Abstract
Purpose
Predicting fluid responsiveness is essential when treating surgical or critically ill patients. When using a pulmonary artery catheter, pulse pressure variation and systolic pressure variation can be calculated from right ventricular and pulmonary artery pressure waveforms.
Methods
We conducted a prospective interventional study investigating the ability of right ventricular pulse pressure variation (PPVRV) and systolic pressure variation (SPVRV) as well as pulmonary artery pulse pressure variation (PPVPA) and systolic pressure variation (SPVPA) to predict fluid responsiveness in coronary artery bypass (CABG) surgery patients. Additionally, radial artery pulse pressure variation (PPVART) and systolic pressure variation (SPVART) were calculated. The area under the receiver operating characteristics (AUROC) curve with 95%-confidence interval (95%-CI) was used to assess the capability to predict fluid responsiveness (defined as an increase in cardiac index of > 15%) after a 500 mL crystalloid fluid challenge.
Results
Thirty-three patients were included in the final analysis. Thirteen patients (39%) were fluid-responders with a mean increase in cardiac index of 25.3%. The AUROC was 0.60 (95%-CI 0.38 to 0.81) for PPVRV, 0.63 (95%-CI 0.43 to 0.83) for SPVRV, 0.58 (95%-CI 0.38 to 0.78) for PPVPA, and 0.71 (95%-CI 0.52 to 0.89) for SPVPA. The AUROC for PPVART was 0.71 (95%-CI 0.53 to 0.89) and for SPVART 0.78 (95%-CI 0.62 to 0.94). The correlation between pulse pressure variation and systolic pressure variation measurements derived from the different waveforms was weak.
Conclusions
Right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation seem to be weak predictors of fluid responsiveness in CABG surgery patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Predicting fluid responsiveness is essential when treating surgical or critically ill patients. Fluid responsiveness can be predicted using variables describing pressure variation in the arterial blood pressure waveform [1, 2]. Pressure variation in the arterial blood pressure waveform caused by positive pressure ventilation can be quantified by calculating pulse pressure variation or systolic pressure variation [3,4,5].
Pulse pressure variation becomes less predictive for fluid responsiveness in the presence of cardiac dysfunction [6], ventilation with low tidal volume [7], or reduced pulmonary compliance [2] that can be frequently found in cardiac surgery and critically ill patients. These patients, however, may particularly benefit from guided fluid management.
Right heart catheterization with a pulmonary artery catheter is routinely performed in cardiac surgery patients. Pulse pressure variation and systolic pressure variation can thus be calculated for right ventricular and pulmonary artery pressure waveforms – similarly as for the radial artery blood pressure waveform.
The right ventricle and pulmonary artery are low pressure high-compliant structures [8]. Nonetheless, their thinner muscle wall – compared to the left ventricle and systemic arteries – as well as their direct connection to the venous system may make them prone to be affected by changes in venous return [9]. From a physiological perspective, it seems reasonable to assume that the right ventricle and pulmonary artery are also sensitive to respiratory cycle-induced changes in cardiac preload [10, 11].
We hypothesized that right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation may predict fluid responsiveness in mechanically ventilated patients.
To test this hypothesis we performed a prospective interventional study investigating the ability of right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation to predict fluid responsiveness in coronary artery bypass (CABG) surgery patients.
2 Methods
2.1 Study design and setting
This prospective interventional study was performed in patients having CABG surgery at the University Medical Center Hamburg-Eppendorf, Hamburg, Germany. The study was approved by the ethics committee (Ethikkomission der Ärztekammer Hamburg, Hamburg, Germany; registration number PV5366) and adhered to the principles outlined in the Declaration of Helsinki. The study was conducted between September 2017 and June 2018. All patients provided written informed consent.
2.2 Study participants
We included adult patients (18 years or older) scheduled for CABG surgery who had pulmonary artery catheters as part of routine care. Patients with cardiac arrhythmia, active pacemaker, high grade tricuspid regurgitation, persistent foramen ovale, pregnancy, or patients who were unable to provide informed consent were excluded from the study.
2.3 Anesthetic management
General anesthesia was maintained with continuous sufentanil infusion and inhaled sevoflurane. All patients were mechanically ventilated using positive pressure ventilation at the discretion of the attending anesthesiologist. Ventilator settings as well as administered medications including norepinephrine and epinephrine were at the discretion of the attending anesthesiologist. A radial artery catheter and a pulmonary artery catheter were part of routine care.
2.4 Study measurements
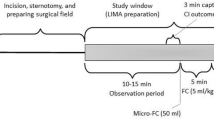
We recorded the right ventricular and pulmonary artery blood pressure waveforms using a 7.5 F 110 cm pulmonary artery catheter (Thermodilution VIP+; Edwards Lifesciences, Irvine, CA, USA) with separate pressure transducers for each pressure output signal. We simultaneously recorded the arterial blood pressure waveform from a radial artery catheter. All pressure transducers were zeroed to the level of the right atrium, which was repeated when the patient’s body position changed. Correct placement and adequate signal quality of the pressure waveforms were assessed prior to the first measurement. All signals were recorded on the patient monitor (Infinity Delta Monitor; Dräger, Lübeck, Germany) and extracted to a personal computer (eData Data Grabber; Dräger). The blood pressure waveforms were recorded continuously at four different time points when the patient’s chest was closed: (1) baseline in supine position; (2) in Trendelenburg position (to induce transfer of venous blood into the intrathoracic compartment); (3) after return to supine position; and (4) after completion of a fluid challenge with 500 mL crystalloid fluid (Sterofundin, B. Braun, Melsungen, Germany) (Fig. 1).
We calculated pulse pressure variation and systolic pressure variation for the right ventricular (PPVRV, SPVRV), pulmonary artery (PPVPA, SPVPA), and radial artery (PPVART, SPVART) blood pressure waveforms post hoc using manual offline calculation (Supplementary Fig. 1). We analyzed the blood pressure waveforms using an adapted beat detection algorithm [12] to determine maxima (Pmax) and minima (Pmin) of pulse pressure and systolic pressure. For calculation of pulse pressure, we used the diastolic pressure and the subsequent systolic pressure (Fig. 2). In each case, pressure variation was calculated as: pressure variation (%) = 100 x (Pmax – Pmin) / (Pmax + Pmin) / 2. After the exclusion of artifacts, we used the first four Pmax/Pmin-pairs corresponding to four respiratory cycles to calculate pulse pressure variation and systolic pressure variation for each measurement time point.
Methods. (a) Simultaneous recording of right ventricular, pulmonary artery, and radial artery blood pressure waveforms. (b) Pulse pressure variation and systolic pressure variation. RV – right ventricle; PA – pulmonary artery; ART – radial artery; PPV – pulse pressure variation; SPV – systolic pressure variation
At each measurement time point, we performed intermittent pulmonary artery thermodilution and calculated the mean cardiac index based on three repeated measurements. Further, we measured the mixed-venous oxygen saturation using point-of-care blood gas analysis.
2.5 Definition of fluid responsiveness
Patients were separated into “fluid-responders”, if the increase in cardiac index was > 15% after the 500 mL crystalloid fluid challenge, or “non-responders”, if the increase in cardiac index was ≤15% after the fluid challenge.
2.6 Statistical analysis
Descriptive data are reported as mean ± standard deviation (SD) for continuous data and as absolute frequency and percentage for categorical data. We used the area under the receiver operating characteristics (AUROC) curve with 95%-confidence interval (95%-CI) to analyze the ability of the different pressure variation variables to predict fluid responsiveness. The optimal threshold to predict fluid responsiveness for each pressure variation variable was determined using the Youden index [sensitivity + (specificity – 1)]. The AUROC was only calculated for measurements obtained directly before the fluid challenge (time point 3: after return to supine position). The relationship between pulse pressure variation and systolic pressure variation obtained from the three pressure waveforms was analyzed using Pearson’s correlation coefficient. We analyzed differences between “fluid-responders” and “non-responders” using Mann-Whitney U tests for independent data pairs. To investigate differences between consecutive measurement points we used Wilcoxon signed-rank tests for paired measurements.
For the sample size calculation we assumed that 50% of the included patients would be fluid responders. Power analysis showed that a sample size of 21 patients per group, thus 42 patients in total, would be necessary to detect an AUROC of 0.75 or higher with the alpha level set at 0.05 and the beta level at 0.20 (i.e., a power of 80%). A P-value of less than 0.05 was considered statistically significant for all tests. No correction for multiple testing was performed. We used SPSS Statistics 25 (IBM, Armonk, NY, USA) and R (version 4.0.5, corrplott-package version 0.9, R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses.
3 Results
A total of 45 patients were included in the study. Four patients were excluded because of cardiac arrhythmia and 8 because of an active pacemaker; thus 33 patients were included in the final analysis. Patients’ characteristics, ventilator settings, and administered medications are shown in Table 1.
Thirteen patients (39%) were fluid-responders with a mean (± SD) increase in cardiac index of 25.3% (± 10.0%) after the fluid challenge. Cardiac index and mixed-venous oxygen saturation were lower in fluid-responders prior to the fluid challenge (Table 2). Before the fluid challenge, there were no important differences in right ventricular and pulmonary artery pressure variation between fluid-responders and non-responders. Radial artery pressure variation, however, was higher in fluid-responders (Table 3). Trendelenburg positioning and fluid challenge-induced hemodynamic changes are shown in Tables 2 and 3.
The AUROC was 0.60 (95%-CI 0.38 to 0.81) for PPVRV, 0.63 (95%-CI 0.43 to 0.83) for SPVRV, 0.58 (95%-CI 0.38 to 0.78) for PPVPA, and 0.71 (95%-CI 0.52 to 0.89) for SPVPA (Fig. 3). The AUROC for PPVART was 0.71 (95%-CI 0.53 to 0.89) and for SPVART 0.78 (95%-CI 0.62 to 0.94). The respective Youden indices with optimal thresholds are shown in Table 4. Scatter plots showing pressure variations and changes in cardiac index are shown in Supplementary Fig. 2.
Receiver operating characteristic curves. Receiver operating characteristic curves for the prediction of > 15% increase in cardiac index by (a) pulse pressure variation and (b) systolic pressure variation derived from the right ventricle, pulmonary artery, and radial artery blood pressure waveforms. RV – right ventricle; PA – pulmonary artery; ART – radial artery
Correlation analyses of the different pressure variation variables are shown in Fig. 4. The correlation between pulse pressure variation measurements of the different pressure waveforms was weak (PPVRV and PPVPA: r = 0.41; PPVRV and PPVART: r = 0.39; PPVPA and PPVART: r = 0.51). In line, the correlation between systolic pressure variation measurements was also weak (SPVRV and SPVPA: r = 0.54; SPVRV and SPVART: r = 0.45; SPVPA and SPVART: r = 0.57). In contrast, correlation analyses of corresponding pulse pressure variation and systolic pressure variation measurements of the same pressure waveform showed a stronger positive correlation (PPVRV and SPVRV: r = 0.75; PPVPA and SPVPA: r = 0.59; PPVART and SPVART: r = 0.88).
Correlation analysis. Correlation analysis between pulse pressure variation and systolic pressure variation obtained from the different blood pressure waveforms. Circle color and size resemble the value of Pearson’s correlation coefficient. PPVRV – right ventricular pulse pressure variation; SPVRV – right ventricular systolic pressure variation; PPVPA –pulmonary artery pulse pressure variation; SPVPA – pulmonary artery systolic pressure variation; PPVART – radial artery pulse pressure variation; SPVART – radial artery systolic pressure variation
4 Discussion
Just contrary to our hypothesis, this study shows that right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation derived from a pulmonary artery catheter seem to be weak predictors of fluid responsiveness in CABG surgery patients – and that their predictive value was lower than that of radial artery pressure variations.
In cardiac surgery and critically ill patients, it is essential to reliably predict fluid responsiveness to optimize fluid administration [13]. Pulse pressure variation and systolic pressure variation from the radial artery blood pressure waveform can predict fluid responsiveness [2, 5]. Pulmonary artery catheters allow for pulmonary artery thermodilution to measure cardiac output [14] and – if catheters have an additional port – continuous recording of both right ventricular and pulmonary artery pressure waveforms. Intermittent changes in cardiac preload during the respiratory cycle, as an indicator for fluid responsiveness, might cause more pronounced pressure alterations in the right ventricle and pulmonary artery compared to systemic arteries [11]. First experimental studies have investigated right ventricular stroke volume [15] and right ventricular stroke volume variation [10, 16] to predict fluid responsiveness with promising results. Further, respiratory variation in right atrial pressure has been previously suggested as a predictor of fluid responsiveness [17].
We therefore hypothesized that right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation may also predict fluid responsiveness in mechanically ventilated patients. Just contrary to our hypothesis, right ventricular and pulmonary artery systolic pressure variation and pulse pressure variation were less predictive for fluid responsiveness than radial artery pressure variation in mechanically ventilated CABG surgery patients. As expected with the low predictive accuracy for fluid responsiveness, our results do not show distinct differences for right ventricular or pulmonary artery pressure variation between fluid-responders and non-responders. The moderate predictive accuracy for radial artery pressure variation was similar to previous studies in patients after cardiac surgery [18, 19]. The low predictive accuracy of right ventricular and pulmonary artery pressure variation may be – in part – explained by high right ventricular and pulmonary artery compliance [8]. Because of the high compliance of the right ventricle and pulmonary artery, changes in volume presumably cause smaller changes in pressure and pressure variation (Supplementary Fig. 1). Further, mechanical ventilation induces changes in intra-thoracic pressure and, thus, in extramural pressure of the right ventricle and pulmonary artery. These changes in extramural pressure may affect pressure variation in the right ventricle and pulmonary artery regardless of volume status.
On a side note, Trendelenburg positioning – to induce a reversible transfer of venous blood into the intra-thoracic compartment – increased right ventricular and pulmonary artery pressure, but had no important effect on pulse or systolic pressure variation. Overall, our results suggest that calculating right ventricular or pulmonary artery pulse pressure variation and systolic pressure variation is possible, but unlikely provides additional information for the prediction of (systemic) fluid responsiveness.
A strength of the study is that cardiac output (used to define fluid responsiveness after a fluid challenge) was measured using the clinical reference method – pulmonary artery thermodilution [20]. However, we performed our study only in cardiac surgery patients – as these patients are routinely monitored with pulmonary artery catheters. Naturally, our results may thus not be generalizable to non-cardiac surgery or critically ill patients – who are rarely monitored with pulmonary artery catheters. Further, investigating patients with right ventricular failure may have yielded different results. Varying times between interventions and measurements may have also affected the results. Tidal volumes less than 8 mL per kilogram predicted body weight may have reduced the predictive accuracy of pressure variations [2] – but reflect current clinical routine [21]. The effect of fluid challenges may have been more pronounced if we had used colloids [22, 23]. Since their use is controversial in critically ill patients [24], we only used crystalloids for fluid challenges. Additionally, the 95%-CI of our results are wide as we included fewer patients than initially planned. Nonetheless it seems unlikely that right ventricular or pulmonary artery pressure variation have a higher predictive value than radial artery pressure variation. Finally, the Trendelenburg positioning was not standardized (and should not be mistaken for a passive leg raising maneuver [25]).
5 Conclusions
Right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation seem to be weak predictors of fluid responsiveness in CABG surgery patients. In our study, right ventricular and pulmonary artery pressure variation had a lower predictive capability for fluid responsiveness than radial artery pressure variation.
References
Teboul JL, Monnet X, Chemla D, Michard F. Arterial Pulse Pressure Variation with Mechanical Ventilation. Am J Respir Crit Care Med. 2019;199(1):22–31. doi:https://doi.org/10.1164/rccm.201801-0088CI.
Alvarado Sanchez JI, Caicedo Ruiz JD, Diaztagle Fernandez JJ, Amaya Zuniga WF, Ospina-Tascon GA, Cruz Martinez LE. Predictors of fluid responsiveness in critically ill patients mechanically ventilated at low tidal volumes: systematic review and meta-analysis. Ann Intensive Care. 2021;11(1):28. doi:https://doi.org/10.1186/s13613-021-00817-5.
Kubitz JC, Forkl S, Annecke T, Kronas N, Goetz AE, Reuter DA. Systolic pressure variation and pulse pressure variation during modifications of arterial pressure. Intensive Care Med. 2008;34(8):1520–4. doi:https://doi.org/10.1007/s00134-008-1114-8.
Mathis MR, Schechtman SA, Engoren MC, Shanks AM, Thompson A, Kheterpal S, Tremper KK. Arterial Pressure Variation in Elective Noncardiac Surgery: Identifying Reference Distributions and Modifying Factors. Anesthesiology. 2017;126(2):249–59. doi:https://doi.org/10.1097/aln.0000000000001460.
Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162(1):134–8. doi:https://doi.org/10.1164/ajrccm.162.1.9903035.
Wyler von Ballmoos M, Takala J, Roeck M, Porta F, Tueller D, Ganter CC, Schröder R, Bracht H, Baenziger B, Jakob SM. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010;14(3):R111. doi:https://doi.org/10.1186/cc9060.
De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517–23. doi:https://doi.org/10.1007/s00134-005-2586-4.
Pinsky MR. The right ventricle: interaction with the pulmonary circulation. Crit Care. 2016;20(1):266. doi:https://doi.org/10.1186/s13054-016-1440-0.
Thenappan T, Prins KW, Pritzker MR, Scandurra J, Volmers K, Weir EK. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann Am Thorac Soc. 2016;13(2):276–84. doi:https://doi.org/10.1513/AnnalsATS.201509-599FR.
Kubitz JC, Annecke T, Kemming GI, Forkl S, Kronas N, Goetz AE, Reuter DA. The influence of positive end-expiratory pressure on stroke volume variation and central blood volume during open and closed chest conditions. Eur J Cardiothorac Surg. 2006;30(1):90–5. doi:https://doi.org/10.1016/j.ejcts.2006.04.008.
Bellofiore A, Chesler NC. Methods for measuring right ventricular function and hemodynamic coupling with the pulmonary vasculature. Ann Biomed Eng. 2013;41(7):1384–98. doi:https://doi.org/10.1007/s10439-013-0752-3.
Zong W, Heldt T, Moody GB, Mark RG An open-source algorithm to detect onset of arterial blood pressure pulses. In: Computers in Cardiology, 2003, 21–24 Sept. 2003 2003. pp 259–262. doi:https://doi.org/10.1109/CIC.2003.1291140.
Miller TE, Mythen M, Shaw AD, Hwang S, Shenoy AV, Bershad M, Hunley C. Association between perioperative fluid management and patient outcomes: a multicentre retrospective study. Br J Anaesth. 2021;126(3):720–9. doi:https://doi.org/10.1016/j.bja.2020.10.031.
Kouz K, Michard F, Bergholz A, Vokuhl C, Briesenick L, Hoppe P, Flick M, Schon G, Saugel B. Agreement between continuous and intermittent pulmonary artery thermodilution for cardiac output measurement in perioperative and intensive care medicine: a systematic review and meta-analysis. Crit Care. 2021;25(1):125. doi:https://doi.org/10.1186/s13054-021-03523-7.
Berger D, Hobi J, Möller PW, Haenggi M, Takala J, Jakob SM. Right ventricular stroke volume assessed by pulmonary artery pulse contour analysis. Intensive Care Med Exp. 2020;8(1):58. doi:https://doi.org/10.1186/s40635-020-00347-7.
Kubitz JC, Richter HP, Petersen C, Goetz AE, Reuter DA. Right ventricular stroke volume variation: a tool to assess right ventricular volume responsiveness. Minerva Anestesiol. 2014;80(9):992–5.
Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76–85. doi:https://doi.org/10.1016/0883-9441(92)90032-3.
Fischer MO, Pelissier A, Bohadana D, Gérard JL, Hanouz JL, Fellahi JL. Prediction of responsiveness to an intravenous fluid challenge in patients after cardiac surgery with cardiopulmonary bypass: a comparison between arterial pulse pressure variation and digital plethysmographic variability index. J Cardiothorac Vasc Anesth. 2013;27(6):1087–93. doi:https://doi.org/10.1053/j.jvca.2013.02.024.
Vistisen ST, Struijk JJ, Larsson A. Automated pre-ejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand. 2009;53(4):534–42. doi:https://doi.org/10.1111/j.1399-6576.2008.01893.x.
Rajaram SS, Desai NK, Kalra A, Gajera M, Cavanaugh SK, Brampton W, Young D, Harvey S, Rowan K. (2013) Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev (2):CD003408. doi:https://doi.org/10.1002/14651858.CD003408.pub3.
Futier E, Constantin J-M, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant J-Y, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin J-E, Pereira B, Jaber S. A Trial of Intraoperative Low-Tidal-Volume Ventilation in Abdominal Surgery. N Engl J Med. 2013;369(5):428–37. doi:https://doi.org/10.1056/NEJMoa1301082.
Ma GG, Hao GW, Yang XM, Zhu DM, Liu L, Liu H, Tu GW, Luo Z. Internal jugular vein variability predicts fluid responsiveness in cardiac surgical patients with mechanical ventilation. Ann Intensive Care. 2018;8(1):6. doi:https://doi.org/10.1186/s13613-017-0347-5.
Cherpanath TG, Geerts BF, Maas JJ, de Wilde RB, Groeneveld AB, Jansen JR. Ventilator-induced central venous pressure variation can predict fluid responsiveness in post-operative cardiac surgery patients. Acta Anaesthesiol Scand. 2016;60(10):1395–403. doi:https://doi.org/10.1111/aas.12811.
Perel P, Roberts I, Ker K. (2013) Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev (2):Cd000567. doi:https://doi.org/10.1002/14651858.CD000567.pub6.
Monnet X, Teboul J-L. Passive leg raising: five rules, not a drop of fluid! Crit Care. 2015;19(1):18. doi:https://doi.org/10.1186/s13054-014-0708-5.
Funding
Support was provided solely from institutional and departmental sources.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the ethics committee (Ethikkomission der Ärztekammer Hamburg, Hamburg, Germany; registration number PV5366).
Informed consent
All patients provided written informed consent.
Conflict of interest
MF has received honoraria for consulting and giving lectures from CNSystems Medizintechnik (Graz, Austria). BS is a consultant for and has received honoraria for giving lectures from Edwards Lifesciences Inc. (Irvine, CA, USA). BS is a consultant for and has received institutional restricted research grants and honoraria for giving lectures from Pulsion Medical Systems SE (Feldkirchen, Germany). BS has received institutional restricted research grants and honoraria for giving lectures from CNSystems Medizintechnik. BS is a consultant for and has received institutional restricted research grants from Retia Medical LLC (Valhalla, NY, USA). BS is a consultant for and has received honoraria for giving lectures from Philips Medizin Systeme Böblingen GmbH (Böblingen, Germany). BS is a consultant for and has received honoraria for giving lectures from GE Healthcare (Chicago, IL, USA). BS was a consultant for and has received institutional restricted research grants from Tensys Medical Inc. (San Diego, CA, USA). DF is an employee of CNSystems Medizintechnik. US, AB, KK, BR, and JCK have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flick, M., Sand, U., Bergholz, A. et al. Right ventricular and pulmonary artery pulse pressure variation and systolic pressure variation for the prediction of fluid responsiveness: an interventional study in coronary artery bypass surgery patients. J Clin Monit Comput 36, 1817–1825 (2022). https://doi.org/10.1007/s10877-022-00830-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-022-00830-4