Abstract
Patients palliated with Total Cavopulmonary Connection have a lower muscle mass and a lower exercise capacity. We assessed calf muscle oxidative metabolism during and after heel raise exercise to exhaustion in young patients with TCPC compared to healthy peers. Near-infrared spectroscopy was used for measuring oxygen metabolism in the medial portion of the gastrocnemius muscle. Forty-three patients with TCPC, aged 6–18 years, were compared with 43 age and sex-matched healthy control subjects. Subgroups were formed to include children (6–12 years) and adolescents (13–18 years) to determine if these age groups influenced the results. During exercise, for the patients compared to controls there was a lower increase in deoxygenated hemoglobin (oxygen extraction) (5.13 ± 2.99au vs. 7.75 ± 4.15au, p = 0.001) and a slower rate of change in total hemoglobin (blood volume) (0.004 ± 0.015au vs 0.016 ± 0.01au, p = 0.001). Following exercise, patients exhibited a slower initial increase in tissue oxygenation saturation index (0.144 ± 0.11au vs 0.249 ± 0.226au, p = 0.007) and a longer half-time to maximum hyperemia (23.7 ± 11.4 s vs 16.8 ± 7.5 s, p = 0.001). On the subgroup level, the adolescents differed compared to healthy peers, whereas the children did not. Young patients with TCPC had impaired oxidative metabolism during exercise and required a longer time to recover. In that the differences were seen in the adolescent group and not in the children group may indicate a declining function with age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the most complex of congenital heart defects are the functional single ventricle defects. These defects consist of several different morphological diagnoses. Earlier, functional single ventricle defects or univentricular hearts (UVH) were incompatible with survival into adulthood. However, the introduction and development of surgical techniques [1] during recent decades have vastly improved survival [2, 3]. With increased survival rates of complex congenital heart defects, much can be learned of the long-term complications and survivability with these defects and their palliation [4,5,6].
For children born with UVH, the most usual procedure is to undergo three stages of palliative surgery with the result being total cavopulmonary connection (TCPC), also called Fontan circulation. With TCPC, blood is redirected from the systemic veins directly to the pulmonary arteries, thus bypassing the heart. Because blood flowing from the lower part of the body has to go against gravity and hence is largely dependent on lower limb muscle contractions for venous return, good skeletal muscle function is crucial [7]. Studies have shown that adults living with TPCP have lower aerobic exercise capacity, lower muscle mass, and lower muscle strength in comparison to healthy peers [5, 8,9,10,11].
The presence of such a different and deviating circulation together with findings of reduced skeletal muscle function (e.g., muscle strength in adults with TCPC) raises questions of whether the peripheral muscle blood flow and metabolism are impaired [12]. Near-infrared spectroscopy (NIRS) is a non-invasive method for continuous monitoring of local muscle oxygenation, i.e., the dynamic balance between oxygen delivery and consumption [13,14,15,16,17,18]. In regard, Sandberg and co-workers reported impaired oxygenation for the deltoid muscle and calf muscle in adults with CHD of mixed complex lesions. This was exhibited as a slower oxygen desaturation at exercise onset and a slower oxygen recovery post-exercise in comparison to control subjects [19, 20]. To our knowledge, there are no NIRS studies on peripheral muscle oxygenation in young patients (pre-adult) with TCPC during exercise and recovery. These type of data would address whether oxygenation impairments are already present at a young age.
Our primary aim was to determine the extent of difference in calf muscle oxygenation kinetics during exercise and recovery between young patients with TCPC and healthy control subjects matched by age and sex. Our study population included both children (6–12 years) and adolescents (13–18 years). Therefore, our secondary aim was to determine if the division into age groups influenced the results.
Materials and Methods
Design
The present study is a collaboration between the pediatric cardiology outpatient clinics at the University Hospital in Umeå and Astrid Lindgren Children’s Hospital in Stockholm, Sweden. Consequently, the experiments were done in these two locations, but with the same staff. The design was cross-sectional to study muscle oxygenation during exercise and recovery in young patients with TCPC and healthy peers.
Study Population
Participants were identified from local registers of patients with functional univentricular heart defects surgically palliated with TCPC that were presently in the follow-up program at three pediatric centers in the northern regions of Sweden (Västernorrland, Västerbotten and Norrbotten) and from the Astrid Lindgren Children’s Hospital in Stockholm. Exclusion criteria were younger than 6 years and younger than 19 years, cognitive impairment, or muscular or motor disorders that would make it difficult to participate.
Initially, 78 patients (49 from Stockholm and 29 from the northern region) were identified as eligible and they or their caregivers were contacted. Of these, two had co-morbidities, eight did not respond, and 25 declined participation (19 from Stockholm and 6 from the northern region). Forty-three patients accepted participation and were included in the study (Fig. 1). Medical records were sourced for each patient to determine the type of congenital heart defect that lead to the TCPC procedure (Table 1). For each patient, a control subject matched by sex and age was recruited (Fig. 1). The criterion for the control subject was no record of any heart or vascular disease; all controls underwent an echocardiographic examination prior to the study. Post hoc analyses showed no differences in gender or age between the patients that were included and those who declined (data not shown). Prior to the study, caregivers provided informed written consent for the subjects younger than 18 years and those who were 18 years gave consent themselves. The study was approved by the Regional Ethical Review Board in Umeå, Sweden (Dnr: 2016-445-31M).
Measurements
Anthropometrics
At the time of the examination, calibrated digital scales (Tanita scale, Tokyo, Japan) measured body weight to the nearest 0.1 kg and wall-mounted stadiometers (Seca, Vogel & Halke, Hamburg, Germany) measured height to the nearest 0.1 cm. The same systems were used in Umeå and Stockholm. BMI was calculated (kg × m−2), and BMI Z-scores were assessed according to the WHO 2007 charts [21] (Table 1).
Peripheral Capillary Oxygen Saturation
Both before and at the end of the exercise test, peripheral capillary oxygen saturation was assessed using a handheld pulse oximeter (GE Data Ohmeda Tuffsat Handheld Pulse Oximeter, GE Healthcare, Sweden).
Heel Raise Exercise
The exercise consisted of unilateral isotonic heel raises with participants standing on one leg facing a wall with the fingertips touching for balance support. The contralateral foot was held slightly above the floor. The participant was then instructed to perform as many heel raises as possible. Prior to the test, an individual’s maximum height of a heel rise was determined and marked by a board fastened to the wall. The test was terminated by the participant due to exhaustion or by the test leader when the participant failed to reach the pre-set maximum height. After this, participant was moved back to the starting position and a probe for NIRS monitoring (see below) was attached on the contralateral leg, and the same procedure was repeated. A rationale for testing both legs was to ascertain whether previous femoral catheterization on one leg for the earlier surgical procedure of TCPC had any impact on our variables of interest [22, 23]. Hence also for each participant a mean of the data from the legs for each measurement and data point was derived. Verbal encouragement was given throughout the test. After termination of the test, the participant sat down and rested for at least 60 s. The total number of heel raises was recorded. This test was used in previous studies on adults with heart failure and with congenital heart disease [20, 24, 25]; we made only minor adjustments to best fit the children.
NIRS Measurements
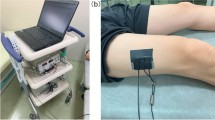
For muscle oxygenation monitoring we used a portable, continuous wave spectrometer (PortaLite mini, Artinis medical systems BV, the Netherlands) and a probe with three linear arranged emitters (emitting wavelengths of 760 mm and 850 nm) and a receiver with emitter-to-receiver distances of 16 mm, 21 mm, and 26mm, respectively. With the probe fastened on the medial head of the gastrocnemius muscle, data were sampled at a frequency of 5Hz. The gastrocnemius was chosen because it is activated during the aforementioned heel raise test. Data were continuously collected with the participant seated at rest for 60 s, during exercise, and during recovery for 60 s. For data collection, software from the manufacturer (PortaSoft, Artinis medical systems BV, the Netherlands) was used.
NIRS Data Management and Calculations
Oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) absorb light of different wavelengths making it possible to measure their different concentrations in relation to each other [26]. These parameters, as well as the tissue saturation index (TOI)—proportion of O2Hb to the total hemoglobin (HbT), and the total amount of hemoglobin (HbT)—O2Hb+HHb were displayed graphically and recorded numerically in real time. An additional parameter of interest was the mean difference in muscle oxygenation (Hbdiff)—O2Hb-HHb that was calculated after the experiment. NIRS assessments of TOI and HHb as an expression for oxygen extraction in lower limb muscles have been shown to correlate to whole body aerobic capacity [18, 27]. For each subject, a baseline for each respective parameter was derived as the mean of the 30 s prior to exercise and changes during exercise were expressed relative to this baseline value.
Because the pace of the exercise was not fixed, we were not able to calculate the rate of changes in parameters at exercise onset. Therefore, variables of interest during exercise were calculated for the last 30 s of the exercise. Hence, the magnitudes of drop in TOI and oxygen extraction during exercise (HHb) were calculated as the second of data with the lowest and highest mean, respectively. Also during exercise, the rate of change in blood volume was calculated as the slope of increase (derived using linear regression) in HbT for the last 30-s window. At the end of exercise, the slope of TOI recovery was calculated for the first 15 s of recovery and the half-time of recovery (T½) was calculated as the time from the start of recovery until TOI reached ½ of the maximum hyperemic value following exercise. We chose both variables since they express different physiological entities of the recovery phase. T½ gives information on the post-exercise hyperemic response, whereas the initial resaturation primarily reflects restoration of vascular components.
Statistical Analyses
Offline analyses and calculations of data were done in MatLab software (MatLab R2018b, The MathWorks, Inc. Natick, Massachusetts, USA) and the Statistical Package for Social Sciences version 25 (SPSS, IBM corp., Armonk, NY, US). All data were presented as means with standard deviations and ratios with percentages. Comparisons between patients and controls were analyzed using Student's t tests. Subgroup analysis between patients and controls was conducted with the participants stratified into two age groups, children (6–12 years) and adolescents (13–18 years). The cut-off 12–13 was set to match the physical and mental development during puberty [28, 29]. The gender distribution between the children and adolescent groups was not significantly different (data not shown). The null hypothesis was rejected for p-values <0.05.
Results
Peripheral Capillary Saturation
The patients with TCPC had lower peripheral capillary oxygen saturation than controls before the start of exercise (93.7±2.7% vs. 98.2±1.1% p<0.001) and after exercise (93.7±2.5% vs. 98.1±1.1% p<0.001). From start to end of exercise the drop in saturation was virtually unchanged within groups and thus did not differ between patients and controls (−0.10±1.95 percentage points vs. 0.05±1.41 percentage points, p=0.69).
Heel Raise Test
There was no difference in the number of heel raises between patients and controls (25.0±14.9 vs. 25.7±12.6, p=0.75).
Muscle Oxygenation During Exercise
Table 2 presents oxygenation data at rest and during exercise. TOI at rest differed between groups with lower values in patients compared to controls. On group level, the drop in TOI did not differ between patients and controls (Table 2). Further, there were no differences on the subgroup levels between patients and controls.
On group level, the increase in HHb and the slope of change in HbT during exercise were lower for the patients compared with controls (Table 2). In subgroup analyses, changes in HHb and HbT did not differ between patients and controls for the children group, but both variables were lower for the adolescent group (Table 2).
On group level, Hbdiff did not differ between patients and controls. In subgroup analyses, there was no difference between patients and controls for the children group, but there was a trend in the adolescent group (p=0.050) with a higher value for the patients (Table 2).
Muscle Oxygenation in Recovery
On group level, the rate of initial increase of TOI was lower and T½ was longer for the patients compared to controls (Table 3). In subgroup analyses, there was no difference between patients and controls for the children group in either variable, but both variables were different for the adolescent group (Table 3).
Discussion
An important finding in the present study was that the calf muscle oxygenation kinetics were impaired in young patients with TCPC. While we found no difference between patients and controls in tissue saturation during exercise, the patient group exhibited a lower magnitude of oxygen extraction and a slower rate of blood volume increase during exercise in comparison to healthy controls. In addition, oxygenation recovery post-exercise was slower for patients than controls. When separating in subgroups of children and adolescents, it was shown that the adolescents with TCPC were primarily responsible for our oxygenation findings.
Peripheral Capillary Saturation and Resting Tissue Oxygenation
As expected, patients with TCPC had a lower peripheral capillary oxygen saturation presumably due to impaired diffusion capacity, ventilation–perfusion mismatch in the lung, and right-to-left shunting of blood [12, 30]. Gewillig et al. reported that venous congestion and reduced pulmonary blood flow may contribute to lower arterial oxygen saturation at rest and that the inability to compensate for the demand of increased cardiac output during exercise could lead to decreases in both exercise capacity and arterial saturation [12].
Muscle Oxygenation During Exercise
Our findings of no differences in TOI drop are consistent of those of Moalla et al. [31]. In contrast to both Moalla et al. and Vandekerckhove et al., we found no differences in the magnitude of TOI decrease during exercise. To our knowledge, the study by Vandekerckhove et al. is the only study of muscle oxygenation measured with NIRS in patients below eighteen years of age with TCPC [32]. The authors reported a lower TOI of the vastus lateralis muscle during exercise for TCPC patients compared to controls. We had no such difference in our population. The discrepancy between studies could be due to differences in muscle groups and exercise modes.
The trend of a smaller drop in relative oxygen saturation, combined with the lower levels of oxygen extraction for the adolescents with TCPC compared to controls, indicates a mismatch of oxygen delivery and consumption for these patients despite equal performance in the heel raise test. This indicates an availability of substrates for oxidative metabolism but a lower rate of aerobic metabolism. Moalla et al. previously investigated the response of muscle oxygenation during exercise using NIRS in children with CHD and found a similar response in HHb [31].
We observed a slower increase of blood volume during the heel raise test in the patient group compared to controls that was mostly influenced by the adolescent subgroup. The typical response to repetitive isotonic exercise in healthy individuals is an increase in blood volume [15, 33]. As described by others, a slower blood volume increase for the patients could indicate that there is an obstruction due to increased intramuscular pressure that was greater for patients than controls. An increased intramuscular pressure during exercise for the patients with TCPC would most likely arise due to the congestion and peripheral pooling of venous blood because of the lack of pumping ventricle to the pulmonary circulation [12].
Muscle Oxygenation in Recovery
Following the heel raise test, muscle oxygen recovery was impaired in patients with TCPC compared to controls. This finding is in line with previous studies that show a slower initial increase in TOI in the recovery phase and a longer time to reach peak of hyperemic phase after exercise in patients with TCPC [19, 34].
Capacity Declining with Age
When performing subgroup analysis, several of our variables differed significantly in the adolescent group of TCPC patients (aged 13–18 years) compared to age-matched controls but did not in the children group (Table 2). Muscle strength is shown to decline with age in patients with TCPC, and adults with complex congenital heart defects have been shown to have a lower muscle mass when compared to healthy controls [11, 35]. A decline in exercise capacity with age has been observed in TCPC patients, and our data suggest that this in part may be due to an impairment in the local muscle metabolism [36].
What Could Be Done?
Patients with TCPC have higher central venous pressure and a limited ability to increase and regulate preload, leading to reduced filling volumes of the systemic ventricle and thus a lower cardiac output during exercise. The limitations of exercise capacity are probably multifactorial and includes both central cardiovascular and peripheral factors (i.e., muscle mass and muscle metabolism) and are consequences of the vast physiological changes that comes with a TCPC [30, 36,37,38,39]. Our findings, of equal tissue saturation drop in combination with signs of intramuscular blood flow congestion during exercise, indicate that this obstruction might play a role in the reduced physical function for this group of patients.
In the subgroup of children with TCPC (6–12 years), we found no signs of differences in muscle microcirculation and oxidative function compared to healthy controls. This finding may suggest that targeted muscle training could be indicated in this younger patient group so as to minimize a decline in physical capacity. An exercise training protocol promoting muscle strength with continuous progression might result in increased muscle mass, improved peripheral vascular function, and thus improved muscular oxygenation [40]. An increased muscle mass might also lead to an increased muscle pump contributing to an improved venous return that possibly could reduce the amount of muscular blood flow congestion as also discussed by Cordina et al. when studying muscle function in adults with TCPC [9]. Cordina et al. have also previously shown the benefit of isolated muscle resistance training on both cardiac output, muscle strength, and exercise capacity for adults with TCPC [41].
Study Limitations
The study was performed in 43 children and adolescents after TCPC procedure compared to 43 healthy controls. This number is limited, but as far as we know, it is still the largest NIRS study on young patients with TCPC. Larger studies are needed to evaluate the findings in different age groups. NIRS is a technique that requires careful interpretation by experienced practitioners. Excessive adipose tissue thickness can influence the NIRS signals, but since our measurements were on the gastrocnemius muscle and all the participants were lean by our observation, we do not expect any such effect.
Conclusion
This study shows that even if younger patients with TCPC have equal muscle endurance capacity during heel raises and no difference in tissue saturation drop in exercise compared to healthy peers, they have an impaired muscle metabolism with impaired oxygen extraction and less increase of muscle blood flow during the exercise performed. We also showed a slower rate of recovery for the patients with TCPC compared to healthy peers and that the leg muscle metabolic activity in patients with TCPC may have a progressive decline, starting already before adolescence. These findings might provide important information for the future design of rehabilitation programs targeting muscle function in children and adolescents with TCPC.
Data Availability
Data available on demand.
Code Availability
Code available on demand.
References
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26:240–248
Hoashi T, Shimada M, Imai K, Komori M, Kurosaki K, Ohuchi H, Ichikawa H (2019) Long-term therapeutic effect of Fontan conversion with an extracardiac conduit. Eur J Cardio-thorac Surg 57
Bezuska L, Lebetkevicius V, Sudikiene R, Liekiene D, Tarutis V (2017) 30-year experience of Fontan surgery: single-centres data. J Cardiothorac Surg 12
Atz AM, Zak V, Mahony L, Uzark K, D’Agincourt N, Goldberg DJ, Williams RV, Breitbart RE, Colan SD, Burns KM, Margossian R, Henderson HT, Korsin R, Marino BS, Daniels K, McCrindle BW, Pediatric Heart Network I (2017) Longitudinal outcomes of patients with single ventricle after the fontan procedure. J Am Coll Cardiol 69:2735–2744
Hock J, Reiner B, Neidenbach RC, Oberhoffer R, Hager A, Ewert P, Muller J (2018) Functional outcome in contemporary children with total cavopulmonary connection - Health-related physical fitness, exercise capacity and health-related quality of life. Int J Cardiol 255:50–54
Wolff D, van Melle JP, Bartelds B, Ridderbos FS, Eshuis G, van Stratum E, Recinos SJ, Willemse BWM, Hillege H, Willems TP, Ebels T, Berger RMF (2017) Fontan circulation over time. Am J Cardiol 120:461–466
Hsia TY, Khambadkone S, Redington AN, Migliavacca F, Deanfield JE, de Leval MR (2000) Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation 102: III148–153
Muller J, Christov F, Schreiber C, Hess J, Hager A (2009) Exercise capacity, quality of life, and daily activity in the long-term follow-up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J 30:2915–2920
Cordina R, O’Meagher S, Gould H, Rae C, Kemp G, Pasco JA, Celermajer DS, Singh N (2013) Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart 99:1530–1534
Brassard P, Bedard E, Jobin J, Rodes-Cabau J, Poirier P (2006) Exercise capacity and impact of exercise training in patients after a Fontan procedure: a review. Can J Cardiol 22:489–495
Sandberg C, Frisk E, Hansson L, Isberg A, Rylander Hedlund E, Sjoberg G, Rydberg A (2020) Impaired knee extension muscle strength in adolescents but not in children with Fontan circulation. Cardiol Young 30:1138–1143
Gewillig M, Brown SC (2016) The Fontan circulation after 45 years: update in physiology. Heart 102:1081–1086
Neary JP (2004) Application of near infrared spectroscopy to exercise sports science. Can J Appl Physiol 29:488–503
Moalla W, Elloumi M, Chamari K, Dupont G, Maingourd Y, Tabka Z, Ahmaidi S (2012) Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Appl Physiol Nutr Metab 37:621–630
Ferrari M, Muthalib M, Quaresima V (2011) The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci 369:4577–4590
Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bulow J, Kjaer M (2001) Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 11:213–222
Boushel R, Piantadosi CA (2000) Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand 168:615–622
Okushima D, Poole DC, Barstow TJ, Rossiter HB, Kondo N, Bowen TS, Amano T, Koga S (2016) Greater V O2peak is correlated with greater skeletal muscle deoxygenation amplitude and hemoglobin concentration within individual muscles during ramp-incremental cycle exercise. Physiol Rep 4: n/a-n/a
Sandberg C, Crenshaw AG, Elcadi GH, Christersson C, Hlebowicz J, Thilen U, Johansson B (2019) Slower skeletal muscle oxygenation kinetics in adults with complex congenital heart disease. Can J Cardiol 35:1815–1823
Sandberg C, Crenshaw AG, Elçadi GH, Christersson C, Hlebowicz J, Thilén U, Johansson B (2021) Patients with complex congenital heart disease have slower calf muscle oxygenation during exercise. Int J Cardiol Congenit Heart Dis 4: 100157
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J (2007) Development of a WHO growth reference for school-aged children and adolescents/Mise au point d'une reference de croissance pour les enfants d'age scolaire et les adolescents/ Elaboracion de valores de referencia de la OMS para el crecimiento de escolares y adolescentes.(Research)(Author abstract). Bull World Health Org 85: 660
Taylor LM, Troutman R, Feliciano P, Menashe V, Sunderland C, Porter JM (1990) Late complications after femoral artery catheterization in children less than five years of age. J Vasc Surg 11: 297–304; discussion 304
Ruud E, Natvig S, Holmstrøm H, Wesenberg F (2002) Low prevalence of femoral venous thrombosis after cardiac catheterizations in children: a prospective study. Cardiol Young 12:513–518
Sandberg C, Thilén U, Wadell K, Johansson B (2015) Adults with complex congenital heart disease have impaired skeletal muscle function and reduced confidence in performing exercise training. Eur J Prev Cardiol 22:1523–1530
Cider Å, Carlsson S, Arvidsson C, Andersson B, Stibrant Sunnerhagen K (2006) Reliability of clinical muscular endurance tests in patients with chronic heart failure. Eur J Cardiovasc Nurs 5:122–126
Hamaoka T, McCully KK (2019) Review of early development of near-infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism. J Physiol Sci 69:799–811
Lagerwaard B, Keijer J, McCully K, Boer V, Nieuwenhuizen A (2019) In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur J Appl Physiol 119:1799–1808
Chulani VL, Gordon LP (2014) Adolescent growth and development. Primary Care Clin Office Pract 41:465–487
Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B (2012) Child—adult differences in muscle activation — a review. Pediatr Exerc Sci 24:2–21
Ohuchi H, Ohashi H, Takasugi H, Yamada O, Yagihara T, Echigo S (2004) Restrictive ventilatory impairment and arterial oxygenation characterize rest and exercise ventilation in patients after fontan operation. Pediatr Cardiol 25:513–521
Moalla W, Dupont G, Costes F, Gauthier R, Maingourd Y, Ahmaidi S (2006) Performance and muscle oxygenation during isometric exercise and recovery in children with congenital heart diseases. Int J Sports Med 27:864–869
Vandekerckhove K, Coomans I, Moerman A, Panzer J, De Groote K, De Wilde H, Bove T, Francois K, De Wolf D, Boone J (2019) Differences in cerebral and muscle oxygenation patterns during exercise in children with univentricular heart after Fontan operation compared to healthy peers. Int J Cardiol 290:86–92
Muthalib M, Millet GY, Quaresima V, Nosaka K (2010) Reliability of near-infrared spectroscopy for measuring biceps brachii oxygenation during sustained and repeated isometric contractions. J Biomed Opt 15: 017008
Danduran MJ, Dixon JE, Rao RP (2012) Near infrared spectroscopy describes physiologic payback associated with excess postexercise oxygen consumption in healthy controls and children with complex congenital heart disease. Pediatr Cardiol 33:95–102
Sandberg C, Johansson K, Christersson C, Hlebowicz J, Thilén U, Johansson B (2019) Sarcopenia is common in adults with complex congenital heart disease. Int J Cardiol 296:57–62
Goldberg DJ, Avitabile CM, McBride MG, Paridon SM (2013) Exercise capacity in the Fontan circulation. Cardiol Young 23:824–830
Takken T, Tacken MH, Blank AC, Hulzebos EH, Strengers JL, Helders PJ (2007) Exercise limitation in patients with Fontan circulation: a review. J Cardiovasc Med (Hagerstown) 8:775–781
Takken T, Giardini A, Reybrouck T, Gewillig M, Hovels-Gurich HH, Longmuir PE, McCrindle BW, Paridon SM, Hager A (2012) Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: a report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur J Prev Cardiol 19:1034–1065
Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafañe J, Bhatt AB, Peng LF, Johnson BA, Marsden AL, Daniels CJ, Rudd NA, Caldarone CA, Mussatto KA, Morales DL, Ivy DD, Gaynor JW (2012) Hypoplastic left heart syndrome. J Am Coll Cardiol 59:S1–S42
Olver T, Ferguson BS, Laughlin M (2015) Molecular mechanisms for exercise training-induced changes in vascular structure and function: skeletal muscle, cardiac muscle, and the brain. Prog Molec Biol Transl Sci: 227–257
Cordina RL, O’Meagher S, Karmali A, Rae CL, Liess C, Kemp GJ, Puranik R, Singh N, Celermajer DS (2013) Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol 168:780–788
Funding
Financial support was provided through the Swedish Heart–Lung Foundation (20160496) and the Swedish Odd Fellow Order.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Participant invitation and informed consent gathering were performed by Magne Sthen Bergdahl and Camilla Sandberg. Data collection was mainly done by Camilla Sandberg and Albert Crenshaw. Data analysis were performed by Magne Sthen Bergdahl and Albert Crenshaw. The first draft of the manuscript was written by Magne Sthen Bergdahl and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Regional Ethical Review Board in Umeå, Sweden (Dnr: 2016-445-31 M).
Consent to Participate
Prior to the study, caregivers provided informed written consent for the subjects younger than 18 years and those who were 18 years gave consent themselves.
Consent for Publication
All authors consent publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bergdahl, M.S., Crenshaw, A.G., Hedlund, E.R. et al. Calf Muscle Oxygenation is Impaired and May Decline with Age in Young Patients with Total Cavopulmonary Connection. Pediatr Cardiol 43, 449–456 (2022). https://doi.org/10.1007/s00246-021-02743-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-021-02743-6