Abstract
Purpose
Fluid overload is a risk factor for poor outcome in intensive care; thus volume loading should be tailored towards patients who are likely to increase stroke volume. We aimed to evaluate the paediatric predictive ability (stroke volume increase of at least 15 % after fluid bolus) of novel and established volumetric and dynamic haemodynamic variables, and assess the influence of baseline contractility on response.
Methods
We assessed 142 volume loading episodes (10 ml/kg crystalloid) in 100 critically ill ventilated children, median (interquartile) weight 10 (5.6–15) kg. Eight advanced haemodynamic variables were assessed using two commercially available devices. Systemic ventricular contractility was measured as the maximum rate of systolic arterial pressure rise.
Results
Overall, predictive ability was poor, with volumetric variables performing better than dynamic (area under receiver operating characteristic curves ranged from 0.53 to 0.67). The best predictor was total end-diastolic volume index; however, this did not increase in a consistent way with volume loading, with change post volume being weakly related to baseline values (r = −0.19, p = 0.02). A multivariable model quantified the importance of contractility in stroke volume response. Children with high baseline contractility (≥75th centile) typically achieved a positive stroke volume response when end-diastolic volume values changed by 10–15 ml/m2.6, whereas patients with low contractility (≤25th centile) typically required end-diastolic volume increases of 35–40 ml/m2.6.
Conclusions
Current paediatric predictors of volume response perform poorly; prediction may be improved if baseline contractility is taken into account.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluid resuscitation may increase cardiac stroke volume in critically ill patients with suboptimal preload [1]. However, this carries risk, as overzealous fluid administration can lead to fluid overload with deleterious consequences on patient outcome [2–4]. Approximately 40–60 % of patients will typically respond to fluid therapy, by increasing cardiac output by more than 10–15 % [5, 6]. Thus, the ability to predict which patients will respond may obviate the need for unnecessary fluid administration.
The past two decades have witnessed development of several haemodynamic variables aimed at predicting fluid responsiveness [1, 5–9]. Broadly speaking, these are classified as static and dynamic. Static variables are typically volumetric and estimate either (1) maximal ventricular volumes, found at end-diastole, or (2) an aspect of intravascular volume; both are commonly derived using indicator dilution techniques [10]. Dynamic variables rely on the principle that cyclical changes in preload occur during the ventilatory cycle, which translate into changes in stroke volume via the Frank–Starling mechanism: the greater the change, the higher the likelihood of volume response.
Early enthusiasm for the predictive ability of these variables has been tempered by later studies, which show a poorer performance when applied in normal clinical practice [11–13]. This is likely for a variety of reasons, including low tidal volume ventilation, spontaneous respirations, arrhythmias, and heart valve regurgitation [11–13]. However, two other haemodynamic factors which can compromise predictive ability require consideration. First, standardized volumes (e.g. 10 ml/kg) of administered fluid may not produce equivalent alterations in preload between patients. This is due to variability in (a) baseline blood volume, (b) mechanics of the venous vasculature (capacitance, compliance and resistance), (c) transthoracic pressure gradients, and (d) myocardial diastolic function [14, 15]. Second, patients with diminished contractility will manifest a flatter gradient of the Frank–Starling curve, yielding smaller changes in stroke volume for a given change in preload [16, 17].
Prediction of fluid responsiveness remains relatively underexplored in the paediatric intensive care unit (PICU), perhaps because of the challenges of cardiac output measurement. To date, most studies are small (typically less than 50 patients), and thus potentially both underpowered and prone to type 1 error [5]. None, to our knowledge, have addressed the two haemodynamic factors alluded to above. Thus, we aimed to evaluate volume responsiveness in a large PICU cohort (n = 100), using an accurate, indicator dilution method for cardiac output measurement. Our aims were threefold:
-
1.
To evaluate the predictive ability (stroke volume increase greater than 15 % after fluid administration) of a range of static and dynamic variables derived using two commercially available devices.
-
2.
To document typical changes in volumetric variables after a constant fluid bolus (10 ml/kg), and investigate factors associated with change in preload (end-diastolic volume).
-
3.
To investigate whether the baseline myocardial contractile status influences stroke volume response and hence predictive ability.
Methods
This prospective, non-randomized study was conducted within a 20-bed multidisciplinary PICU, after ethics committee approval and parental informed consent. Detailed methodology is available in the electronic supplement. Briefly, haemodynamic measurements were made within 24 h of admission, timed to coincide with fluid bolus administration. Fluid boluses (10 ml/kg of crystalloid over 20 min) were administered at the discretion of the attending clinicians, who were blinded to the advanced haemodynamic measurements. Variables were measured less than 15 min before and up to 30 min after fluid administration.
Standard haemodynamic variables included heart rate, arterial and central venous pressures. Advanced haemodynamic variables (Table 1) were measured using two commercially available devices. The first, CO-Status™ (Transonic systems, Ithaca, NY), uses an indicator dilution method: transpulmonary ultrasound velocity [18]. Cardiac output is calculated via the Stewart–Hamilton equation after venous injection of a small volume of 0.9 % saline; several static volumetric variables are also calculated from the curve properties (Table 1 and online supplement) [10]. The second device, Mostcare® (Vytech®, Padova, Italy) is a continuous system utilizing arterial pulse contour analysis (pressure recording analytical method, PRAM) [19]. This calculates beat-to-beat stroke volume via a custom algorithm, as well as a range of dynamic variables (Table 1). Variability measures (e.g. pulse pressure variability) were averaged over 30 s.
A positive fluid response was described as an increase in stroke volume index (SVI) of at least 15 % after fluid bolus. We chose arterial dp/dt MAX as a measure of systemic ventricular contractility, as this correlates closely with intraventricular dp/dt MAX [20]. Arterial load was as expressed as effective arterial elastance, using the Segers formula [21]. Cardiac volumetric data were allometrically scaled to body surface area using a power of 1.38 [i.e. (m2)1.38 = m2.6]; hence volumes are expressed as ml/m2.6 [22, 23].
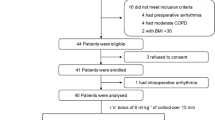
Inclusion criteria were (1) weight ≥2 kg, (2) age ≤16 years, (3) pre-existing arterial and central venous lines. Exclusion criteria were (1) significant valvular regurgitation, (2) large anatomical shunts, (3) residual left-sided obstructive lesions (e.g. aortic stenosis, coarctation), (4) extreme haemodynamic instability, (5) arrhythmias. Exclusions 1–3 were screened using transthoracic echocardiography. Patients with repaired single ventricle physiology (i.e. post Fontan operation) were included, as this should not compromise indicator dilution-based cardiac output assessment. However, the effect on intravascular volume estimation (which relies on mean transit times) is unknown; thus, a sensitivity analysis was anticipated (with and without Fontan patients).
Statistical methods
Data are expressed as mean (±SD) or median (IQR). Bivariate comparisons were via unpaired t tests. Models evaluating factors predicting and explaining fluid response were constructed using multilevel logistic and linear regression, respectively (adjusted for multiple measurements within patients). Model fit was quantified by the area under the receiver operating characteristic curve (AUROC) for logistic models, and adjusted r 2 for linear. Collinearity was quantified via the variance inflation factor. The statistical software was Stata v13.1 (StataCorp Texas).
Results
Study population and measurements
One hundred mechanically ventilated children, median (IQR) age 18 (6–48) months and weight 10 (5.6–15) kg, were enrolled between September 2010 and May 2012. The majority were admitted following cardiac surgery (supplement Table E2). Two subsets of this population have been published elsewhere [24, 25], and abstract results containing the first 47 patients are reported in a systematic review [5].
A total of 169 paired (pre- and post-fluid) measurements were taken; no patient had more than four measurement pairs. On review of the raw data from the dilution curves and PRAM outputs, 27 measurements were rejected because of poor signal quality or data capture issues, leaving 142 measurements for final analysis. Of these, 116 (82 %) were taken while patients were receiving inotropic agents (predominantly milrinone), and 19 (13 %) were receiving vasopressors. All patients were mechanically ventilated in SIMV mode (see Table E3). Muscle relaxants were not routinely used; however, sedation level was titrated to minimise spontaneous respirations, as evidenced by concordance of the set SIMV and measured patient respiration rates (Table E3). None received renal replacement therapy. The overall response rate (SVI increase at least 15 %) was 45.1 % (64/142).
Prediction of fluid responsiveness
There were no significant differences in baseline, basic haemodynamic data between responders and non-responders (Table 2). However, responders demonstrated lower stroke volumes and cardiac indices and higher systemic vascular resistance and effective arterial elastance.
Responders had significantly different values than non-responders for all baseline static haemodynamic variables, but for none of the dynamic variables (Table 2). Of relevance to the dynamic variables, there were no differences between the two groups in terms of expiratory tidal volumes: 12.0 ± 3.3 versus 11.8 ± 3.8 ml/kg (p = 0.73) or respiratory rate 18.4 ± 2.2 versus 18.6 ± 2.2 (p = 0.69).
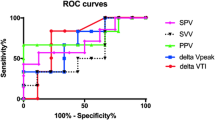
Despite these statistical differences, overall prediction was poor, with static volumetric variables generally performing better than dynamic variables in terms of AUROC (Table 2). The highest predictive value was demonstrated for TEDVI (AUROC 0.67), with optimal prediction occurring at TEDVI of 240 ml/m2.6. This yielded sensitivity 45.3 %, specificity 85.9 %, and correct classification rate 67.6 %. Prediction improved significantly when dp/dt MAX was added as a second predictor to TEDVI (AUROC increased from 0.67 to 0.71, p = 0.02).
Factors associated with change in preload (total end-diastolic volume)
Fluid bolus increased TEDVI, on average by 34.6 ± 34.2 ml/m2.6 for responders and 8.4 ± 34.7 ml/m2.6 for non-responders (p < 0.001). There were similar inter-group differences for other volumetric variables: delta CBVI (2.2 ± 2.3 versus −0.11 ± 2.4 ml/kg, p < 0.001), delta ACVI (6.3 ± 5.4 versus 0.4 ± 5.4 ml/kg, p < 0.001). The correlation between delta TEDVI and delta CBVI was r = 0.71, p < 0.001, which was higher than that for delta TEDVI and delta ACVI, r = 0.42, p < 0.001. Delta TEDVI did not have a significant correlation with delta central venous pressure, r = 0.14, p = 0.10, nor delta heart rate, r = 0.08, p = 0.37. Also, there was no difference in heart rate change after volume between responders and non-responders (mean change −4.7 versus −3.2 beats/min, p = 0.24).
There was a weak inverse relationship between delta TEDVI and baseline TEDVI (r = −0.19, p = 0.02; Fig. 1), but none between delta TEDVI and baseline central venous pressure (r = −0.03, p = 0.72), expiratory tidal volume (r = −0.01, p = 0.89), dp/dt MAX (r = 0.10, p = 0.24) or arterial load (r = −0.02, p = 0.83, see supplement Fig. E2).
Factors influencing fluid responsiveness
We investigated the joint influence of change in preload (delta TEDVI), baseline systemic ventricular contractility, and arterial load on the change in stroke volume (delta SVI) after fluid bolus using multivariable linear regression (Table 3; Fig. 2). The model explained 45 % of the variability in delta SVI after fluid bolus, with delta TEDVI being the dominant factor, exhibiting a partial R 2 value approximately five times greater than that for contractility (dp/dt MAX). Of note, arterial load was not related to delta SVI.
Scatter plot demonstrating the relationship between absolute change in stroke volume index and absolute change in total end-diastolic volume index after fluid administration. TEDVI total end-diastolic volume index. Grey circles represent patients who did not respond to fluid bolus, red squares represent responders
Although delta TEDVI explained the majority of the variability in delta SVI, baseline contractility was nonetheless an important factor. This is shown in Fig. 3, which was constructed using the regression coefficients from Table 3. Here the grey horizontal bar represents the cut-off whereby a positive delta SVI response is defined as at least 15 %. Children with high baseline contractility, defined as a dp/dt MAX ≥75th centile (≥1300 mmHg/s), typically achieved a positive SVI response with delta TEDVI values of 10–15 ml/m2.6, whereas patients with low contractility (dp/dt MAX ≤25th centile, ≤800 mmHg/s), typically required delta TEDVI values of 35–40 ml/m2.6.
Regression-derived multivariable relationship between percentage change in stroke volume index and absolute change in total end-diastolic volume index after fluid administration, taking into account baseline contractile state. TEDVI total end-diastolic volume index. Isobars represent centiles of contractility (dp/dt MAX) for the study population. The grey horizontal bar represents the cut-off when a positive delta stroke volume index response is defined as at least 15 %
Sensitivity analysis
Analyses were repeated (n = 114) after excluding single ventricle patients (post Fontan procedure). This produced a significant improvement in predictive ability for dp/dt MAX, with AUROC increasing from 0.61 to 0.68 (see supplement Table E4). For other variables, the predictive ability changed only slightly and not consistently. For example, the AUROC increased for TEDVI (from 0.67 to 0.69), but decreased for PPV (from 0.54 to 0.51). In terms of “factors associated with change in preload” (see above), exclusion of Fontan patients did not produce major qualitative changes in the relationship between delta TEDVI and any variable. For “factors influencing fluid responsiveness”, Fontan exclusion improved regression model fit (R 2 increased from 0.45 to 0.53); however, the coefficients and p values for individual variables changed little.
Discussion
Our first aim was to evaluate potential predictors of volume response using two novel, commercially available devices. Overall the predictive ability of most variables was poor, with static variables performing better than dynamic (see Table 2). This is largely concordant with Gan’s systematic review (12 studies, 438 children), which demonstrated limited predictive ability across a broader range of static and dynamic variables using different measuring devices to those in our study [5]. Our AUROC values compared to GanFootnote 1 are as follows: central venous pressure 0.53 versus 0.54, cardiac end-diastolic volumetric variables 0.67 versus 0.62, systolic pressure variability 0.59 versus 0.63, pulse pressure variability 0.54 versus 0.71, stroke volume variability 0.53 versus 0.69. The larger between-study AUROC differences for the last two variables have possible explanations. For pulse pressure variability, Gan’s higher AUROC is influenced by one outlier study; when this is excluded the value falls to 0.61 [5]. For stroke volume variability, our lower AUROC may be due to different methods of calculation. We used PRAM, which was not assessed by Gan; this technology may provide an inaccurate estimate of paediatric stoke volume [24], and predicts volume response poorly in adult studies, yielding an AUROC of 0.60 [26].
It is interesting to note that the predictive ability for the dynamic, arterial waveform-derived variables is markedly lower in children compared to adults, where typical AUROC values range from 0.84 to 0.96 [27, 28]. This may be due to age-related differences in vascular mechanical properties, affecting arterial waveform behaviour. There are dramatic (at least twofold) changes in both total arterial compliance and aortic characteristic impedance (normalized to body surface area) from birth to adulthood [29, 30], associated with changes in arterial vessel wall thickness and collagen fibre quantity/length [31]. There are also inherent differences in vascular properties within paediatric pathologies. For example, in congenital cardiovascular disease, average arterial elastance may exhibit twofold differences, dependent upon the anatomic cardiac lesion, being both higher and lower than that for normal children [32].
However, arterial mechanical factors would likely only explain a proportion of the differences between paediatric and adult studies. Thus, our second and third aims involved investigating factors that may affect predictive ability. Unsurprisingly, the largest determinant of change in SVI with volume loading was change in preload, as measured by TEDVI. However, preload did not change in a consistent fashion after volume loading, with delta TEDVI being only weakly correlated with baseline TEDVI (Fig. 1). This relationship was three to four times weaker in our patients compared to adults with sepsis [33], and in an animal haemorrhagic model [34], yielding correlation coefficients of −0.19 (current study), versus −0.65 and −0.73, respectively. We do not know the reason for this, but speculate that it may be due to variable degrees of systemic inflammation and capillary leak, differences in diastolic function [35, 36] and venous mechanics [37, 38] in paediatrics. Of note, very little is known about the mechanics of venous return in paediatric critical illness [39].
Our third finding emphasized the importance of considering systemic ventricular contractility when assessing predictive ability of end-diastolic volume. Although highlighted by others, this has only been quantified in terms of change in AUROC when contractility is dichotomized as “poor” versus “preserved” [40, 41]. We have extended these findings, by considering contractility as a continuous variable (Table 3; Fig. 3), allowing for a more precise quantification. This shows that a patient with poor baseline contractility needs an absolute increase in end-diastolic volume approximately 3–4 times greater than a patient with good contractility (i.e. 35–40 versus 10–15 ml/m2.6) to achieve SVI “responder” status (i.e. SVI increment of at least 15 %). This obviously requires higher volumes of fluid loading: Reuter showed that critically ill adult patients with reduced contractility require on average 25 ml/kg of 6 % hetastarch to reach the top of their Starling curves [17]. The clinical implications are important for patients with reduced contractility, who will require increased volume administration if a stroke volume increase of greater than 15 % is targeted, but are also at higher risk of inadequate fluid clearance and hence fluid overload [42]. Earlier inotrope usage with judicious fluid administration may be a preferable strategy for such patients.
Study limitations
-
1.
SVI and the volumetric variables (TEDVI, ACVI and CBVI) were derived from aspects of the ultrasound dilution curve, raising the possibility of mathematical coupling. Reassuringly, however, several clinical and animal studies have shown that coupling is unlikely with transpulmonary dilution curves, as volumetric variables remained constant when stroke volume was altered with beta agonists/blockers [33, 43, 44].
-
2.
We may not have evaluated stoke volume variability accurately, as PRAM has been found to be an inaccurate measure of SVI [24]. This may explain the discrepancy between our findings and the good prediction (AUROC 0.85) for aortic peak velocity, a variable closely related to stroke volume variability [45]. However, there is no reason to believe a similar error is present with PPV or SPV, as these were calculated directly from the arterial line.
-
3.
Spontaneous ventilation can compromise the predictive value of dynamic variables. Although our patients were ventilated in SIMV mode, the concordance of the set SIMV and measured patient respiration rates (Table E3) suggests that very little spontaneous respiration was occurring. Also, our AUROC for the dynamic variables were similar to other paediatric studies using CMV (see the first paragraph of “Discussion”).
-
4.
We used only one indirect measure of contractility (dp/dt MAX) derived from the arterial rather than the left ventricular waveform. However, this correlates closely with invasively measured intraventricular dp/dt MAX in children [20]. Also, the PRAM estimate of dp/dt MAX appears valid (despite being inaccurate for SVI) when compared to echocardiographically derived dp/dt MAX in adults [46]. It also tracks changes in inotropic state accurately in adults [47]. A limitation of this measure is its sensitivity to changes in preload [47]. However, this potential collinearity did not appear to be significant in our study, given the low regression variance inflation factors (Table 3).
-
5.
We did not assess the influence of venous mechanics on TEDVI response to volume loading. Bedside techniques for this are still relatively novel in adult practice [48], and pose distinct challenges in terms of paediatric application.
-
6.
Clinical indications for fluid administration were not recorded. The likelihood of volume response may possibly differ according to clinical indication or perhaps clinician seniority. Interestingly, a recent multicentre study has highlighted enormous variability with fluid challenges, in terms of indication, type, volume and assessment of response [49].
We suggest that these limitations are unlikely to change the three fundamental findings of our study. First, haemodynamic variables predict response to volume poorly when SVI response is dichotomized as less than or at least 15 %. Second, volume loading does not produce consistent changes in ventricular end-diastolic volumes. Third, baseline contractility plays an important role in influencing volume response. Paediatric studies investigating the role of venous mechanics in volume response are needed. We also suggest that more information would be gained from future studies if SVI response is expressed as a continuous variable.
Notes
Calculated using pooled, weighted data from relevant studies within Gan [5].
References
Marik PE (2009) Techniques for assessment of intravascular volume in critically ill patients. J Intensive Care Med 24:329–337
Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, Brett M, Convery M, Christie LM, Karamessinis L, Randolph AG, Pediatric Acute Lung Injury and Sepsis Investigator’s (PALISI) Network, Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet) (2012) Fluid balance in critically ill children with acute lung injury. Crit Care Med 40:2883–2889
Willson DF, Thomas NJ, Tamburro R, Truemper E, Truwit J, Conaway M, Traul C, Egan EE, Pediatric Acute Lung and Sepsis Investigators Network (2013) The relationship of fluid administration to outcome in the pediatric calfactant in acute respiratory distress syndrome trial. Pediatr Crit Care Med 14:666–672
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325
Gan H, Cannesson M, Chandler JR, Ansermino JM (2013) Predicting fluid responsiveness in children: a systematic review. Anesth Analg 117:1380–1392
Michard F, Teboul J (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 121:2000–2008
Michard F (2005) Changes in arterial pressure during mechanical ventilation. Anesthesiology 103:419–428
Monnet X, Teboul JL (2006) Invasive measures of left ventricular preload. Curr Opin Crit Care 12:235–240
Pinsky MR (2015) Understanding preload reserve using functional hemodynamic monitoring. Intensive Care Med 41:1480–1482
Stewart GN (1921) The pulmonary circulation time, the quantity of blood in the lungs and the output. Am J Physiol 58:20–44
Lansdorp B, Lemson J, van Putten MJ, de Keijzer A, van der Hoeven JG, Pickkers P (2012) Dynamic indices do not predict volume responsiveness in routine clinical practice. Br J Anaesth 108:395–401
de Waal EE, Rex S, Kruitwagen CL, Kalkman CJ, Buhre WF (2009) Dynamic preload indicators fail to predict fluid responsiveness in open-chest conditions. Crit Care Med 37:510–515
Rex S, Schälte G, Schroth S, de Waal EE, Metzelder S, Overbeck Y, Rossaint R, Buhre W (2007) Limitations of arterial pulse pressure variation and left ventricular stroke volume variation in estimating cardiac pre-load during open heart surgery. Acta Anaesthesiol Scand 51:1258–1267
Funk DJ, Jacobsohn E, Kumar A (2013) Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med 41:573–579
Lansdorp B, Hofhuizen C, van Lavieren M, van Swieten H, Lemson J, van Putten MJ, van der Hoeven JG, Pickkers P (2014) Mechanical ventilation-induced intrathoracic pressure distribution and heart–lung interactions. Crit Care Med 42:1983–1990
Kronas N, Kubitz JC, Forkl S, Kemming GI, Goetz AE, Reuter DA (2011) Functional hemodynamic parameters do not reflect volume responsiveness in the immediate phase after acute myocardial ischemia and reperfusion. J Cardiothorac Vasc Anesth 25:780–783
Reuter DA, Kirchner A, Felbinger TW, Weis FC, Kilger E, Lamm P, Goetz AE (2003) Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med 31:1399–1404
Krivitski NM, Kislukhin VV, Thuramalla NV (2008) Theory and in vitro validation of a new extracorporeal arteriovenous loop approach for hemodynamic assessment in pediatric and neonatal intensive care unit patients. Pediatr Crit Care Med 9:423–428
Romano SM, Pistolesi M (2002) Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med 30:1834–1841
Masutani S, Iwamoto Y, Ishido H, Senzaki H (2009) Relationship of maximum rate of pressure rise between aorta and left ventricle in pediatric patients. Implication for ventricular-vascular interaction with the potential for noninvasive determination of left ventricular contractility. Circ J 73:1698–1704
Segers P, Stergiopulos N, Westerhof N (2002) Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol 282:H1041–H1046
Lytrivi ID, Bhatla P, Ko HH, Yau J, Geiger MK, Walsh R, Parness IA, Srivastava S, Nielsen JC (2011) Normal values for left ventricular volume in infants and young children by the echocardiographic subxiphoid five-sixth area by length (bullet) method. J Am Soc Echocardiogr 24:214–218
Buechel EV, Kaiser T, Jackson C, Schmitz A, Kellenberger CJ (2009) Normal right- and left ventricular volumes and myocardial mass in children measured by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 11:19
Saxena R, Durward A, Puppala NK, Murdoch IA, Tibby SM (2013) Pressure recording analytical method for measuring cardiac output in critically ill children: a validation study. Br J Anaesth 110:425–431
Saxena R, Krivitski N, Peacock K, Durward A, Simpson JM, Tibby SM (2015) Accuracy of the transpulmonary ultrasound dilution method for detection of small anatomic shunts. J Clin Monit Comput 29(3):407–414
Biais M, Cottenceau V, Stecken L, Jean M, Ottolenghi L, Roullet S, Quinart A, Sztark F (2012) Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med 40:1186–1191
Marik PE, Cavallazzi R, Vasu T, Hirani A (2009) Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 37:2642–2647
Zhang Z, Lu B, Sheng X, Jin N (2011) Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth 25:904–916
Senzaki H, Akagi M, Hishi T, Ishizawa A, Yanagisawa M, Masutani S et al (2002) Age associated changes in arterial elastic properties in children. Eur J Pediatr 161:547–551
Sharp MK, Pantalos GM, Minich L, Tani LY, McGough EC, Hawkins JA (2000) Aortic input impedance in infants and children. J Appl Physiol 88:2227–2239
Roach MR, Burton AC (1959) The effect of age on the elasticity of human iliac arteries. Can J Biochem Physiol 37:557–570
Senzaki H, Chen CH, Masutani S, Taketazu M, Kobayashi J, Kobayashi T, Sasaki N, Asano H, Kyo S, Yokote Y (2001) Assessment of cardiovascular dynamics by pressure–area relations in pediatric patients with congenital heart disease. J Thorac Cardiovasc Surg 122:535–547
Michard F, Alaya S, Zarka V, Bahloul M, Richard C, Teboul JL (2003) Global end-diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest 124:1900–1908
Vigani A, Shih A, Queiroz P, Pariaut R, Gabrielli A, Thuramalla N, Bandt C (2012) Quantitative response of volumetric variables measured by a new ultrasound dilution method in a juvenile model of hemorrhagic shock and resuscitation. Resuscitation 83:1031–1037
Klitsie LM, Hazekamp MG, Roest AA, Van der Hulst AE, Gesink-van der Veer BJ, Kuipers IM, Blom NA, Ten Harkel AD (2013) Tissue Doppler imaging detects impaired biventricular performance shortly after congenital heart defect surgery. Pediatr Cardiol 34:630–638
Raj S, Killinger JS, Gonzalez JA, Lopez L (2014) Myocardial dysfunction in pediatric septic shock. J Pediatr 164:72–77
Geerts BF, Maas JJ, Aarts LP, Pinsky MR, Jansen JR (2010) Partitioning the resistances along the vascular tree: effects of dobutamine and hypovolemia in piglets with an intact circulation. J Clin Monit Comput 24:377–384
Scott-Douglas NW, Robinson VJB, Smiseth OA, Wright CI, Manyari DE, Smith ER, Tyberg JV (2002) Effects of acute blood volume changes on intestinal vascular capacitance: a mechanism whereby capacitance regulates cardiac output. Can J Cardiol 18:515–522
Macé L, Dervanian P, Bourriez A, Mazmanian GM, Lambert V, Losay J, Neveux JY (2000) Changes in venous return parameters associated with univentricular Fontan circulations. Am J Physiol Heart Circ Physiol 279:H2335–H2343
de la Oliva P, Menéndez-Suso JJ, Iglesias-Bouzas M, Álvarez-Rojas E, González-Gómez JM, Roselló P, Sánchez-Díaz JI, Jaraba S, Spanish Group for Preload Responsiveness Assessment in Children (2015) Cardiac preload responsiveness in children with cardiovascular dysfunction or dilated cardiomyopathy: a multicenter observational study. Pediatr Crit Care Med 16:45–53
Trof RJ, Danad I, Reilingh MW, Breukers RM, Groeneveld AB (2011) Cardiac filling volumes versus pressures for predicting fluid responsiveness after cardiovascular surgery: the role of systolic cardiac function. Crit Care 15:R73
Bagshaw SM, Cruz DN (2010) Fluid overload as a biomarker of heart failure and acute kidney injury. Contrib Nephrol 164:54–68
McLuckie A, Bihari D (2000) Investigating the relationship between intrathoracic blood volume index and cardiac index. Intensive Care Med 26:1376–1378
Buhre W, Kazmaier S, Sonntag H, Weyland A (2001) Changes in cardiac output and intrathoracic blood volume: a mathematical coupling of data? Acta Anaesthesiol Scand 45:863–867
Durand P, Chevret L, Essouri S, Haas V, Devictor D (2008) Respiratory variations in aortic blood flow predict fluid responsiveness in ventilated children. Intensive Care Med 34:888–894
Scolletta S, Bodson L, Donadello K, Taccone FS, Devigili A, Vincent JL, De Backer D (2013) Assessment of left ventricular function by pulse wave analysis in critically ill patients. Intensive Care Med 39:1025–1033
De Hert SG, Robert D, Cromheecke S, Michard F, Nijs J, Rodrigus IE (2006) Evaluation of left ventricular function in anesthetized patients using femoral artery dP/dt(max). J Cardiothorac Vasc Anesth 20:325–330
Maas JJ, Geerts BF, van den Berg PC, Pinsky MR, Jansen JR (2009) Assessment of venous return curve and mean systemic filling pressure in postoperative cardiac surgery patients. Crit Care Med 37:912–918
Cecconi M, Hofer C, Teboul JL et al (2015) Fluid challenges in intensive care: the FENICE study. Intensive Care Med 41:1529–1537
Acknowledgments
The manufacturers of the advanced haemodynamic monitors used in this study (Transonic Systems, Ithaca, New York and Vytech, Padova, Italy) provided hardware and consumables free of charge. Rohit Saxena received an educational grant from Transonic Systems, Ithaca, NY to partially cover his student fees with Kings College London. The current work forms part of his submission for the research degree MDres (Doctor of Medicine: Research).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest are declared for any author.
Additional information
Take-home message: The majority of advanced haemodynamic variables (volumetric and dynamic) have poor to moderate predictive ability in children in terms of the stroke volume response to fluid boluses. Baseline contractility is an important factor influencing patients’ response to fluid volume loading, such that a patient with poor baseline contractility will need to increase their end-diastolic volume by an increment that is 3–4 times greater than that of a patient with good contractility to be able to increase stroke volume by more than 15 %.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saxena, R., Durward, A., Steeley, S. et al. Predicting fluid responsiveness in 100 critically ill children: the effect of baseline contractility. Intensive Care Med 41, 2161–2169 (2015). https://doi.org/10.1007/s00134-015-4075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4075-8