Abstract
Device selection and procedural guidance for percutaneous ductal closure strongly rely upon angiographic and echocardiographic imaging. Current literature recognises 2D echocardiography as an essential tool for diagnosis and assessment but does not define a consistent methodology to optimise ductal measurement. There is little research comparing echocardiography with gold standard angiography for ductal measurement. Proving 2D echocardiographic ductal measurement to be equivalent to angiography could pave the way for its use as the primary modality in image guidance for percutaneous closure of the ductus. This was a retrospective study of 100 consecutive paediatric patients who underwent percutaneous ductal closure. Echocardiographic images were studied to determine ductal (a) morphology (b) dimensions (length, aortic ampulla, pulmonary end, minimum diameter) (c) size of device that would be appropriate for closure. These data were compared to corresponding measurements generated by angiographic images. Inter and intra-observer ratings were calculated to assess levels of agreement. There were significant differences between the imaging methods in classifying the morphological sub-type and ductal measurements (p < 0.005), except for length which was not found to be significantly different between modalities. Prediction of device selection from angiographic images showed excellent agreement (weighted k = 0.81). Predictions based on echocardiographic images showed a poor level of agreement (weighted k = 0.14). We found poor correlation between echocardiography and angiography for measurement, morphological assessment and device selection. Based on our findings, percutaneous arterial duct occlusion without angiographic guidance in this age group cannot be advocated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Clinical evaluation and echocardiography are currently considered the key diagnostic tools in the initial assessment of patent ductus arteriosus (PDA) [1–3].
Accurate assessment of ductal dimensions and morphology is crucial in determining percutaneous closure suitability, device choice and procedural guidance, hence at the time of intervention, the vast majority of operators choose to measure the PDA angiographically [4, 5]. Recent work, extolling echocardiography’s diagnostic and guidance roles for ductal occlusion has called into question the need for routine angiography for ductal delineation [6, 7]. The potential benefits of this approach are obvious, including decreased contrast and radiation exposure and decreasing procedure time.
As literature describing a consistent correlation between two-dimensional echocardiographic and angiographic assessment is still limited [8, 9] we planned to assess the degree of correlation between these modalities in a preliminary effort to determine circumstances where echocardiography could replace angiography.
Methods
Study Population and Data Collection
We reviewed 100 consecutive patients who underwent percutaneous PDA closure at a single tertiary referral centre for congenital heart disease—Evelina London Children’s Hospital (ECH)—between August 2011 and January 2016.
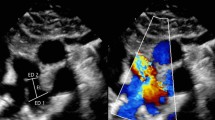
Echocardiograms recorded within seven days of catheterisation as part of a preassessment and procedural angiography were reviewed. All procedures analysed were successful with no clinically significant residual leaks, pulmonary artery or aortic stenosis on predischarge echocardiography. Patients older than 16 years or with other associated significant congenital cardiac defects were excluded. Demographic and procedural data were collected from our departmental database, Heartsuite™ (Systeria, Glasgow, UK). All echocardiograms were reviewed by two experienced observers using Philips Xcelera™ software (Philips 5680 DA Best, The Netherlands). Angiograms were reviewed by two experienced operators using GE-PACS (General Electrics, Milwaukee, USA) software. Imaging was anonymised, and observers were blinded to previous measurements. For both echocardiography and angiography, ductal measurements made were those thought to have the most influence on the choice of the closure device: aortic ampulla diameter (mm), narrowest diameter (mm), pulmonary end diameter (mm) and length (mm).
Ductal morphology was also categorised according to the angiographic classification outlined by Krichenko et al. [10]. Type and size of the devices used were also recorded for comparison.
Echocardiographic Imaging Analysis
Reviewed echocardiographic images included standard parasternal views optimised to define the duct. These were usually the high parasternal window for the ductal length and the traditional “ductal view” to measure the narrowest portion, the pulmonary end and aortic ampulla. Colour Doppler mapping was also reviewed when 2D scans were insufficient for ductal characterisation as in ducts of small dimensions or unusual anatomy.
Angiography Imaging Analysis
Biplane angiograms were available for review in all cases, and measurements were attempted in both planes using the angles selected by the procedural operator. In the vast majority of cases a straight lateral projection offered most information about ductal morphology, length and diameters.
Reproducibility, Intra- and Inter-observer Variability Evaluation
Analysis of angiography versus echocardiography also included ten datasets randomly selected from the dataset and two separate experienced raters performed repeated measurements in each modality using the same protocol criteria for original ductal measurements.
Predicting Device Selection from Echocardiography and Angiography
The level of agreement between the different imaging modalities was also tested by predicting device choice from echocardiography and angiographic findings. The devices chosen to serve as references were the Amplatzer® Ductal Occluder (ADOI®) and the Ceraflex™ PDA occluder for their similarity of design and sizing criteria as well as their wide and well-known applicability in PDA closure. An experienced interventionist first reviewed the echocardiograms and was asked to predict if the ductus was suitable for device closure with ADOI/Ceraflex and if so, what size of device would be chosen. The dataset was then shuffled before the same interventionist analysed the angiography with the same set if questions [11]. These hypothetical device selections were then compared with the actual device choice. Forty ducts had been closed with ADOI® (n = 24) or Ceraflex™ (n = 16) allowing direct comparison with 40 hypothetical device choices. The complete process of data analysis is summarised in Fig. 1.
The study was approved by the institutional board of the Evelina London Children’s Hospital, and need for individual consent was waived due to the anonymised retrospective nature of the study.
Statistical Analysis
Analysis was performed using Minitab® statistical software 17, SPSS® 22 and MedCalc®. Results are presented as mean ± standard deviation or median with range. For comparison of angio- and echocardiographic measurements, a paired t test was used and Pearson’s product moment correlation coefficient was used for analysis of correlation of agreement between measurements with both imaging techniques. Bland–Altman plots were used to compare the two measurement techniques by evaluating the bias between the mean differences leading to an estimate agreement interval, within which 95% of the differences between echo and angiography measurements fall [12, 13].
Inter- and intra-operator measurements reliability was analysed using interclass correlation coefficient (ICC): a Cohen’s Kappa analysis result of >0.6 and >0.8 was considered good and excellent correlations, respectively [14, 15].
The analysis of the predicted device choice from different imaging modalities was graded depending on whether the hypothetical device choice matched or was under or overestimated. A weighted Cohen’s Kappa was performed to determine the level of agreement. All measurements were rounded to the closest millimetre to allow effective data analysis.
Results
Demographics
Data were collected on 100 consecutive patients who underwent percutaneous ductal closure. The median age was 1.6 years (range 4 months to 15.3 years), 23% were infants. Median weight was 10.75 kg (range from 5 to 75.2 kg). There was an even male to female split with 56% female patients.
Inter- and Intra-observer Agreement
Echocardiographic and angiographic measurements were analysed for intra- and inter-observer agreement using an ICC. There was excellent intra- and inter-observer agreement for both modalities (Table 1).
Ductal Measurement Correlation between Echocardiography and Angiography
Globally, no agreement was found between echocardiography and angiography measurements. There was a tendency for echocardiography to significantly overestimate the size of both the pulmonary end and narrowest diameter and to underestimate the size of the aortic ampulla diameter. Length diameter was the exception with no significant difference between the two modalities found (Table 2).
Ductal Morphology
There was a significant difference between the echocardiographic and angiographic morphological classification. Although there was no correlation between imaging modalities, type A was most common morphology on both echocardiography and angiography (Fig. 2).
Predicting Device Selection from Echocardiography and Angiography
For those patients who received ADOI® or Ceraflex™ device (n = 40), there was a perfect match in 29 cases (73%) with the angiographically predicted device size (Table 3) and excellent overall agreement: weighted k = 0.81 (95% CI 0.68–0.93) (Table 4). In seven cases the predictions were one device size different, and in the other four cases the reviewing interventionist did not feel that the reviewed angiographic images could be used to predict suitability and/or sizing for a device.
When using echocardiography there was a poor level of agreement with only 11 patients (28%) correctly predicted (Table 3). In 16 cases the device size was overestimated, and in five cases the size was underestimated. In eight cases the reviewing interventionist did not feel that the echocardiograms could be used to predict suitability and sizing for an ADOI®. weighted k = 0.14 (95% CI −0.03 to 0.31) (Table 4).
Discussion
The indication for ductal closure should be determined by symptoms and hemodynamic assessment based on echocardiography. Although angiography is currently considered the gold standard for precise delineation of the duct, integration of echocardiography and angiography could help to decrease radiation exposure [16].
Previous work aiming to find a significant correlation between echocardiographic and angiographic ductal assessment has been done, but the available data are limited and findings conflicting. Wong et al. [8] found no correlation between echocardiographic and angiographic measurements in a study of 27 patients. This contrasts to a 36 patient study from Ramaciotti et al. [9] who found a significant correlation between 2D echocardiography measurements of PDA minimal diameter and aortic end when assessing suitability for transcatheter closure before angiography; however, it was felt that a larger prospective series would be necessary to determine accuracy. Chen et al. [17] reported that 2D echocardiography combination with colour coded flow could be used as an alternative major guidance to angiography for first occluding attempt in a 296 patient study using a defined protocol to achieve ductal imaging prior and during the procedure. They focused on proving that device closure was feasible with echocardiographic guidance alone and there was no attempt to systematically correlate angiographic characteristics with echocardiography. In fact our observation that the echocardiogram tended to overestimate the minimal duct diameter in comparison with angiography does not contradict Chen’s findings. One could postulate that this overestimation may lead to modest oversizing of the chosen device, which would in most cases still facilitate a safe and effective closure.
To our knowledge, our data represent the largest study in a paediatric population to evaluate echocardiography’s potential to replace aortic angiography in percutaneous duct closure by systematically comparing both methods. We failed to demonstrate agreement between echocardiographic and angiographic measurements other than the length of the PDA. Whilst measurements of the narrowest diameter and the pulmonary diameter tended to be overestimated when using echocardiography, the aortic ampulla diameter tended to be underestimated. Ductal length measurement was an exception with good correlation shown between the two imaging methods. However, despite being an important feature in device selection, ductal length needs to be considered alongside the other more key measurements, in particular the minimum diameter.
No evidence of a predictable correlation or trend was found in order to predict the angiographic measurements from echo measurements. That is to say; not only did the measurements not agree, but the variation was not predictable. Considering ductal morphology; unsurprisingly, more type A ducts were found by both methods, but no agreement on characterisation was found between the two methods.
Inter- and intra-operative observer agreement in both modalities measurements was excellent (ICC > 0.8). Despite the data being correlated retrospectively and without using defined prospective imaging protocols, there were no significant systematic errors, inferring a high level of imaging quality and internal consistency.
Device selection predictions from echocardiography were inaccurate when compared with angiographic images (weighted k = 0.14 vs. 0.81, respectively) demonstrating not only that echo could not predict the device size but also that the “direction” of the error was not consistent.
The lack of agreement found between the two methods could lead to miss sizing a device and possibly affect operator’s confidence if echocardiographic measurements were exclusively used.
Study Limitations
Echocardiographic quantification for the study was obtained retrospectively, from imaging acquired during routine preassessment of patients attending for percutaneous duct occlusion. There was no specific echo protocol designed to assess device size selection or procedure guidance. It is likely that data acquired prospectively with sophisticated acquisition and measurement protocols would improve accuracy and increase the level of agreement between the imaging methods. A study involving blinded, prospective, standardised angiographic and echocardiographic assessment would help to further test our hypothesis.
Conclusion
Overall, our data do not suggest that accurate correlation exists between routine pre-operative echocardiography and angiographic imaging. Angiographic assessment of ductal anatomy, despite information gleaned from diagnostic echocardiography, remains the gold standard method for assessment and guidance at the time of device closure. Based on our data, an appropriate device selection cannot be based on echocardiographic measurements alone.
References
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD (2008) ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2008(118):e714
Schneider DJ, Moore JW (2006) Patent ductus arteriosus. Circulation 24(114):1873–1882
Stevenson JG, Kawabori I, Guntheroth WG (1980) Pulsed Doppler echocardiographic diagnosis of patent ductus arteriosus: sensitivity, specificity, limitations, and technical features. Catheter Cardiovasc Diagn 6:255–263
Galal MO, Hussain A, Arfi AM (2006) Do we need the surgeon to close the persistently patent arterial duct? Cardiol Young 16:522–536
Rao PS (2003) Summary and comparison of patent ductus arteriosus closure methods. In: Rao PS, Kern MJ (eds) Catheter based devices for the treatment of non-coronary cardiovascular disease in adults and children. Lippincott Williams & Wilkins, Philadelphia, pp 219–228
Alkamali AM (2015) Patent ductus arteriosus closure. In: Butera G, Chessa M, Eicken A, Thomson J (eds) Cardiac catheterization for congenital heart disease from fetal life to adulthood. Springer, Italy, pp 489–506
Ruiz AF, del Cerro Marín MJ, Vidal DR, Gussoni MCC, Granados FM, Ruiz AF et al (2002) Transcatheter closure of patent ductus arteriosus using the Amplatzer duct occluder: initial results and mid-term follow-up. Rev Esp Cardiol 55:1057–1062
Wong JA, Shim D, Khoury PR, Meyer RA (1998) Validation of color Doppler measurements of minimum patent ductus arteriosus diameters: significance for coil embolization. Am Heart J 136:714–717
Ramaciotti C, Lemler MS, Moake L, Zellers TM (2002) Comprehensive assessment of patent ductus arteriosus by echocardiography before transcatheter closure. J Am Soc Echocardiogr 15:1154–1159
Krichenko A, Benson LN, Burrows P, Moes CA, McLaughlin P, Freedon RM (1989) Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol 63:877–879
Instructions for Amplatzer Duct Occluder use. https://professional.sjm.com/products/sh/heart-occluders/pda-closure-devices/amplatzer-duct-occluder. Accessed July 2016
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 8(1):307–310
Giavarina D (2015) Understanding Bland Altman analysis. Biochem Med (Zagreb). 5(25):141–151
Di Eugenio B, Glass M (2004) The kappa statistic: a second look. Comput Linguist 30:95–101
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 1(20):37–46
Smith BG, Tibby SM, Qureshi SA, Rosenthal E, Krasemann T (2012) Quantification of temporal, procedural, and hardware-related factors influencing radiation exposure during pediatric cardiac catheterization. Catheter Cardiovasc Interv 80:931–936
Chen W, Yan X, Huang Y, Sun X, Zhong L, Li J, Chen H, Zhong H, Chen L (2015) Transthoracic echocardiography as an alternative major guidance to angiography during transcatheter closure of patent ductus arteriosus: technical feasibility and clinical relevance. Pediatr Cardiol 36:14–19
Acknowledgements
The authors thank Alexandra Savis BSc (Department of Pediatric Cardiology, Evelina London Children’s Hospital, Guys and St Thomas’ NHS Foundation Trust, London, UK) and Kings College University Statistics Department for their collaboration.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Carmo Mendes, I., Heard, H., Peacock, K. et al. Echocardiographic Versus Angiographic Assessment of Patent Arterial Duct in Percutaneous Closure: Towards X-ray Free Duct Occlusion?. Pediatr Cardiol 38, 302–307 (2017). https://doi.org/10.1007/s00246-016-1513-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-016-1513-5