Abstract
Purpose of Review
To review the most recent literature on pediatric transcatheter ductal intervention including ductus arteriosus occlusion and stenting.
Recent Findings
With the development and FDA approval of smaller ductal devices, including most recently the Amplatzer Piccolo Occluder (Abbott, Abbott Park, IL), transcatheter ductus arteriosus device closure is now being safely performed in premature infants and patients < 6 kg using a transvenous approach. In patients with ductus-dependent pulmonary blood flow, ductal stenting with pre-mounted coronary artery stents has been shown to be an acceptable alternative to the surgically placed Blalock-Taussig shunt. Centers with experience in ductal stenting have demonstrated success, even with the tortuous ductus.
Summary
Innovation in transcatheter device technology and procedural practices have allowed for significant advances. Transcatheter ductal device closure is a reasonable alternative to surgical ligation even in premature, low-birthweight infants. Ductal stenting is also an accepted alternative to the modified Blalock-Taussig shunt. We anticipate continued advancement and procedural refinement over the next several years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Percutaneous catheter-based intervention on the ductus arteriosus represents an area of interventional cardiology that has evolved significantly over the last decade. With known surgical complications such as recurrent laryngeal nerve injury and chylothorax, percutaneous ductus arteriosus device closure to treat pulmonary overcirculation and prevent endocarditis has become the standard of care [1]. In 2013, a large, prospective multi-center study demonstrated an adverse event rate of percutaneous ductus arteriosus closure of 9%, with only 2% classified as high severity [2]. High-severity events were more likely to occur in patients smaller than 6 kg. In patients with ductus-dependent pulmonary blood flow, the modified Blalock-Taussig shunt (BTS) has been performed since 1944. A report published by The Society of Thoracic Surgeons Congenital Heart Surgery showed a 7.2% in hospital mortality rate for a cohort of neonates undergoing modified BTS placement and 13.1% of patients had serious adverse events [3]. After recent advances, percutaneous stenting of the ductus arteriosus to maintain patency for ductus-dependent pulmonary blood flow has been shown to be non-inferior with regard to major outcomes and superior with regard to other important outcomes to BTS placement [4••]. With significant innovation in transcatheter procedures and technology, these less invasive percutaneous interventions are a viable alternative to surgical procedures.
Ductus Arteriosus Closure
History of Ductus Arteriosus Closure
The first ductus arteriosus device occlusion using an Ivalon plug was described by Porstmann in 1971 [5]. In the late 1970s and early 1980s, Rashkind published his use of ductus arteriosus closure devices, first with a single-disk device, followed by a double-disk device in 1987 [6, 7]. After the development of smaller delivery systems, rapid advancement of device technology occurred. Successful occlusion has been reported both using coils and devices. In 1995, a large series including 535 patients in the Ductus Arteriosus Coil Registry demonstrated a 95% occlusion success rate [8]. With the U.S. Food and Drug Administration approval of the Amplatzer Ductal Occluder (ADO- Abbott, Abbott Park, IL) device in 2003, operators are now able to close larger ducts as well. With the ADO device, moderate to large defects can be closed with 98% complete occlusion in follow-up [9].
Ductal Morphology
A careful pre-procedural and peri-procedural assessment of ductal morphology guides selection of the most appropriate device. Often, echocardiographic images are helpful, but the gold standard is angiography of the ductus. The most widely used schema of angiographic morphologic classification was described by Krichenko et al. in 1989 [10]. Type A, the most common, is a conical-shaped ductus with a larger aortic end that tapers down to the pulmonary end. Type B, the least common, is a short window-like duct. Type C is a long, tubular ductus without any areas of restriction. Type D or the complex ductus has multiple areas of restriction. Type E, which is commonly seen in premature infants, features an elongated ductus and often has areas of narrowing. Although not included in Krichenko’s original morphologic classification, the type F, or fetal type, ductus has recently been described [11•]. The type F ductus is found more often in premature infants and is generally larger and longer than the aforementioned types. Careful attention to ductal morphology is essential in procedural planning and device selection.
Indications for Closure
According to the 2011 AHA guidelines, transcatheter ductus arteriosus device closure to treat a moderate to large ductus arteriosus with left to right shunting that results in CHF, failure to thrive, pulmonary overcirculation, or left heart enlargement is a class I indication (level of evidence: B) [1]. Additionally, ductus arteriosus device closure of an audible ductus with a small left to right shunt and normal cardiac size is a class IIa indication (level of evidence: C) [1]. There remains a debate regarding the closure of an inaudible ductus arteriosus with a small left to right shunt and normal heart sizes; therefore, this remains a class IIb indication (level of evidence: C) [1].
Ductus Arteriosus Closure in Preterm Infants
Ductus arteriosus occlusion in patients greater than 6 kg has a well-established safety and success profile. Data from the IMPACT registry published in 2017 demonstrated a 94% success rate of ductal occlusion in patients less than 6 kg [12•]. A large meta-analysis of ductus arteriosus occlusion published in 2017 reviewed 38 studies documenting 635 procedures [13•]. Overall, 48% of patients weighed between 3 and 6 kg while 19% weighed less than 3 kg. There was a 92% success rate with an adverse event rate of 10%. Notably, adverse events rates were 2–3 times higher in patients smaller than 6 kg.
Ductus arteriosus closure in smaller and even premature patients has been the subject of a recent study. The rate of spontaneous ductal closure in premature (< 24 weeks) and extremely low-birthweight infants is less than 15% [14]. In addition to the low rate of spontaneous closure in this population, unrepaired patients can develop significant morbidity and mortality related to a large left to right shunt at the level of the ductus arteriosus. This includes chronic lung disease, pulmonary hypertension, intraventricular hemorrhage, and necrotizing enterocolitis. In a study published in Pediatrics, the mortality rate for infants born at less than 29 weeks who had an open ductus arteriosus was increased 8-fold [15]. Over the last several years, there has been ongoing work to improve the safety and technical success of ductal occlusion in premature infants. In 2001, the Japanese group at Shimane Medical University reported a successful transcatheter ductal closure in an infant weighing 1180 g [16]. The ductus was occluded with a Cook coil using an antegrade approach. The patient had no residual shunt at 1-month follow-up. More recently, there have been reports of bedside echocardiography-guided duct occlusion [17]. While these techniques are favorable in that they can be performed in the NICU and do not require transporting a critically ill patient, they were performed using arterial access, which introduces risk of limb ischemia due to arterial thrombus. In 2016, the group at Cedars-Sinai described their technique for transcatheter ductal occlusion using femoral venous access [18••]. Using this approach in 24 extremely low-birthweight infants, ductal occlusion was performed using the Amplatzer Vascular Plug II (AVP II, Abbott, Abbott Park, IL). Fluoroscopy was used to obtain access and wire position in the descending aorta. Echocardiographic guidance was then used to guide device placement. Success rate was 88% in their cohort of patients with a median weight of 1249 g [18••]. Two patients required intraprocedural device repositioning due to descending aorta obstruction. One patient developed left pulmonary artery stenosis requiring stent placement after the procedure. Recently, the Medtronic Micro Vascular Plug (MVP) (Medtronic, Minneapolis, MN), originally designed for peripheral and neurological interventions in adults, has been successfully used to occlude the ductus arteriosus in premature infants [19•, 20••].
Several reports from European groups have looked at the Amplatzer Ductal Occluder in Additional Sizes (ADO II AS, Abbott, Abbott Park, IL). The most recent study by Baspinar et al. included a cohort of 69 patients, all of whom weighed less than 6 kg. The authors demonstrated an implant success rate of 94% overall and 81% for premature infants (n = 16) [21]. Complications included one death and three device embolizations. Patient age, but not patient weight, was related to complication rate. The ADO II AS has also been studied in the USA and in January 2019, the newest Amplatzer device gained Food and Drug Administration approval under the name the Amplatzer Piccolo Occluder (Abbott, Abbott Park, IL). The approval of a device specifically designed for the treatment of the premature infant ductus (approved for patients as small as 700 g) represents a significant innovation allowing for lower morbidity and mortality rates when compared to surgical intervention. With the use and development of devices that can be used in premature infants, virtually all types of ductus arteriosus can now be closed in the cardiac catheterization laboratory, although a learning curve is involved (Fig. 1). In addition, recognizing and promptly treating complications described above is important. This procedure can be done via a transvenous approach without any arterial access and has been reported in premature infants as small as 640 g [20••]. In addition, transcatheter ductal closure may result in less days of respiratory assistance compared to surgical ductal ligation in premature infants [20••].
Ductus arteriosus closure in a premature infant. a Descending aortic angiogram through a 4-Fr-long sheath that has been advanced transvenously through the ductus arteriosus (arrow) of a 2-month-old, ex-30-week premature infant weighing 1.2 kg. Note that no arterial access was obtained in the patient, in order to avoid the risk of arterial trauma. b Angiogram in the main pulmonary artery after device positioning, but before full release showing good position of a 4-mm AVP II device (Abbott, Abbott Park, IL) (arrow) in the ductus arteriosus. c Transthoracic echocardiography (2D and corresponding color images) performed in the cardiac catheterization laboratory before ductus arteriosus device deployment showing the large ductus arteriosus (between markers). The right pulmonary artery (RPA) and left pulmonary artery (LPA) are also visualized. d Transthoracic echocardiography (2D and corresponding color images) performed in the cardiac catheterization laboratory after ductus arteriosus device deployment (arrow) showing unobstructed flow in the descending aorta (Ao). e Transthoracic echocardiography (2D and corresponding color images) performed in the cardiac catheterization laboratory after ductus arteriosus device deployment showing unobstructed flow in right pulmonary artery (RPA) and left pulmonary artery (LPA)
Ductus Arteriosus Stent
History of Ductus Arteriosus Stent
While ductal device occlusion has been practiced for 50 years, stenting of the ductus arteriosus is a relatively new transcatheter intervention. The procedure was first reported in 1992 by Gibbs et al. [22]. In this report, ductal stenting was performed in two neonates with ductus-dependent pulmonary blood flow. Although both patients died remote from the procedure, the ductus was patent at autopsy, thus validating ductal stenting as a means of securing pulmonary blood flow as a viable alternative to BTS placement. Given the significant risks of BTS placement, including thrombosis, chylothorax, and recurrent laryngeal nerve injury, ductal stenting has become an appealing alternative. Over the last decade, significant technical advances have been made to improve clinical outcomes of this procedure. The most notable advance is the use of pre-mounted stents designed for coronary arteries, which allow operators to use much smaller delivery systems than the ones used by Gibbs.
Indications for Ductal Stent
According to the 2011 AHA guidelines, transcatheter stenting of an anatomically suitable ductus arteriosus in a patient with cyanotic congenital heart disease and a single source of pulmonary blood flow, but who requires additional pulmonary blood flow for a short period of time, is a class IIa indication (level of evidence: B) [1]. Transcatheter stenting of an anatomically suitable ductus arteriosus in patients with cyanotic congenital heart disease and a single source of pulmonary blood flow is a class IIb indication (level of evidence: C) [1]. Although ductal stenting in patients who have proximal pulmonary artery stenosis near the ductal insertion is considered class III, multiple centers have performed this procedure successfully using the technique of jailing a branch pulmonary artery, with dilation and often stenting through a stent side cell [4, 23•].
Ductal Stent Vs. Blalock-Taussig Shunt
While modified BTS placement has long been the standard of care for patients with ductus-dependent pulmonary blood flow, recent studies comparing the two have demonstrated similar outcomes. A large study by the Congenital Catheterization Research Collaborative (CCRC) in 2018 compared three-hundred and fifty-seven patients with ductus-dependent pulmonary blood flow [4]. Between 2008 and 2015, 106 patients underwent ductal stenting and 251 patients underwent BTS, with the primary outcome being death or unplanned reintervention for cyanosis. No difference in the hazard ratio was seen between the two groups [4]. Additionally, while the overall reintervention rate was higher in the ductal stent group, they also had a shorter adjusted intensive care unit stay, lower procedure-related complications, and larger and more symmetric pulmonary arteries at surgical repair or last follow-up [4]. A similar study performed in the UK enrolled 254 patients over a 4-year period [24••]. Patients who underwent BTS or ductal stenting were compared. Similar to the CCRC study, patients who underwent ductal stenting were more likely to require reintervention, but were less likely to require ECMO support. Patients in the ductal stenting group also had improved survival to surgical intervention.
Ductal Morphology and Ductal Stent Procedure
Similar to ductus arteriosus occlusion, close attention to the ductal morphology and origin is of critical importance when pre-procedure planning for ductal stent placement. In addition to the morphologic classification proposed by Krichenko, a tortuosity index has recently been described [23•]. Tortuosity index type I ducts are relatively straight, type II ducts have one turn, and type III ducts have multiple turns. Additionally, the use of echocardiographic images to determine ductal origin is a critical step in planning arterial access. If echocardiographic images are not adequate to delineate origin of the ductus, CT imaging may be necessary. Newer flexible coronary stent platforms, operator/center experience, and other special techniques beyond the scope of this text have all resulted in higher rates of technical success of this procedure in the recent years, even with a highly tortuous ductus [23•, 25,26,27,28,29]. Careful consideration in choosing the straightest trajectory to the ductus is of utmost importance [25]. Standard femoral arterial access may be used for a ductus which takes off from the descending aorta. However, ductus arteriosus which arises from the underside of the aortic arch may be best approached via the carotid artery or the axillary artery (or via a transvenous approach in some instances). While in the past access to these vessels would occur via surgical cutdown, recent studies have demonstrated the safety of percutaneous access [30, 31]. In 2016, a retrospective study reviewed 42 patients who underwent percutaneous carotid access (PCA) at two different institutions. Of the 47 access attempts, there were 45 successful PCAs with sheath placement [30]. Follow-up carotid ultrasound was documented in each patient. Two patients had complete occlusion of their carotid artery within 24 h of the procedure and one patient had a nonocclusive thrombus. All three patients were treated with Lovenox and experienced resolution of thrombus. Another recent study by Polat compared femoral venous access and axillary arterial access for ductus arising from the underside of the aortic arch [32]. Successful stenting was completed in 67% of cases using femoral access and in all cases using axillary arterial access. In addition, axillary access procedure times and fluoroscopy times were significantly lower. Two other recent series demonstrated high success rates of stent deployment using a percutaneous axillary artery approach [33•, 34•] (Fig. 2). Vascular complications, though mostly minor, may be encountered using this approach [34•]. Another more recent development has been the use of drug-eluting stents designed for percutaneous coronary artery intervention. Although some early data suggest that neonatal clearance of sirolimus is lower than in older children, there were no negative clinical outcomes reported [35]. Our institution has used drug-eluting stents and noted decreased reintervention rates, less in-stent restenosis, and no negative outcomes related to immunosuppression as compared to older bare-metal stents [36•].
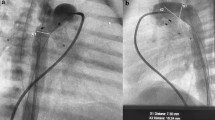
Ductus arteriosus stenting via a percutaneous axillary artery approach. a Angiogram in the ascending aorta of a 3-week-old, ex-35-week premature infant with tetralogy of Fallot and severe cyanosis. A left axillary arterial sheath (solid arrow) has been advanced via a percutaneous approach. Note the tortuous patent ductus arteriosus (dotted arrow) originating from the underside of the aortic arch and supplying the pulmonary arteries. The ductus arteriosus has been crossed with a soft 0.014-in. coronary guidewire. b After ductus arteriosus stent placement (arrow), there is good filling seen through the stent to both branch pulmonary arteries
It is important for operators to be able to promptly manage complications such as ductal spasm, dissection, thrombosis within the ductal stent, and stent embolization. A learning curve is also involved with this procedure and appropriate surgical backup and collaboration is essential.
Conclusion
Although surgical intervention has historically been the preferred method for ductal closure, particularly in small infants and in patients with ductus-dependent pulmonary blood flow (with a BTS), recent advances in procedural techniques and device technology have allowed percutaneous intervention to be a successful alternative. While catheter-based intervention is not without risk, severe morbidity and mortality rates have been shown to be no greater than those associated with surgical intervention. Additionally, many important outcomes have recently been shown to favor percutaneous procedures. It is anticipated that further advances in the field will allow for safer and more successful procedures, especially on premature and lower-birthweight infants.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Feltes TF, Bacha E, Beekman RH 3rd, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123(22):2607–52. https://doi.org/10.1161/CIR.0b013e31821b1f10.
El-said HG, Bratincsak A, Foerster SR, Murphy JJ, Vincent J, Holzer R, et al. Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience. J Am Heart Assoc. 2013;2(6):e000424. https://doi.org/10.1161/JAHA.113.000424.
Petrucci O, O’Brien SM, Jacobs ML, Jacobs JP, Manning PB, Eghtesady P. Risk factors for mortality and morbidity after the neonatal Blalock-Taussig shunt procedure. Ann Thorac Surg. 2011;92(2):642–51; discussion 651–2. https://doi.org/10.1016/j.athoracsur.2011.02.030.
•• Glatz AC, Petit CJ, Goldstein BH, et al. A comparison between patent ductus arteriosus stent and modified Blalock-Taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: insights from the congenital catheterization research collaborative. Circulation. 2018;137(6):589–601. https://doi.org/10.1161/circulationaha.117.029987 This multicenter retrospective review of patients who underwent either PDA stent or Blalock-Taussig shunt for ductus-dependent pulmonary blood flow demonstrated no difference in death or unplanned reintervention rates. A shorter ICU length of stay, lower procedural complication rate, and larger and more symmetric pulmonary arteries were found in the PDA stent group.
Porstmann W, Wierny L, Warnke H, Gerstberger G, Romaniuk PA. Catheter closure of patent ductus arteriosus: 62 cases treated without thoracotomy. Radiol Clin N Am. 1971;9(2):203–18.
Rashkind WJ, Cuaso CC. Transcatheter closure of a patent ductus arteriosus: successful use in a 3.5 kg infant. Pediatr Cardiol. 1979;1:3–7. https://doi.org/10.1007/BF02307335.
Rashkind WJ. Therapeutic interventional procedures in congenital heart disease. Radiol Diagn (Berl). 1987;28:449–60.
Lloyd TR, Beekman RH, Moore JW, Hijazi ZM, Hellenbrand WE, Sommer RJ, Wiggins JW, Zamora R, Vincent RN; for the PDA Coil Registry Investigators. The PDA Coil Registry: report of the first 535 procedures.
Pass RH, Hijzi Z, Hsu DT, Lewis V, Hellenbrand WE. Multicenter USA Amplatzer Patent Ductus Arteriosus Occlusion Device Trial: initial and one-year results. J Am Coll Cardiol. 2004;44(3):513–9. https://doi.org/10.1016/j.jacc.2004.03.074.
Krichenko A, Benson LN, Burrows P, et al. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. 1989;63(12):877–80. https://doi.org/10.1016/0002-9149(89)90064-7.
• Philip R, Rush Waller B III, Agrawal V, Wright D, Arevalo A, Zurakowski D, et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. 2015;87:310–7. https://doi.org/10.1002/ccd.26287 The authors describe a fetal type or type F ductus arteriosus as an addition to the classic A–E Krichenko classification. This duct morphology is commonly found in premature infants.
• Backes CH, Kennedy KF, Locke M, et al. Transcatheter occlusion of the patent ductus arteriosus in 747 infants <6 kg: insights from the NCDR IMPACT Registry. JACC Cardiovasc Interv. 2017;10(17):1729–37. https://doi.org/10.1016/j.jcin.2017.05.018 Findings from this multicenter study found that transcatheter PDA occlusion in infants less than 6 kg is technically feasible. Major adverse events including arterial injury and device embolization are more common in infants less than 30 days old and of extremely low weight (< 2 kg).
• Backes CH, Rivera BK, Bridge JA, et al. Percutaneous patent ductus arteriosus (PDA) closure during infancy: a meta-analysis. Pediatrics. 2017;139(2):e20162927. https://doi.org/10.1542/peds.2016-2927 This meta-analysis includes 38 studies with a technical success rate of 92.2% with a clinically significant adverse event incidence of 10.1%. Lack of standardized adverse event reporting limits the interpretation.
Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36(2):123–9. https://doi.org/10.1053/j.semperi.2011.09.022.
Noori S, Mccoy M, Friedlich P, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–44. https://doi.org/10.1542/peds.2008-2418.
Haneda N, Masue M, Tasaka M, Fukui C, Saito K, Yamaguchi S. Transcatheter closure of patent ductus arteriosus in an infant weighing 1180 g. Pediatr Int. 2001;43(2):176–8.
Bentham J, Meur S, Hudsmith L, Archer N, Wilson N. Echocardiographically guided catheter closure of arterial ducts in small preterm infants on the neonatal intensive care unit. Catheter Cardiovasc Interv. 2010;77(3):409–15. https://doi.org/10.1002/ccd.22637.
•• Zahn EM, Peck D, Phillips A, et al. Transcatheter closure of patent ductus arteriosus in extremely premature newborns: early results and midterm follow-up. JACC Cardiovasc Interv. 2016;9(23):2429–37 The authors review outcomes for extremely premature infants who underwent transcatheter echocardiographically guided PDA closure. The success rate was 88% with no procedural deaths or device embolizations.
• Sathanandam S, Justino H, Waller BR 3rd, Radtke W, Qureshi AM. Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc Interv. 2017;89(6):1051–8. https://doi.org/10.1002/ccd.26878 The authors describe a multicenter experience using the Medtronic Micro Vascular Plug in 15 premature infants with a median weight of 1210 g. The successful occlusion rate was 93% with no complications related to the procedure.
•• Sathanandam S, Balduf K, Chilakala S, Washington K, Allen K, Knott-Craig C, et al. Role of transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv. 2019;93(1):89–96. https://doi.org/10.1002/ccd.27808 This study compares outcomes between surgical ductal ligation and transcatheter PDA closure in extremely low-birthweight infants. The findings demonstrate that transcatheter PDA closure may be associated with faster weaning of respiratory support post-procedure.
Baspinar O, Sahin DA, Sulu A, Irdem A, Gokaslan G, Sivasli E, et al. Transcatheter closure of patent ductus arteriosus in under 6 kg and premature infants. J Interv Cardiol. 2015;28(2):180–9. https://doi.org/10.1111/joic.12196.
Gibbs JL, Rothman MT, Rees MR, Parsons JM, Blackburn ME, Ruiz CE. Stenting of the arterial duct: a new approach to palliation for pulmonary atresia. Br Heart J. 1992;67:240–5. https://doi.org/10.1136/hrt.67.3.240.
• Qureshi AM, Goldstein BH, Glatz AC, et al. Classification scheme for ductal morphology in cyanotic patients with ductal dependent pulmonary blood flow and association with outcomes of patent ductus arteriosus stenting. Catheter Cardiovasc Interv. 2019;93(5):933–43. https://doi.org/10.1002/ccd.28125 The authors propose a new classification for ductal morphology in patients with ductus-dependent pulmonary blood flow based on the tortuosity of the vessel. A higher tortuosity index was associated with pulmonary artery jailing and unplanned reintervention, but pulmonary artery jailing did not affect pulmonary artery size at follow-up.
•• Bentham JR, Zava NK, Harrison WJ, et al. Duct stenting versus modified Blalock Taussig shunt in neonates with duct-dependent pulmonary blood flow: associations with clinical outcomes in a multicenter national study. Circulation. 2018;137(6):589–601. https://doi.org/10.1161/circulationaha.117.028972 The authors compare post-procedural outcomes between patients who underwent a modified Blalock-Taussig shunt or a ductal stent. The findings demonstrate a discharge survival advantage for patients in the ductal stent group. Long-term outcomes show a reduced risk of death prior to repair in the ductal stent group, but the reintervention rate was slightly higher.
Aggarwal V, Petit CJ, Glatz AC, Goldstein BH, Qureshi AM. Stenting of the ductus arteriosus for ductal dependent pulmonary blood flow—current techniques and procedural considerations. Congenit Heart Dis. 2019;14:110–5. https://doi.org/10.1111/chd.12709.
Rehman R, Marhisham MC, Alwi M. Stenting the complex patent ductus arteriosus in tetralogy of Fallot with pulmonary atresia: challenges and outcomes. Futur Cardiol. 2018;14(1):55–73. https://doi.org/10.2217/fca-2017-0053.
Celebi A, Yucel IK, Bulut MO, Kucuk M, Balli S. Stenting of the ductus arteriosus in infants with functionally univentricular heart disease and ductal-dependent pulmonary blood flow: a single-center experience. Catheter Cardiovasc Interv. 2017;89(4):699–708. https://doi.org/10.1002/ccd.26796.
Santoro G, Gaio G, Giugno L, Capogrosso C, Palladino MT, Iacono C, et al. Ten-years, single-center experience with arterial duct stenting in duct-dependent pulmonary circulation: early results, learning-curve changes, and mid-term outcome. Catheter Cardiovasc Interv. 2015;86(2):249–57. https://doi.org/10.1002/ccd.25949.
Udink Ten Cate FE, Sreeram N, Hamza H, Agha H, Rosenthal E, Qureshi SA. Stenting the arterial duct in neonates and infants with congenital heart disease and duct-dependent pulmonary blood flow: a multicenter experience of an evolving therapy over 18 years. Catheter Cardiovasc Interv. 2013;82(3):E233–43. https://doi.org/10.1002/ccd.24878.
Justino H, Petit CJ. Percutaneous common carotid artery access for pediatric interventional cardiac catheterization. Circulation: Cardiovasc Interv. 2016;9(4):e003003. https://doi.org/10.1161/circinterventions.
Choudhry S, Balzer D, Murphy J, Nicolas R, Shahanavaz S. Percutaneous carotid artery access in infants < 3 months of age. Catheter Cardiovasc Interv. 2016;87(4):757–61. https://doi.org/10.1002/ccd.26310.
Polat TB. Stenting the vertical ductus arteriosus via axillary artery access using “wire-target” technique. Congenital Heart Dis. 2017;12(6):800–7. https://doi.org/10.1111/chd.12512.
• Lee J, Ratnayaka K, Moore J, El-Said H. Stenting the vertical neonatal ductus arteriosus via the percutaneous axillary approach. Congenital Heart Dis. 2019. https://doi.org/10.1111/chd.12786 The authors provide a brief series of patients with vertical ducts who underwent ductal stenting via the axillary artery approach. No procedural mortality or complications were reported.
• Breatnach CR, Aggarwal V, Al-Alawi K, CJ MM, Franklin O, Prendiville T, et al. Percutaneous axillary artery approach for ductal stenting in critical right ventricular outflow tract lesions in the neonatal period. Catheter Cardiovasc Interv. 2019;93(7):1329–35. https://doi.org/10.1002/ccd.28302 The authors describe the axillary artery approach as a viable option for ductal stenting in neonates with ductus-dependent pulmonary blood flow. Three access-related complications were reported with no long-term sequelae.
Lee KJ, Seto W, Benson L, Chaturvedi RR. Pharmacokinetics of sirolimus-eluting stents implanted in the neonatal arterial duct. Circ Cardiovasc Interv. 2015;8(5). https://doi.org/10.1161/circinterventions.
• Aggarwal V, Dhillon GS, Penny DJ, Gowda ST, Qureshi AM. Drug eluting stents compared to bare metal stents for stenting the ductus arteriosus in infants with ductal dependent pulmonary blood flow. Am J Cardiol. 2019;124(6):952–9. https://doi.org/10.1016/j.amjcard.2019.06.014 The findings suggest that infants undergoing ductal stenting for ductus-dependent pulmonary blood flow with a drug-eluting stent as opposed to a bare-metal stent have decreased luminal loss and lower unplanned reintervention rates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lindsay Eilers declares no conflict of interest.
Athar M. Qureshi reports personal fees from W. L. Gore and Associates, and from Edwards Lifesciences.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Congenital Heart Disease
Rights and permissions
About this article
Cite this article
Eilers, L., Qureshi, A.M. Advances in Pediatric Ductal Intervention: an Open or Shut Case?. Curr Cardiol Rep 22, 14 (2020). https://doi.org/10.1007/s11886-020-1266-x
Published:
DOI: https://doi.org/10.1007/s11886-020-1266-x