease. J Pediatr 128:220–224
Priest JR, McDermott MB, Bhatia S, Watterson J, Manivel JC, Dehner LP (1997) Pleuropulmonary blastoma: a clinicopathologic study of 50 cases. Cancer 80:147–161
Priest JR, Hill DA, Williams GM et al (2006) Type I pleuropulmonary blastoma: a report from the International Pleuropulmonary Blastoma Registry. J Clin Oncol 24:4492–4498
Priest JR, Magnuson J, Williams GM et al (2007) Cerebral metastasis and other central nervous system complications of pleuropulmonary blastoma. Pediatr Blood Cancer 49:266–273
Priest JR, Williams GM, Hill DA, Dehner LP, Jaffe A (2009) Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol 44:14–30
Priest JR, Andic D, Arbuckle S, Gonzalez-Gomez I, Hill DA, Williams G (2011a) Great vessel/cardiac extension and tumor embolism in pleuropulmonary blastoma: a report from the International Pleuropulmonary Blastoma Registry. Pediatr Blood Cancer 56(4):604–609
Priest JR, Williams GM, Manera R et al (2011b) Ciliary body medulloepithelioma: four cases associated with pleuropulmonary blastoma–a report from the International Pleuropulmonary Blastoma Registry. Br J Ophthalmol 95(7):1001–1005
Radulescu VC, Gerrard M, Moertel C et al (2008) Treatment of recurrent clear cell sarcoma of the kidney with brain metastasis. Pediatr Blood Cancer 50:246–249
Rio Frio T, Bahubeshi A, Kanellopoulou C et al (2011) DICER1 mutations in familial multinodular goiter with and without ovarian sertoli-leydig cell tumors. JAMA 305:68–77
Scott RH, Stiller CA, Walker L, Rahman N (2006) Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 43:705–715
Seitz H (2010) siRNAs: the hidden face of the small RNA world. Curr Biol 20:R108–R110
Slade I, Bacchelli C, Davies H et al (2011) DICER1 syndrome – clarifying the diagnosis, clinical features and management implications of a pleiotropic tumor predisposition syndrome. J Med Genet 48(4):273–278
Stocker JT (2002) Congenital pulmonary airway malformation – a new name for and an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathology 41:424–431
Acknowledgments
The International PPB Registry has been supported by the Pine Tree Apple Tennis Classic since 1987 and by the Theodora H. Lang Charitable Trust and Children’s Hospitals and Clinics of Minnesota foundation. Appreciation is expressed to Gretchen Williams and Marsha Finkelstein for the help in preparation of this material.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Priest, J.R. (2012). Pleuropulmonary Blastoma. In: Schneider, D., Brecht, I., Olson, T., Ferrari, A. (eds) Rare Tumors In Children and Adolescents. Pediatric Oncology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-04197-6_25
Download citation
DOI: https://doi.org/10.1007/978-3-642-04197-6_25
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-04196-9
Online ISBN: 978-3-642-04197-6
eBook Packages: MedicineMedicine (R0)