Abstract
The conventional transcatheter closure of patent ductus arteriosus (PDA) requires femoral artery puncture and angiography for duct anatomic and shunting estimation. If such estimation can be replaced by transthoracic echocardiography (TTE), the procedure will be further simplified, with fewer invasions. This study aimed to examine whether TTE can serve as an alternative to aorta angiography and as a major guidance for transcatheter duct closure. The study enrolled 298 consecutive patients (71 males and 227 females) with PDA. In the study, TTE with combined two-dimensional echocardiography (2DE) imaging and color-coded flow imaging (CDFI) was performed to measure the minimal shunting width (MSW) as the estimated minimal duct size for selection of an Amplatzer duct occluder (ADO) and to monitor the transcatheter duct closure intraprocedurally. The MSW was validated against the duct-stretched diameter (SDD), against the minimal waist diameter of the conical part of a released occluder measured by X-ray spot picture after successful duct closure (SDC), and against the size of the finally used ADO (SADO). Good correlation was found between MSW and SDD [SDD (mm) = 1.31 MSW; r = 0.89; p < 0.01] and between MSW and SADO [SADO (mm) = 1.71 MSW; r = 0.88; p < 0.01]. Of 296 patients who received occlusion using MSW as the reference for selection of the occluder, SDC was attained in 288 (97.3 %), 5 (1.7 %), and 2 (0.7 %) patients, respectively, at the first, second (1 ADO replacement), and third (2 ADO replacements) occluding attempt. Acute occluder dislodgement occurred in one patient (0.3 %). At the 12-month follow-up assessment, no major complications were found, and the total immediate or 12-month SDC was 99.7 %. Echocardiography as an alternative major guidance to angiography for transcatheter duct closure is technically feasible, and TTE guidance can further simplify the procedure, with fewer invasions and potential complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The Amplatzer duct occluder (ADO) has been broadly used clinically, and both its efficacy and safety for transcatheter closure of patent ductus arteriosus (PDA) have been confirmed by numerous studies [3, 4, 9, 11, 16–18, 21]. The ADO is manufactured in various sizes, and the suitable size of an occluder usually is selected according to aorta angiographic findings when the conventional transcatheter duct closure is used [4, 11, 16]. In addition to increasing the procedural time, femoral artery puncturing for angiography will increase the potential risks of vascular complications [1, 6, 7, 14, 23], X-ray radiation, and contrast agent toxicity [2, 5, 8, 10, 12, 15, 19, 22].
This study aimed to determine whether transthoracic echocardiography (TTE) can serve as an alternative to aorta angiography, particularly for selecting of an appropriate occluder and monitoring transcatheter duct closure, and to test whether TTE as a major guidance can further simply the procedure, with more safety and less invasion.
Methods
Patients
The study enrolled 298 consecutive patients (71 males and 227 females) who underwent transcatheter duct closure from January 2006 to December 2012. The patients had a median age of 17 years (range 0.75–64.50 years) and a weight of 50.57 kg (range 7.52–71.20 kg). All the patients had clinical indications and were suitable for duct closure without any contraindications.
The study protocol was approved by the Ethics Committee of Fujian Medical University and conformed to the principles outlined in the Declaration of Helsinki. Signed informed consent was obtained from the patients or their legal guardians.
Echocardiographic Studies
For this study, TTE with imaging modalities of two-dimensional echocardiography (2DE), color-coded Doppler flow imaging (CDFI), and continuous wave Doppler (CW) was performed pre-, intra-, and postprocedurally at discharge and at follow-up visits using commercially available echocardiograph systems (Vivid-1, Vivid-9; GE Medical System, Wis, USA; IE-33, Philip Medical System, Andover, Massachusetts, USA).
The parasternal short-axis view at the level of great vessels and its modified views were obtained to image the whole duct structure. For more accurate assessment of duct size, it was essential to optimize gain setting and imaging resolution, and to properly select a measurable phase from a cardiac cycle, and 2DE combined with CDFI imaging (2DE–CDFI) was crucial.
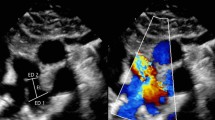
Usually, when PDA was sufficiently large, 2DE was capable of visualizing the duct anatomy (Fig. 1c), but in case of a smaller PDA, an unusual duct type, or poor image quality, 2DE might not clearly show the duct anatomy (Fig. 1a, e). In most situations, 2DE–CDFI was capable of detecting the duct-shunting signals (Fig. 1b, d, f). Accordingly, two parameters for estimation of the minimal duct diameter (MDD) were assessed respectively with 2DE and 2DE–CDFI on mid- to end-diastolic images: (1) the minimal duct width (MDW) measured with 2DE at the narrowest duct segment approximately at the duct constriction (Fig. 1a, c, e) and (2) the minimal shunting width (MSW) measured with 2DE–CDFI at the narrowest shunting jet approximately in the vena-contracta zone [24] (Fig. 1b, d, f).
Two-dimensional echocardiographic (2DE) imaging estimating the MDD from three patients with respectively a small, b medium, and c large patent ductus arterioses (PDA) and the corresponding 2DE and CDFI in b, d, f. a because 2DE cannot easily investigate a small PDA, estimation of MDW is impossible. b however, combined 2DE and CDFI (2DE–CDFI) easily show a small shunt, with MSW estimated to be 1 mm. Either 2DE or 2DE–CDFI can clearly detect duct anatomy and shunting, with c MDW estimated to be 4 mm and d MSW estimated to be 4.2 mm. e It is difficult for 2DE to visualize a large PDA well due to its unusual connection and suboptimal imaging quality. f however, 2DE–CDFI can well map a broad shunting, with MSW estimated to be 10 mm
TTE as the Main Procedural Guidance During Transcatheter Duct Closure
We used a simplified method rather than the conventional standard method for transcatheter duct closure with ADO [5]. Briefly, the femoral vein was punctured to build up a device delivery track under brief fluoroscopy, whereas the femoral artery puncture and angiography necessary for the standard method could be ignored. Except for brief fluoroscopy, TTE was used as the major guidance intraprocedurally in the following four steps: (1) to initially select an occluder according to MDD measured preprocedurally by TTE (1.5–2.0 mm, with MSW or MDW used as the reference), (2) to check whether the initially selected occluder was appropriate for the duct occluding (Fig. 2a, b), (3) to detect whether any significant residual shunting (Fig. 2c, d) or ADO-caused obstruction in the aorta (Fig. 2e–h) or pulmonary artery (Fig. 2i–l) was present, and (4) to decide whether a selected occluder should be replaced before release due to improper size (too large or too small), which could be judged by checking the aforementioned steps.
Transthoracic echocardiography (TTE)-guidance of transcatheter duct closure, with TTE showing an occluder positioned in the duct. Normal recovery of its memorized configuration (a) without residual shunting (b) indicate that the occluder is suitable in size. An occluder is well positioned and normally recovered (c), but a larger marginal residual shunt (>2 mm) is visible (d), indicating that the occluder might be too small. No significant Amplatzer duct occluder (ADO)-caused obstruction is found in the aorta (e, f) or the pulmonary artery (g, h). A significant ADO-caused obstruction is found in the aorta (i, j) or the left pulmonary artery (k, l). Upper panel two-dimensional echocardiographic (2DE) imaging. Lower panel combined 2DE and CDFI corresponding to the upper panel
Validation for Echocardiographic Estimation of Duct Size
Immediately after successful duct occlusion by adjustment of X-ray projection to show the occluder waist optimally, a spot picture was taken to measure the stretched duct diameter (SDD), defined as the minimal waist diameter of the conical part of a finally used occluder that had already released (Fig. 3).
To test whether TTE was technically feasible for the selection of an occluder, the correlation and difference between MSW and SDD or the size of a finally used Amplatzer duct occluder (SADO) was examined.
Follow-up Evaluation
Clinical and echocardiographic follow-up assessment was indexed at discharge and at 1, 6, and 12 months. The major procedure-related complications were recorded including hemolysis, thrombocytopenia, thrombosis, thromboembolism, infectious endocarditis, and ADO dislodgment or embolization, as well as any complications leading to ADO retrieved surgically. Echocardiography was performed to assess the duct-occluding results including duct residual shunting or reopening (shunting width and velocity), ADO-caused obstruction at the sides of the aorta or the left pulmonary artery (transobstruction velocity or pressure gradient), and ADO dislodgement.
Definitions of Occluding Result
Incomplete Duct Closure
Any existence of CDFI-detectable residual shunting at any time after the procedure was regarded as persistent residual shunting, and any existence of persistent residual or reopening shunting was regarded as incomplete duct closure.
Successful Duct Closure
An effective shunting occlusion after the procedure or at follow-up visits was defined as successful duct closure (SDC) including complete closure without significant ADO-caused aortic or pulmonary artery obstruction (CW-derived peak velocity of 2 m/s at the obstructed site, if any) or incomplete closure with some persistent residual shunting but no hemodynamic relevance (CDFI-derived shunting width of 2 mm or CW-derived shunting peak velocity of 2 m/s, if any). Otherwise, the result was considered a duct-occluding failure.
Statistical Analysis
Continuous variables with normal distribution are expressed as means ± SD. Otherwise, they are expressed as medians. For a comparison between MSW and SDD or SADO, the difference was examined by paired t test or t test, and the relationship was compared by the linear regression technique. A p value lower than 0.05 was considered statistically significant.
Results
Echocardiographic Estimation of Duct Size
For evaluation of the duct size, 2DE alone could not visualize PDA in 65 patients (21.8 %) and thereby failed to estimate the duct size for these patients, whereas 2DE–CDFI could detect PDA in all the patients, thus making estimation of the duct size for all patients possible. Regardless of the 2DE-detectable PDA in 233 patients (78.2 %), MDW remained significantly lower than MSW in the paired measurement in corresponding patients (5.14 and 2.18 mm vs. 5.75 and 1.99 mm; p < 0.001). These results clearly showed that 2DE did not identify all PDAs and significantly underestimated the duct size.
Although MSW was significantly lower than SDD (5.19 and 2.10 mm vs 6.80 and 3.14 mm; p < 0.001), a highly linear relationship existed between them. By setting the intercept at zero, the simplified equation of SDD (mm) equaled 1.31 MSW (r = 0.89; p < 0.01). Because SDD is a representative of the duct-stretched dimension, the data suggested that MSW could be a reliable measurement of the MMD.
Additionally, although MSW also was significantly lower than SADO (5.19 and 2.10 mm vs 8.99 and 3.90 mm; p < 0.001), a highly linear relationship existed between them. By setting the intercept at zero, the simplified equation of SADO (mm) equaled 1.71 MSW (r = 0.88; p < 0.01), indicating that MSW could be used as reference for choosing an ADO. Taking them together, TTE could be used as an alternative to angiography to estimate the minimal duct size in the selection of suitable occluders for duct closure.
Immediate Outcomes for TTE-Guided Transcatheter Duct Closure
Of 298 patients, 296 patients underwent a duct-occluding attempt, with the attempt waived for 2 patients because of a non–hemodynamically relevant small PDA (<1.5 mm). Of the 296 patients who received duct occluding with MSW used as the major reference for the initial selection of an occluder, SDC was attained in 288 (97.3 %), 5 (1.7 %), and 2 (0.7 %) patients respectively at the first, second (1 ADO replacement), and third (2 ADO replacements) occluding attempt. Acute occluder dislodgement occurred in one patient (0.3 %) due to intraprocedural trouble unscrewing the occluder, resulting in right pulmonary artery embolism. Subsequently, the dislodged occluder was retrieved surgically.
Thrombocytopenia was observed in 15 patients, but all had a mild to moderate decrease in platelet count and completely recovered within 7 days. No other major procedure-related complications were noted periprocedurally.
Follow-up Evaluation and Long-Term Clinical Outcomes
At the 12-month follow-up visit, echocardiography did not detect any defined significant residual shunting, duct reopening, ADO dislodgement, or ADO-caused obstruction at the sides of the aorta or the pulmonary artery. Clinical follow-up assessment showed no major procedure-related complications. As a result, the immediate and 12-month SDC rate was 99.7 %.
Discussion
The well-accepted guidance for transcatheter duct closure has been X-ray aorta angiography [1, 3, 4, 6, 7, 9, 11, 14, 16–18, 21, 23]. Despite the potential capability of echocardiography, using TTE as a major guidance for transcatheter duct closure remains uncertain. Our study was the first to show that TTE with the combined imaging of 2DE and CDFI was technically feasible for accurate evaluation of duct size and monitoring of the procedure, suggesting that TTE could serve as an alternative to aorta angiography for choosing a suitable ADO and guiding transcatheter duct closure.
Feasibility and Accuracy of Echocardiographic Estimation of Duct Size
Both MDW and MSW could be used to estimate MDD. Whereas MDW directly measures MDD at its narrowest site, MSW indirectly estimates MDD by measuring the shunting width in the vena-contracta zone. However, in the real world of clinical practice, MDW frequently is not measurable in the settings of an excessively small a PDA (<2 mm) beyond 2DE resolution, a poor acoustic window, or suboptimal image quality. Actually, in our study, 2DE failed to detect PDA in 21.8 % of the patients and also significantly underestimated duct size using SDD as the reference standard. Combined 2DE and CDFI was especially helpful in the interrogation of duct morphologic and shunting characteristics, particularly in the settings of small PDA or usual types of ductus. As indicated in our study, 2DE–CDFI detected PDA in all patients. As shown by our findings, MSW derived by 2DE–CDFI was closer to and better correlated with SDD than MDW.
For initial selection of an occluder, the reference standard of 1.7 MSW was suitable for occluding most types of PDA despite the fact that a larger occluder might be required for the window-like PDA and a smaller occluder for the conical type of PDA. In fact, by using this standard, SDC at the first occluding attempt was achieved for 97.3 % of the patients, with an occasional ADO replacement and a second or third occluding attempt, suggesting that choosing an adequate occluder based on MSW was technically feasible. However, for better fixation and avoidance of ADO dislodgement, a window-like PDA usually required a much larger ADO than usual compared with the conical type of PDA [14].
Technical Points of Echocardiographic Estimation of Size
First, the parasternal short-axis view and some modified views at the level of great vessels were the best to show the full duct anatomy of the proximal, middle, and distal segments. The supersternal window could be helpful for investigation of the PDA with the usual connections and an abnormal shunting direction.
Second, optimization of spatial and temporal resolution and adjustment of gain setting were essential. Particularly when measuring MDW or MSW, the scanning section should be adjusted to the smallest possible size to increase the imaging frame rate and spatial resolution, and Niquist’s limit usually should be set at a higher level to inhibit aliasing and overflow artifacts during flow mapping.
Third, for more accurate assessment of the duct size, combined 2DE and CDFI instead of 2DE alone was crucial. Usually, when PDA was large enough (>2 mm), 2DE was capable of visualizing the duct anatomy. However, in many settings such as a smaller PDA, an unusual duct type with an abnormal duct distal connection, or suboptimal image quality, 2DE might not show the duct anatomy clearly, making estimation of duct types and size impossible or inaccurate. In most situations, 2DE–CDFI was capable of detecting the duct-shunting signal, particularly the vena-contracta jet and the abnormal shunting direction, thereby rendering accurate estimation of the duct types and sizes possible.
Finally, the measurements of MDW and MSW should be conducted at mid and late diastole because the duct picture or shunting signal is shown most clearly at the phase with fewer artifacts.
Clinical Relevance of Echocardiography-Guided Transcatheter Duct Closure
Adequately choosing an occluder is pivotal for transcatheter closure of PDA. Generally, the suitable occluder size is chosen based on aorta angiographic findings when the conventional method for transcatheter duct closure is used [4, 11, 16]. Performing aorta angiography increases the procedural time and complexity, may induce vascular complications such as from the femoral artery puncture [1, 6, 7, 14, 23], and increases the risk of radiation injury and contrast agent toxicity such as from multiple angiographies intraprocedurally [2, 5, 8, 10, 12, 15, 19, 22].
The aforementioned problems associated with aorta angiography may not be true for adult patients but is true for young patients, particularly for children with low body weight, infants, and neonates. Therefore, if angiographic duct anatomy and shunting can be precisely assessed by TTE, the procedure for transcatheter duct closure will be further simplified, with more safety and less invasion.
Study Limitations
Although we demonstrated that TTE as a major guidance for transcatheter duct closure was technically feasible, accurate evaluation for the configuration of all PDA types was somehow difficult due to suboptimal imaging quality and complex anatomy of types D and E ductus [13, 20]. Additionally, because our data were from a single-center observational study, bias could not be avoided completely. Therefore, a multicenter, randomized, comparative trial is warranted to validate our results.
In conclusion, TTE with 2DE–CDFI is technically feasible for evaluation of duct size and shunting and can serve as an alternative to aorta angiography for selection of an appropriate occluder and as a major guidance during transcatheter duct closure. The study findings show that TTE-guidance can further simplify the procedure, with fewer invasions and potential complications.
Abbreviations
- PDA:
-
Patent ductus arteriosus
- ADO:
-
Amplatzer duct occluder
- TTE:
-
Transthoracic echocardiography
- 2DE:
-
Two-dimensional echocardiography
- CDFI:
-
Color-coded flow imaging
- MDW:
-
The minimal duct width measured by 2DE
- MSW:
-
The minimal shunting width measured by 2DE-CDFI
- SDD:
-
The duct stretched diameter measured by fluoroscopic technique
- SDC:
-
The successful duct closure defined as effective occlusion of duct shunting
References
Ammar RI, Hegazy RA (2012) Percutaneous closure of medium and large PDAs using Amplatzer duct occluder (ADO) I and II in Infants: safety and efficacy. J Invasive Cardiol 24:579–582
Bellin MF, Stacul F, Webb JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Clement O, Heinz-Peer G, Reimer P, van der Molen A, Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR) (2011) Late adverse reactions to intravascular iodine-based contrast media: an update. Eur Radiol 21:2305–2310
Brunetti MA, Ringel R, Owada C, Coulson J, Jennings JM, Hoyer MH, Everett AD (2010) A multi-institutional registry comparing multiple devices. Cathet Cardiovasc Interv 76:696–702
Butera G, De Rosa G, Chessa M et al (2004) Transcatheter closure of persistent ductus arteriosus with the Amplatzer duct occluder in very young symptomatic children. Heart 90:1467–1470
Chen L, Lin C, Peng Y, et al (2007) A new simplified catheter technique for the occlusion of patent ductus arteriosus. Chin J Interv Cardiol 15(1):41–42
Chen ZY, Wu LM, Luo YK, Lin CG, Peng YF, Zhen XC, Chen LL (2009) Comparison of long-term clinical outcome between transcatheter Amplatzer occlusion and surgical closure of isolated patent ductus arteriosus. Chin Med J 122:1123–1127
Chen Z, Chen L, Wu L (2009) Transcatheter amplatzer occlusion and surgical closure of patent ductus arteriosus: comparison of effectiveness and costs in a low-income country. Pediatr Cardiol 30:781–785
Cousins C, Miller DL, Bernardi G, Rehani MM, Schofield P, Vañó E, Einstein AJ, Geiger B, Heintz P, Padovani R, Sim KH; International Commission on Radiological Protection (2013) ICRP publication 120: radiological protection in cardiology. Ann ICRP 42:1–125
Faella HJ, Hijazi ZM (2000) Closure of the patent ductus arteriosus with the Amplatzer PDA device: immediate results of the international clinical trial. Cathet Cardiovasc Intervent 51:50–54
Fetterly KA, Mathew V, Lennon R, Bell MR, Holmes DR, Rihal CS (2012) Radiation dose reduction in the invasive cardiovascular laboratory: implementing a culture and philosophy of radiation safety. JACC Cardiovasc Interv 5:866–873
Fischer G, Stieh J, Uebing A et al (2001) Transcatheter closure of persistent ductus arteriosus in infants using the Amplatzer duct occluder. Heart 86:444–447
Justino H (2006) The ALARA concept in pediatric cardiac catheterization: techniques and tactics for managing radiation dose. Pediatr Radiol 36(Suppl 2):146–153
Krichenko A, Benson LN, Burrows P, Moes CA, McLaughlin P, Freedon RM (1989) Angiographic classification of the isolated persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol 63:877–879
Kumar SM, Subramanian V, Bijulal S, Krishnamoorthy KM, Sivasankaran S, Tharakan JA (2013) Percutaneous closure of a moderate to large tubular or elongated patent ductus arteriosus in children younger than 3 years: is the ADO II appropriate? Pediatr Cardiol 34:1661–1667
Laville M, Juillard L (2010) Contrast-induced acute kidney injury: how should at-risk patients be identified and managed? J Nephrol 23:387–398
Masura J, Kevin P, Thanopoulos B et al (1998) Catheter closure of moderate-to-large-sized patent ductus arteriosus using the new Amplatzer Duct Occluder: immediate and short-term results. J Am Coll Cardiol 31:878–882
Masura J, Tittel P, Gavora P, et al (2006) Long-term outcome of transcatheter patent ductus arteriosus closure using Amplatzer duct occluders. Am Heart J 151:755.e7–755.e10
Pass RH, Hijazi Z, Hsu DT et al (2004) Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol 44:513–519
Pasternak JJ, Williamson EE (2012) Clinical pharmacology, uses, and adverse reactions of iodinated contrast agents: a primer for the non-radiologist. Mayo Clin Proc 87:390–402
Schneider DJ, Moore JW (2006) Patent ductus arteriosus. Circulation 114:1873–1882
Thanopoulos BD, Hakim FA, Hiari A et al (2000) Further experience with transcatheter closure of the patent ductus arteriosus using the Amplatzer duct occluder. J Am Coll Cardiol 35:1016–1021
Valentin J (2000) Avoidance of radiation injuries from medical interventional procedures. Ann ICRP 30:7–67
Vijayalakshmi IB, Chitra N, Praveen J, Prasanna SR (2013) Challenges in device closure of a large patent ductus arteriosus in infants weighing less than 6 kg. J Interv Cardiol 26:69–76
Weyman AE (1994) Principles and practice of echocardiography, 2nd edn. Lea & Febiger, Philadelphia, p 510
Acknowledgments
The authors thank Miss Hong Zheng for her participation in the collection of data. They also thank all the staff of the Medical Library, Catheter Lab, and Department of Cardiology at Union Hospital for their kind support and enthusiasm for this study.
Conflict of interest
All authors have no relation with industry or interest conflict associated with the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Yan, X., Huang, Y. et al. Transthoracic Echocardiography as an Alternative Major Guidance to Angiography During Transcatheter Closure of Patent Ductus Arteriosus: Technical Feasibility and Clinical Relevance. Pediatr Cardiol 36, 14–19 (2015). https://doi.org/10.1007/s00246-014-0956-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-014-0956-9