Abstract
Dynamic parameters of fluid responsiveness (FR), namely aortic blood flow velocity variation (delta V peak), left ventricular velocity-time integral variation (delta VTI), stroke volume variation, and pulse pressure variation (PPV) have demonstrated good diagnostic performance for the prediction of response to fluid loading in mechanically ventilated critically ill adult patients. We aimed to evaluate these parameters in children undergoing cardiac surgery. A retrospective observational study of mechanically ventilated patients weighing less than 20 kg who received a volume expansion (VE) of 10 ml/kg after sternal closure was conducted. A 10% cardiac index (CI) increase divided patients into 7 responders (R) and 9 non-responders (NR). Transesophageal echocardiography and Pressure Recording Analytical Method data were retrieved. The percentage CI increase was 18.6 (12)% in R and 2.9 (5.7)% in NR (p = 0.037). Prior to VE, delta V peak, delta VTI, PPV, and SPV differed between R and NR (p = 0.045, 0.043, 0.048, 0,037 and 0.044, respectively). Systolic (p = 0.004), diastolic (p = 0.002), mean blood pressure (p = 0.003), delta V peak (p = 0.03), delta VTI (p = 0.04), CI (p = 0.01), PPV (p = 0.04), SPV (p = 0.04), and dP/dt max (maximal pressure-to-time ratio) (p = 0.02) changed the following VE in R patients. Delta V peak decreased after VE in NR patients (p = 0.004). Delta VTI and PPV showed the highest predictive values, with area under receiver operator characteristic curves of 0.76 (p = 0.049) and 0.76 (p = 0.045), respectively. Delta VTI and PPV were revealed to be potential predictors of FR in ventilated children after cardiac surgery. Their combined evaluation could be useful for fluid management after sternal closure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluid handling is crucial for oxygen delivery optimization in children with congenital heart malformations after corrective or palliative surgery [1]. In particular, the evaluation of the adequate amount of fluid for administration is a central issue in neonates and infants affected by LCOS related to cardiopulmonary bypass (CPB). The aim is to minimize the fluid overload and tissue edema that is associated with worse outcomes in these patients [2].
Several hemodynamic indexes have been investigated as fluid responsiveness (FR) parameters [3]. A debate regarding the role of dynamic preload indexes (pulse pressure variation (PPV), stroke volume variation (SVV), systolic pressure variation (SPV)), and static parameters (blood pressure, heart rate (HR) central venous pressure (CVP), left atrial pressure) in mechanically ventilated children [4–6] is ongoing. Echocardiographic parameters of FR—namely respiratory variations of aortic blood flow velocity (delta V peak) and aortic velocity-time integral (delta VTI)—have been described as reliable predictors of FR in both adults and children [4, 7]. Moreover, a recent meta-analysis confirmed the ability of delta V peak to predict the response to volume expansion (VE) in the pediatric population in various clinical settings. However, echocardiography requires expert clinicians to assess delta V peak and its optimal cut-off value differentiating responders (R) and non-responders (NR) to fluid loading, with exact values yet to be determined [8].
Powered by the Pressure Recording Analytical Method (PRAM), MostCare™ (Vygon, Vytech, Padova, Italy) is a continuous minimally invasive hemodynamic monitoring system based on arterial waveform pulse contour analysis sampled at a high rate (1000 points per second). PRAM estimates the stroke volume (SV) by analyzing the morphology and the area under the pressure waveform, including the post-dicrotic notch phase, and delivers averaged or beat-by-beat data [9]. The method also provides dynamic indexes of FR (PPVmc, SPVmc, dicrotic pressure variation, and SVVmc). In addition, PRAM calculates the dP/dt max (maximal pressure-to-time ratio or maximal slope of systolic upstroke (mmHg/ms) between two pressure points). The dP/dt max value depends on both ventricular contractility and vascular impedance (ventriculo-arterial coupling) [10].
The aim of our study was to test the ability of the dynamic indexes estimated by PRAM and measured with transesophageal echocardiography (TEE) to predict FR in a cohort of infants undergoing heart surgery with CPB soon after sternal closure.
Patients and Methods
A retrospective study was conducted. Data were retrieved from charts of patients scheduled for elective congenital heart surgery with CPB between February 2016 and May 2016. In our unit, PRAM and TEE monitoring are routinely applied for CPB weaning and the hemodynamic management of intraoperative care [10]. Data were analyzed for patients meeting the following inclusion criteria:
-
1.
Weight below 20 kg;
-
2.
Biventricular anatomy after surgery;
-
3.
Absence of spontaneous breathing;
-
4.
Volume expansion of 10 ml/kg of blood products or crystalloids after chest closure, as per the management of clinically suspected hypovolemia in our institution; and
-
5.
Recorded TEE measurements and MostCare™ data before and after a VE bolus administered after sternal closure and clearly identified from clinical charts.
Exclusion Criteria
-
1.
Postoperative supraventricular or ventricular arrhythmias;
-
2.
Residual left ventricular outflow tract (LVOT) obstruction;
-
3.
Moderate or severe valve stenosis or regurgitation;
-
4.
Need to change inotrope/vasopressor doses during the study period or severe myocardial dysfunction;
-
5.
Presence of arterial waveform artifacts (inappropriate identification of dicrotic notch, overdamping or underdamping of the transduced signal);
-
6.
Delayed sternal closure;
-
7.
Residual intracardiac shunts; and
-
8.
Peak inspiratory pressure (PIP) above 30 cmH2O and positive end expiratory pressure (PEEP) above 10 cmH2O.
Anesthesia was induced with midazolam (0.1 mg/kg), rocuronium (0.6 mg/kg), and fentanyl (5–10 mcg/kg) and maintained by respective continuous infusions. Mechanical ventilation was set with a tidal volume of 8–10 ml/kg and a respiratory rate (RR) appropriate to age (between 15 and 40 breaths/min). Following the induction of anesthesia, a peripheral arterial catheter (24–22 G) was placed in the left or right radial, humeral, or femoral artery. At the end of anesthesia induction, the MostCare™ monitor retrieved the arterial waveform signal directly from the patient’s monitor (Philips MX800) through an analogic output of the pressure transducer. A neonatal (bodyweight ≤3 kg) or pediatric (bodyweight between 3.5 and 20 kg) echocardiographic transesophageal multiplane probe was inserted and connected to the echocardiography machine (Philips iE33). Using a longitudinal transgastric view at 110–130°, with an angle between the beam and the blood flow always inferior to 20°, pulse-wave Doppler on the LVOT was performed to measure velocity-time integral (VTI) and aortic peak velocity (V peak). Minimal and maximal values over a single respiratory cycle were recorded.
Demographic data (age, weight, height, diagnosis, type of surgery, hours of mechanical ventilation and ICU stay), mechanical ventilation setting data, and hemodynamic data were retrieved from clinical charts. PRAM data (cardiac index (CImc), SVVmc, PPVmc, SPVmc, dP/dt max) were obtained directly from a device that stores up to 50 patient recordings. TEE data (delta VTI and delta V peak) were retrieved from an institutional database. Delta VTI was calculated as follows: delta VTI = (VTImax − VTImin)/[(VTImax + VTImin)/2] × 100. In the same way, delta V peak = (V peakmax − V peakmin)/[(V peakmax + V peakmin)/2] × 100.
Hemodynamic data were analyzed at two time points following sternal closure: (1) before VE, with stable hemodynamics and controlled bleeding; (2) within 10 min after VE.
The primary objective of the study was to describe the performance of delta V peak, delta VTI, and PRAM dynamic indexes as FR predictors.
Secondary Objectives
-
Evaluate static indexes (CVP, HR, invasive blood pressure) as FR predictors;
-
Evaluate the modification of each index before and after VE in R and NR; and
-
Compare the different behavior of each index between R and NR by comparing parameters collected before and after VE.
Statistical Analysis
Results are expressed as mean values (standard deviation). Unpaired and paired t tests were used to compare unmatched or matched data, respectively. Receiver operator characteristic (ROC) curves were generated and the area under each ROC (AUROC) curve was calculated to demonstrate the ability of delta V peak, delta VTI, SVVmc, PPVmc, and SPVmc to predict FR. A logistic multivariable regression was used to combine the best performers and to calculate the cumulative AUROC. A p-value ≤0.05 was considered statistically significant. Statistical analysis was performed using the GraphPad Prism 7.0 software package (GraphPad Software Inc., San Diego, CA, USA).
Results
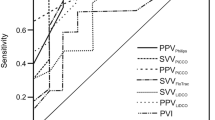
Overall, 16 patients were enrolled in the analysis. Of these, 7 (44%) showed an increase in CImc ≥10% after VE and were defined as R, while 9 (56.25%) showed an increase in CImc <10% and were defined as NR to VE. Table 1 describes the demographic characteristics of the included patients. Mean age was 1182 (1681) days in the R group and 371 (414) days in NR (p = 0.18). Mean Aristotle score was 7.7 (2) in R and 8.8 (2.2) in NR (p = 0.3). CPB duration was 153 (67) min in R and 131 (52) min in NR (p = 0.47). Vasoactive inotropic score (VIS) was 11.6 (0.8) in R and 14.1 (7.8) in NR (p = 0.56). Table 2 describes all the considered hemodynamic variables before and after VE in R and NR patients. VE significantly increased systolic (p = 0.004), diastolic (p = 0.002), and mean blood pressure (p = 0.003), dP/dt max (p = 0.02), CImc (p = 0.01), and decreased delta V peak (p = 0.03), delta VTI (p = 0.04), PPVmc (p = 0.04), and SPVmc (p = 0.04) in R. Only delta V peak significantly changed after VE in NR (p 0.004). The comparison of variables before VE (dP/dt max, delta V peak, delta VTI, PPVmc, and SPVmc) showed significant differences between R and NR (p = 0.045, 0.043, 0.048, 0,037, and 0.044, respectively). After VE, only delta CImc differed between the two groups (p = 0.049) (Table 2). Table 3 and Fig. 1 illustrate ROC curves analysis. In our population, FR was not predicted by SVVmc and SPVmc, whereas delta V peak, delta VTI, and PPVmc were able to predict a 10% increase in CImc after VE. Delta VTI and PPVmc presented the highest predictive values. Delta VTI presented an AUROC of 0.76, a sensitivity of 83%, and a specificity of 77%, while PPVmc presented an AUROC of 0.76, a sensitivity of 67%, and a specificity of 100% (p = 0.049 and 0.045, respectively). The optimal cut-off values were 30% for PPVmc and 16.5% for delta VTI. The combined application of PPVmc and delta VTI significantly improved FR prediction: AUROC 0.83, p: 0.03 (Table 3).
Discussion
This study is the first assessment of FR indexes in a cohort of small children (bodyweight less than 20 kg) undergoing complex heart surgery (mean Aristotle score about 8). The main findings of our study were as follows:
-
I.
Respiratory variation in left ventricular outflow tract velocity-time integral (delta VTI) measured with TEE is a sensitive predictor of FR in ventilated children after the correction of congenital heart disease.
-
II.
Pulse pressure variation (PPVmc) measured using a pulse contour analysis method (PRAM) is a specific predictor of FR.
-
III.
The combination of a sensitive and a specific parameter further improved the accuracy of FR prediction in our cohort.
-
IV.
Pre-VE absolute values of delta V peak, delta VTI, PPVmc, and SPVmc showed a statistically significant difference between R and NR patients, and these might also be considered for a quick evaluation of FR.
-
V.
The modification of FR parameters after VE (essentially present only in the R group) can be considered as a positive response to fluid administration.
FR assessment in critically ill children is currently considered a challenging task. There is certainly a lack of documented information regarding pediatric cardiac surgery patients undergoing correction for congenital heart diseases. Uncertainty regarding the reliability and accuracy of FR predictors has not been clarified, despite several studies, systematic reviews, and meta-analyses having demonstrated the superiority of dynamic—as opposed to static—parameters for FR prediction [4–6]. The study of these parameters is based on the principle of heart–lung interaction in patients under general anesthesia and positive pressure mechanical ventilation. Positive pressure ventilation induces cyclical respiratory variations of systemic venous blood return and, consequently, inspiratory reduction or expiratory increases in left ventricular stroke volume. These variations depend upon the portion of the Frank–Starling curve on which the ventricles are working. They explain whether the patient will be a responder (CI increase after fluid loading) or a non-responder to VE.
Respiratory variation-derived parameters obtained by arterial waveform analysis (SVV, PPV, SPV) or by echocardiographic estimation (delta V peak, delta VTI) have been widely validated as predictive parameters of FR in adult patients in different clinical settings [7]. Recently, fluid therapy based on FR parameters in adult patients under general anesthesia showed a decrease in both post-surgical morbidity (infections, cardiac and abdominal complications) and the length of stay in ICU [11].
Currently, there are not enough data to demonstrate the potential positive clinical impact of this advanced hemodynamic monitoring strategy both in optimizing fluid administration in post-cardio-surgical children and in preventing fluid accumulation. In the pediatric population, the main difficulties relate to ventricular systolic and diastolic function, which change markedly from the early neonatal period to the first year of life [12]. Cardiac function can be affected by the immaturity of neonatal myocardium that has a higher basal contractility, a reduced compliance and a greater sensitivity to changes in HR and afterload [13]. Furthermore, arterial elastic properties in neonates/infants significantly decrease with age. In this context, we can speculate that SVV or SPV, obtained by pulse contour analysis, may fail to predict FR due to the complex interaction between “immature” myocardium and arterial compliance. Conversely, PPV calculation (the variation in the difference between systolic and diastolic pressures) may be less affected by such issues. However, both peripheral and proximal arterial wall elastance in children decline after birth [14], suggesting that there is an increase in arterial wall stiffness as children grow older. It is possible that neonates have significantly different FR indexes and cut-offs to older children, which may explain why the younger patients in our cohort tended to be enrolled in the NR group.
Lung compliance and elastic properties of the chest wall also change with age, leading to surfactant increase, alveolar maturation, improved compliance of lung parenchyma, and modification of the chest wall structure (rib ossification, diaphragm and abdominal wall tension, intraabdominal pressure) [15]. This is certainly another reason why patients’ age can affect dynamic indexes’ ability to predict FR. For all these reasons, a minimum tidal volume of 8 ml/kg is necessary to induce meaningful intrathoracic pressure swings and respiratory variation in LV stroke volume. In our study, to avoid falsely positive FR detection, we hence only included patients ventilated with a mean tidal volume of 8–10 ml/kg, PIP <30 cmH2O, and PEEP <10 cmH2O [16]. In an adult setting, it has also been demonstrated that delta VTI values are less affected by inspiratory tidal volume than are PPV values, and that the transesophageal route is less affected that the transthoracic approach in terms of the respiratory cycle [17].
Acute changes in afterload and hemodynamic instability can compromise the predictability of FR parameters. According to Desranges and coworkers, the use of inotropes or vasopressors may affect heart–lung interactions and aortic blood flow, altering the ability of delta V peak to predict FR [8]. However, a recent investigation by Pinsky and colleagues showed that both SVV and PPV appear to be unaffected by varying doses of vasopressors and inotropic agents for post-cardiac surgery patients [18]. In our population, no significant differences in VIS were found and no change was made to vasopressor or inotrope doses during the study period. Vasoactive drug use in all patients of our population should hence not have affected FR indexes.
Baseline systemic ventricular contractility in cardiac surgery children recently weaned from CPB is another important factor influencing FR. In a recent study [19], Saxena and colleagues reported that children with low contractility reach a positive delta SV in response to VE after a significantly higher fluid administration compared to patients with normal contractility. As reported elsewhere [8], we administered a fluid replacement bolus of 10 ml/kg to all our patients, and it is possible that the amount of fluid administered might alter FR in patients with significantly different baseline contractility. Interestingly, in our population, we found a statistically significant difference in dP/dt max before VE between R and NR patients and in R patients before and after VE. We speculated that FR patients with low contractility improved cardiac function after volume loading. In this context, dP/dt max, integrated into a panel of hemodynamic data, may provide information that further assists clinicians in handling fluids and inotropes–vasopressors [20].
Limitations
The present study had several limitations. First, the cohort was small and heterogeneous involving different diagnoses and procedures. We limited enrollment to patients with biventricular anatomy weighing less than 20 Kg in order to include only neonates and patients below 2 years old—an original population by the standards of current literature. We specifically wanted to extend Choi and coworkers’ [21] observation regarding FR prediction in cardiac surgery patients. In their preliminary study, only simple surgical procedures were included (ventricular septal defect correction), and we aimed to show that FR can be evaluated in different biventricular anatomies. Second, PRAM is not a validated technique for CI monitoring in the pediatric setting [10]. However, our group make extensive use of it on a routine basis, and dynamic indexes are not subject to algorithm errors. Third, over- or underdamping artifacts of the transduced signal can compromise the reliability of all the derived parameters. Even if we routinely check and carefully prevent waveform artifacts to alter hemodynamic monitoring, these may sometimes be difficult to identify or correct. Some of the enrolled patients’ measurements may therefore have been affected by such bias (which is difficult to verify retrospectively) [22]. Nevertheless, we decided to use CImc to distinguish R from NR patients instead of TEE stroke volume measurement to avoid a potential source of bias being VTI, V peak, and SV estimated by echo (LVOT area × LVOT-VTI), as these are mathematically coupled [8]. Furthermore, our intention was to reduce intra- and inter-observer variability and echocardiographic measurement errors among clinicians dedicated to data collection. Finally, we decided to use an increase of 10% from the pre-VE value as a FR CImc cut-off, whereas other authors have used 15%. According to institutional protocol, however, we administer relatively low-volume boluses to our patients when fluid replacement is indicated. This is mainly due to patients’ relatively limited tolerance to greater fluid boluses that, as discussed above, may not be able to increase CI above the threshold of 10%. Finally, no unequivocal cut-off for CI increase to define FR has been recommended in the literature to date.
Conclusions
Echocardiographic dynamic indexes (delta VTI) and PPV measured by MostCare™ have revealed themselves to be reliable predictors of FR in ventilated children after correction of cardiac malformations with CPB. Integrated multi-instrumental and multi-parametric hemodynamic monitoring combining echocardiographic measurement and pulse contour analysis may be helpful in achieving a high predictive performance for these variables.
References
Wessel DL (2001) Managing low cardiac output syndrome after congenital heart surgery. Crit Care Med 29:S220–S230
Ricci Z, Iacoella C, Cogo P (2011) Fluid management in critically ill pediatric patients with congenital heart disease. Minerva Pediatr 63:399–410
Bendjelid K, Romand JA (2003) Fluid responsiveness in mechanically ventilated patients: a review of indices used in intensive care. Intensive Care Med 29:352–356
Durand P, Chevret L, Essouri S, Haas V, Devictor D (2008) Respiratory variations in aortic blood flow predict fluid responsiveness in ventilated children. Intensive Care Med 34:888–894
de Souza Neto EP, Grousson S, Duflo F, Ducreux C, Joly H, Convert J, Mottolese C, Dailler F, Cannesson M (2011) Predicting fluid responsiveness in mechanically ventilated children under general anaesthesia using dynamic parameters and transthoracic echocardiography. Br J Anaesth 106:856–864
Renner J, Broch O, Duetschke P, Scheewe J, Höcker J, Moseby M, Jung O, Bein B (2012) Prediction of fluid responsiveness in infants and neonates undergoing congenital heart surgery. Br J Anaesth 108:108–115
Michard F (2005) Changes in arterial pressure during mechanical ventilation. Anesthesiology 13:419–428
Desgranges FP, Desebbe O, de Souza Neto EP, Raphael D, Chassard D (2016) Respiratory variation in aortic blood flow peak velocity to predict fluid responsiveness in mechanically ventilated children: a systematic review and meta-analysis. Paediatr Anaesth 26:37–47
Romano SM, Pistolesi M (2002) Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 30:1834–1841
Favia I, Rizza A, Garisto C, Haiberger R, Di Chiara L, Romagnoli S, Ricci Z. (2016) Cardiac index assessment by the pressure recording analytical method in infants after paediatric cardiac surgery: a pilot retrospective study. Interact Cardiovasc Thorac Surg. Epub ahead of print
Benes J, Giglio M, Brienza N (2014) The effect of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care 18:584
Akao M, Katsube Y, Kamisago M, Watanabe M, Abe M, Fukazawa R, Ogawa S, Itoh Y (2013) Developmental changes in left and right ventricular function evaluated with color tissue Doppler imaging and strain echocardiography. J Nippon Med Sch 80:260–267
Rowland DG, Gutgesell HP (1995) Noninvasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am J Cardiol 75:818–821
Senzaki H, Akagi M, Hishi T, Ishizawa A, Yanagisawa M, Masutani S, Kobayashi T, Awa S (2002) Age-associated changes in arterial elastic properties in children. Eur J Pediatr 161:547–551
Loosli CG, Potter EL (1959) Pre- and postnatal development of the respiratory portion of the human lung with special reference to the elastic fibers. Am Rev Respir Dis 80:5–23
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL (2005) Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med 31:517–523
Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL (2005) Esophageal doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med 31:1195–1201
Hadian M, Severyn DA, Pinsky MR (2011) The effects of vasoactive drugs on pulse pressure and stroke volume variation in postoperative ventilated patients. J Crit Care 26:328
Saxena R, Durward A, Steeley S, Murdoch IA, Tibby SM (2015) Predicting fluid responsiveness in 100 critically ill children: the effect of baseline contractility. Intensive Care Med 41:2161–2169
Kim JW, Bang JY, Park CS, Gwak M, Shin WJ, Hwang GS (2016) Usefulness of the maximum rate of pressure rise in the central and peripheral arteries after weaning from cardiopulmonary bypass in pediatric congenital heart surgery: a retrospective analysis. Medicine (Baltimore) 95:e5405
Choi DY, Kwak HJ, Park HY, Kim YB, Choi CH, Lee JY (2010) Respiratory variation in aortic blood flow velocity as a predictor of fluid responsiveness in children after repair of ventricular septal defect. Pediatr Cardiol 31:1166–1170
Romagnoli S, Ricci Z, Quattrone D, Tofani L, Tujjar O, Villa G, Romano SM, De Gaudio AR (2014) Accuracy of invasive arterial pressure monitoring in cardiovascular patients: an observational study. Crit Care 18:644
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no financial or non-financial conflict of interest.
Informed Consent
Formal consent is not required because we conducted a retrospective study.
Research Involving Human and Animal Participants
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Favia, I., Romagnoli, S., Di Chiara, L. et al. Predicting Fluid Responsiveness in Children Undergoing Cardiac Surgery After Cardiopulmonary Bypass. Pediatr Cardiol 38, 787–793 (2017). https://doi.org/10.1007/s00246-017-1582-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1582-0