Abstract
The aim of this paper was to determine the moisture desorption isotherms and essentials thermodynamic properties of two Oak wood varieties. Desorption isotherms were measured using a static gravimetric method at 50, 60, 70 and 80 °C within the range of 5–90 % relative humidity. The equilibrium moisture content decreased with increasing temperature and decreased with decreasing relative humidity at a constant temperature. The ‘Thermodynamic’ sorption equation was found to be the best for describing the experimental moisture sorption isotherms of woods within the range of temperature and water activity investigated. The Fiber saturation point, deduced from the ‘Thermodynamic’ model parameters, depends on the temperature and varying from 22.6 to 54.4 (% kg water/kg dry matter). Isosteric heat of desorption and differential entropy were calculated by applying Clausius–Clapeyron equation to the desorption data fitted by the ‘Thermodynamic’ model. The isosteric heat of desorption and the differential entropy decreased with increasing moisture content according to an exponential law equation and varying from 2.03 to 31.14 kJ/mol and from 73.98 to 4.34 J/(mol K), respectively. The linear relationship between differential enthalpy and entropy satisfied the enthalpy–entropy compensation theory. The sign of Gibbs free energy was found to be positive (+283 J/mol) and (+97 J/mol) for Quercus robur and Quercus canariensis, respectively. The isokinetic temperature was found to be greater than the harmonic temperature. Based on the enthalpy–entropy compensation theory, it could be concluded that the moisture desorption isotherm of Oak wood is a non-spontaneous and enthalpy-controlled process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Wood is an important biodegradable and renewable material used all over the world as an energy source, building and packing material, furniture and tools, decoration purposes and artistic medium due to its high strength-to-weight ratio, unique porous structure, and aesthetic characteristics [1, 2]. Freshly cut wood contains large quantity of water in cell walls and liquid–vapour mixture or vapour in cell lumens [3]. Drying of wood stabilizes the dimensions and shape of wooden components and protect them from biological attack by reducing the water activity [4, 5]. The International Plant Protection Convention (IPPC) specifies heating process under controlled climate to sanitize wood packing material, until a well-known moisture content, in order to prevent the circulation of the forest pests around the world [6, 7]. Controlled, handling and storage of wood material minimize moisture content changes after drying when physical, chemical and microbiological change become undesirable [8]. Wood is a hygroscopic product, which tends towards equilibrium moisture content with its environment [4, 9–12]. Therefore, the heat supplied to wood accelerates the water migration and affects the dynamic equilibrium between the vapor and adsorbed phases during adsorption and desorption processes [13].
The better knowledge moisture desorption isotherms and essential thermodynamic properties (net isosteric heat, differential enthalpy, differential entropy) of wood under controlled climate is necessary for the prediction of the drying time, reduction of the heating energy coast and designing of drying apparatus [14, 15] and for calculating moisture changes that may occur during drying, storage and use under various climatic conditions [16].
Numerous reports in the literature demonstrated that the sorption isotherm was the most solution to determine the equilibrium moisture content of wood and various biological products under controlled operating conditions (temperature and relative humidity) by using static-gravimetric method [10–12, 17–23]. The sorption isotherm was the curve representing the equilibrium moisture content of wood versus water activity at a constant temperature [10, 12]. According to [16], the concept of water activity quantifies the presence of water in the product and was commonly defined as the ratio of the water vapor pressure exerted by the product to the vapour pressure of pure water, at the same temperature. Rawat and Khali [24] demonstrate that the changes in free energy, enthalpy, and entropy during water sorption in Klinky Pine wood have a strong dependence on moisture content, and have a weak dependence on temperature. Themelin [17] demonstrates that the increase of temperature and the decrease of relative humidity decrease the equilibrium moisture content of tropical woods of angelica (dicorynia guianensis), iroko (milicia excels) and Eucalyptus (Eucalyptus grandis) and that the degradation of hemicelluloses in torrefied wood reduces the sorption sites of water vapour than in non torrefied wood. Nkolo Meze’e et al. [20] demonstrated that the thermodynamic properties (Gibbs energy, enthalpy and entropy) of tropical wood (Bubinga) have a great dependence to moisture content and no significant variation with temperature. Ouertani et al. [11] demonstrate that the increase of temperature decreases the moisture content of Jack pine (pinus bankisiana) and palm wood and that the corresponding thermodynamic properties depend on temperature and moisture content and that the moisture desorption reactions is a non-spontaneous and enthalpy controlled process.
Mathematical models that describe the experimental sorption data aid to determine the thermodynamic interactions of wood-water system, the useful equilibrium moisture content at the end of drying operation and were important tools in modeling approach of heat and mass transfer operation [5, 25]. Thus, thermodynamic properties are readily deduced from data fitted by the sorption isotherm equations. There are more than 77 mathematical equations with two or more parameters, having a physical meaning, were proposed in the literature to fit the sorption isotherms in a wide range of temperature and relative humidity. The most used equation for lingo-cellulosic products, in particular wood, were Thermodynamic model [26], Dent model [27], GAB and Henderson models [28].
Thermodynamic properties such as isosteric heat of desorption, differential enthalpy, Gibbs free energy and differential entropy may be calculated by applying Clausius–Clapeyron equation to fitted sorption data [22, 29]. The isosteric heat of desorption, measure the energy required to break the intermolecular forces between the water molecules and the surface of adsorbent. The better knowledge of the isosteric heat of desorption has more accurate information when designing drying apparatuses [12]. Enthalpy variation measures the energy changes that occur upon mixing of water molecules with wood during sorption and may be associated with binding forces of the water-wood system [20]. The free energy variation may be indicative of the sorbent’s affinity for water based on its sign and provide a criterion whether water sorption is a spontaneous process or not [30]. Spontaneous reaction occurs naturally without continuous external source energy. However, non-spontaneous reaction takes place as a result of continuous external intervention [31]. The concept of enthalpy controlled desorption process means that the main driving force is the release of heat [30]. The entropy changes during sorption process may be associated with the spatial arrangements occurring in the water–wood system in a defined state [32]. The enthalpy–entropy compensation theory has been widely investigated in different physical and chemical processes, in particular, in water desorption reactions under controlled temperature and relative humidity [11, 33, 34]. The theory specifies that in order to minimize free energy variation due to these phenomena, compensation arises from the nature of the interaction between the solute and solvent causing the reaction and that the relationship between the enthalpy and entropy for a specific reaction is linear [20].
Oak wood (Quercus robur and Quercus canariensis) is an important crop grown in the North-East of France and in the Northern part of Tunisia, respectively. This wood crop plays a very important role in the socioeconomic and ecological levels of France and Tunisia. It used in various specific industries, such as plywood, decorative veneers, furniture, crafts and packaging. However, moisture desorption isotherms and thermodynamic properties of Quercus robur and Quercus canariensis wood under controlled climate stills remains unclear. Therefore, the objective of this work was (1) to determine the desorption isotherms of two Oak wood varieties, (2) to determine the suitable model fitting the desorption isotherms, (3) to determine the dependence of thermodynamic properties with temperature and moisture content and to check the existence of the enthalpy–entropy compensation theory (4) and to verify if the desorption isotherm reaction in this wood materials is spontaneous or non-spontaneous and enthalpy-driven or entropy-controlled process.
2 Materials and methods
2.1 Wood sample preparation
Oak wood samples of Quercus robur and Quercus canariensis are freshly harvested from the North-East of France (Vosges) and from the Northern part of Tunisia, respectively [35, 36]. To focus the attention on the influence of temperature and relative humidity on the water desorption process, wood samples were sawn from a one green tree in order to limit the wood variability [18]. The dimensions of the test pieces were 15 mm long, 10 mm wide and 5 mm thick in longitudinal (L), radial (R) and Tangential (T) directions, respectively. The size of the wood samples was small enough to obtain uniform equilibrium moisture content (Fig. 1).
2.2 Experimental procedure: moisture desorption isotherms and data analysis
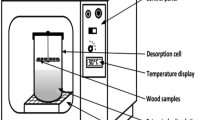
The equilibrium moisture content of wood at 50, 60, 70 and 80 °C was measured using the static gravimetric method. Standard saturated salt solutions (KOH, LiCl, MgCl2, K2CO3, NaBr, NaCl, KCl and BaCl2) were used to control the relative humidity in a closed space. In this method, diffusion is the only mass transfer mechanism inside the product. Values of relative humidity of saturated salt solutions are obtained from the works of [11, 16, 37–39] and presented in Table 1. These solutions are prepared by dissolving the necessary quantity of salt in distilled water up to super-saturation, at a given temperature, covering a water activity range of 0.058–0.891. These solutions were placed inside hermetic jars with insulated lids. Wood samples are suspended to jars lids without touching the solutions. Finally, hermetic jars were placed inside heating oven (DHG-9053A) with a temperature range of +10 to 250 °C and temperature accuracy of ±1 °C (Fig. 2). The thermodynamic equilibrium of wood was considered when the mass of the samples showed a difference less than 1 mg after three consecutive measurements at intervals of 24 h [11, 40–42]. Wood samples were weighed on a balance (Mettler Toledo, Mississauga, Canada) at 0.001 g precision [43]. When equilibrium was reached and based on standard test method to obtain dry matter content, the wood samples were dried using oven method at 103 ± 2 °C for 24 h [44]. The equilibrium moisture content is given by the following Eq. (1):
2.3 Moisture desorption isotherms modeling
The sorption isotherm equations used in the present study for fitting the experimental data are presented in Table 2. The four selected sorption models are mostly used to describe the moisture sorption of wood [11, 17, 18, 27]. To select the most appropriate equation that adequately describes the sorption data of two varieties of Oak wood, models parameters were identified by non-linear least square regression analysis, using CurvExpert 1.3 software with two statistical criteria, namely the standard error (s) and the correlation coefficient (r), defined in the “Appendix” [45].
3 Thermodynamic properties of desorption
3.1 Net isosteric heat of desorption-differential enthalpy
The isosteric heat of desorption (differential enthalpy) was determined by applying the Clausius–Clapeyron equation (Eq. 2), that relates water activity and temperature at a constant moisture content to equilibrium data obtained from the best fitting desorption equation [11, 21, 38].
Assuming that the isosteric heat of desorption is invariant with temperature [46], the integration of equation (Eq. 2) gives the following equation (Eq. 3):
The net isosteric heat of desorption was determined from the slope of the curve Ln(aw) versus 1/T (K−1). This approach was repeated for several equilibrium moisture content values obtained from the best fitting equation. This procedure determines the evolution of isosteric heat of desorption with equilibrium moisture content. Therefore, this procedure was recommended for sorption isotherms measured at more than two temperatures [11, 21, 42].
To describe the relationship between the isosteric heat of desorption and the equilibrium moisture content, Tsami [21] proposed the following empirical exponential expression (Eq. 4):
3.2 Differential entropy
Differential entropy of desorption (∆S) changes may be associated with the binding or repulsive forces of water molecules in the product and is proportional to the number of available sorption sites at a specific energy level. It defines the degree of order or randomness existing in the water-sorbent system and can help interpreting processes such as dissolution, crystallization and swelling [47, 48]. Gibbs free energy (∆G) sign may be indicative of the affinity of sorbents for water, and provides a criterion to determine whether water sorption occurs as a spontaneous (∆G < 0) or non-spontaneous (∆G > 0) process [49]. The differential entropy of desorption is calculated using the equation of Gibbs–Helmholtz [50]:
where ∆G is the Gibbs free energy, calculated from Eq. (6):
The relationship between the differential enthalpy (Qst,n) and entropy of desorption (∆S) is given by Eq. (7), which was deduced from Eqs. (5) and (6):
For given moisture content, differential desorption entropy (∆S) is determined from the intercept of the line of Ln (aw) versus 1/T (K)−1 [34].
3.3 Enthalpy–entropy compensation theory
Enthalpy–entropy compensation theory is used to evaluate physical and chemical phenomena such as moisture sorption reactions. Sharp [51] explained that the entropy-enthalpy compensation term was applied in literature and different usages are discussed. However, the most precise and interesting case was a linear relationship between enthalpy and entropy for some series of perturbations or changes in experimental variables. In a true equilibrium, assumed in the Clausius–Clapeyron equation, entropy is maximized, hence the differential entropy is zero and the differential Gibbs free energy is equal to the isosteric heat of adsorption [52]. For a specific thermodynamic reaction, the enthalpy–entropy compensation theory proposes a linear relationship between the differential enthalpy (Qst,n) and entropy of desorption (∆S) [Eq. (8)] [30, 53]:
In a true equilibrium, assumed in the Clausius–Clepeyron equation, entropy is maximized, hence the differential entropy is zero and the differential Gibbs free energy is equal to the isosteric heat of adsorption [52].
The isokinetic temperature Tβ represents the temperature at which all reactions in the series proceed at the same rate. ΔGβ represent the Gibbs free energy at the specific temperature Tβ. The sign of ΔGβ indicates whether water desorption reaction is a spontaneous (negative) or a non-spontaneous process (positive).
To confirm the enthalpy–entropy compensation theory a statistical analysis test was proposed by Krug et al. [54]. The isokinetic temperature should be compared with the harmonic mean temperature (Thm) [Eq. (9)]. The enthalpy–entropy compensation theory applies if Tβ # Thm . Moreover, if Tβ > Thm the process is enthalpy driven, while if Tβ < Thm, the process is entropy-controlled [30, 48, 53].
Based on [34, 55] works, parameter (ΔGβ) can be neglected for its small contribution in the enthalpy change. The enthalpy–entropy compensation theory was further used to evaluate the impact of temperature on the sorption behaviour by rearranging Eqs. (7) and (8) to obtain the following Eq. (10).
The influence of temperature on moisture equilibrium properties can be considered by re-expressing Eq. (10) in the following form (Eq. 11):
Where ΨT = ((1/T) − (1/Tβ))−1 is the temperature correction factor in the desorption isotherms and Φ (X) is an empirical function of the equilibrium moisture content. The equilibrium data can be represented by plotting ln (ΨT ln(aw)) as a function of the equilibrium moisture content.
Aguerre et al. [34] asserted that if this correction factor is adequate, the equilibrium data of a given product, at different temperatures, present the same mathematical form when plotted in accordance with Eq. (10). The dependence of ΨT ln(aw) with moisture content (X) follows an exponential law (Eq. 12):
After plotting ln (ΨT ln(aw)) versus equilibrium moisture content, the constants K1 and K2 were calculated by linear regression.
4 Results and discussion
4.1 Moisture desorption isotherms
The experimental moisture desorption isotherms for the Oak wood of Quercus canariensis and Quercus robur at 50, 60, 70 and 80 °C are shown in Figs. 3 and 4, respectively. Curves analysis demonstrates that the equilibrium moisture content increases with the water activity at constant temperature. Moisture desorption isotherms behaviour of two wood varieties showed the typical sigmoid shape of type II, according to the BET classification [56]. This shape is typical of tropical wood [10, 57], Jack pine and palm wood [11], Maritime pine wood [18] and Japanese cypress wood (hinoki: Chamaecyparis obtusa) [58]. The curves shape informs on the nature of the water-wood sorption process. The Type II isotherms correspond to process which initially follows ‘Type I’ form but then it shows a buildup of a monolayer. After the monolayer region, the increase of vapour pressure results in a successive multilayer adsorption and a strong interaction between the molecules of adsorbate [59]. Desorption isotherms curves analysis lead to the identification of two different regions. At low and intermediate water activities, multilayer sorption region is localized. It is characterized by linear increases of moisture content with water activity. While, at high water activities, capillary condensation region is localized. It is characterized by rapid increasing of water content with water activity [60].
At constant water activity, the equilibrium moisture content of two types of wood decreases as the temperature increases. This behavior explains that the increase of temperature can reduce the total number of active sites for water binding as a result of physical and/or chemical changes in the product [61]. The increase of temperature promotes the migration of water molecules from the water binding sites [11]. When temperature increases the water molecules become activated due to an increase in their energy level, causing them to become less stable and to break away from the water-binding sites of the material, which consequently decreases the monolayer moisture content [62].
Comparative aspect between moisture desorption isotherms of Figs. 3 and 4 shows that Quercus canariensis wood is less hygroscopic at all operating conditions than Quercus robur. The difference in adsorption capacity, under same controlled climate, between two Oak varieties becomes from various parameters that affect the physical, anatomical structure and tissue of wood such as species, growth conditions, site and climate [9, 63, 64]. The Kelvin equation gives the relationship between the radius of the cell and the equilibrium relative vapor pressure [65]. According to the distribution of the cells size of the product, the capillary condensation can change the part of the sorption isotherm at the high humidity. So we could assume that the samples selected for this work for Quercus canariensis and Quercus robur have a contrasted structure. On the other hand, the average density of dried wood of Quercus robur and Quercus canariensis was equal to 584.3 kg m−3 [66] and 708.5 kg m−3 (determined experimentally), respectively. However, we could assume that Quercus robur wood have a large cells (vessels and fibers) than Quercus canariensis which favorite the capacity of water adsorption under the same operating conditions (Table 3).
4.2 Isotherm predictive models
The experimental desorption data were fitted with GAB, Dent, Henderson, and Thermodynamic models. The results of non-linear regression of desorption isotherms of wood, at various temperatures, are shown in Table 3. According to our results, the ‘Thermodynamic’ model presents the lowest values of standard error (equal to 2.61 × 10−3 and 6.1 × 10−3) and the highest value of correlation coefficients (equal to 0.994 and 0.999), respectively, and gives the best fit as shown in Figs. 3 and 4. Based on these results, Thermodynamic model was selected to describe the desorption isotherms of Quercus robur and Quercus canariensis wood. The ‘Thermodynamic’ equation is based on thermodynamic approach of heat sorption of bound water which depends on the moisture content state, the superficial layer energy state and the environment properties [26]. A plot of predicted (using Thermodynamic model) versus measured equilibrium moisture content, between 50 and 80 °C was presented in Figs. 3 and 4. According to [17] the better knowledge of the desorption data and corresponding mathematical models under controlled operating conditions can be integrated directly in drying schedules and industrial kilns supervision software.
The fiber saturation point (FSP) of Quercus robur and Quercus canariensis wood varying from 54.4 to 45.1 and 34.1 to 22.6 % kg/(kg DM), respectively. Results shows that an increase in temperature decreases the FSP. This result illustrates the impact of temperature on the water adsorption–desorption capacity of Oak wood and gives information about the best fitting of desorption data. Similar results were obtained by [26] for Douglas fir, [18] for Maritime pine and [11] for Jack pine and Palm wood. Fundamentally, the fiber saturation point is the amount of water that the composite polymers of the cell wall can hold at a particular temperature and pressure [67]. Varying bound water bellow FSP causes dimensional changes (shrinkage or swelling) of wood. Therefore, the knowledge of the FSP has many practical or industrial applications, it gives crucial information on the beginning of the shrinkage mechanism during drying and it can aid to optimize the processing parameters, to avoid the cracking, collapse, twist, etc.
4.3 Isosteric heat and entropy of desorption
The isoteric heat of desorption or differential enthalpy and entropy change were calculated from the desorption data determined throughout ‘Thermodynamic’ model. The desorption isosters of Quercus robur and Quercus canariensis wood are presented in Figs. 5 and 6.
Isosteric heat of desorption was calculated from the slope of the plot of Ln(aw) versus 1/T (K)−1 (Eq. 3) for each equilibrium moisture content. Figure 7 shows that the isosteric heat of desorption (Qst,n) of Oak wood, determined by applying Clausius–Clapeyron equation, decreases continuously with the increase of the equilibrium moisture content. At high moisture content, isosteric heat of desorption is close to the mean value of latent heat of vaporization of pure water (Lv) [12, 21]. As seen from Fig. 7, the isosteric heat of desorption (Qst,n) decreased with the increase of the moisture content from 31.15 to 2.03 kJ/mol and from 22.02 to 3.96 kJ/mol with a variation of equilibrium moisture content ranging from 0.02 to 0.18 kg/(kg DM) and from 0.02 to 0.14 kg/(kg DM) for Quercus robur and Quercus canariensis, respectively. The high value of isosteric heat of desorption (Qst,n) showed at low moisture content is due to the existence of highly active polar sites on the surface on the product, which are covered with water molecules forming a mono-molecular layer [68]. According to [69] at increasing moisture content, the most active sites become occupied and sorption occurs on the less active site giving lower heats of sorption. Similar results were reported for wood of Klinky Pine [24], Western hemlock, Douglas fir, Western red cedar, Sitka spruceand Lodgepole pine [19], tropical wood (Bubinga) [20] and Jack pine and Palm wood [11]. The high value of isosteric heat of desorption (Qst,n) at low moisture content indicates the strong link between water molecules and the solid matrix of wood [20, 70]. This requires supplementary heat to the latent heat of vaporization of pure water to evaporate the bound water molecules in the hygroscopic region. This trend supported the fact that as moisture content increases, the forces of water molecules attraction by wood decrease [71]. The water sorption mechanism occurs initially in the most active sites giving rise to the greatest interaction energy [72]. When the moisture content increases the sorption occurs on the less active sites resulting in lower heats of sorption and a negligible influence of solid matrix of wood on adsorbed water. The variation of the isosteric heat of desorption with moisture content was fitted by an exponential function, according to the typical Tsami [21] equation, for Quercus robur and Quercus canariensis as following:
The parameters of Tsami [21] equation (q0; X0) for desorption data were (48.65 kJ/mol; 0.045 kg/kg DM) and (28.53 kJ/mol; 0.074 kg/kg DM) for Quercus robur and Quercus canariensis, respectively. The q0 and X0 parameters defines the necessary isosteric heat to evaporate the monolayer equilibrium water content of the product. Similar results were reported for Jack pine and Palm wood [11] and for prickly pear seeds [42]. However, power law equation has been proposed to describe the isosteric desorption heat with moisture content of olive leaves (Olea europaea L.) [22] and Polynomial law were also proposed for peppermint tea (Mentha piperita) [68].
At low moisture content (<7 %), the isoteric heat of desorption is higher in Quercus robur than in Quercus canariensis. However, at high moisture content (>7 %) it is higher in Quercus canariensis than in Quercus robur. This behaviour can be related to the variability between Oak varieties in anatomical structure, growth conditions, site and climate of the North-East of France and the Northern part of Tunisia [9].
The variation of the differential entropy of desorption of Quercus robur and Quercus canariensis versus equilibrium moisture content is shown in Fig. 8. The curve shows a continuous decrease of the value of differential entropy of desorption with the increase of equilibrium moisture content. It ranged from 73.98 to 4.345 J/(mol K) and from 55.23 to 8.25 J/(mol K) with moisture content ranging from 0.02 to 0.18 kg/kg DM and from 0.02 to 0.14 kg/(kg DM), for Quercus robur and Quercus canariensis, respectively. Similar trends of desorption entropy were found for topical wood and Klinky Pine wood [20, 24]. This results is expected because the differential entropy of desorption measures the molecule ordering change. It is lower when water molecular movement is more restricted at high moisture content [42, 72].
The variation of desorption entropy (∆S) with moisture content for Quercus robur and Quercus canariensis was fitted by an exponential function similar to the behaviour exhibited for the isosteric heat of desorption:
Similar exponential trend for desorption entropy was reported for Palm and Jack pine wood [11]. However, McMinn et al. [55] Reported power law for potato.
4.4 Enthalpy–entropy compensation theory
A linear relationship related the isosteric heat of desorption to differential entropy of desorption for two wood varieties was shown is Fig. 9. The results confirm the enthalpy–entropy compensation theory. The isokinetic temperature (Tβ) and free energy (ΔGβ) were determined by linear regression of Eq. (8). The characteristic parameters for enthalpy–entropy relationship for selected wood varieties were presented by the following fit curves:
The sign of (ΔGβ) was found to be positive (+283 J/mol) and (+97 J/mol) for Quercus robur and Quercus canariensis, respectively, indicating a non-spontaneous desorption process (Table 4). These positive values of Gibbs free energy (ΔGβ) is totally in agreement with thermodynamic laws because the desorption is a non-spontaneous process. The Quercus robur and Quercus canariensis samples are exposed to high relative humidity degree, then there samples are placed at low relative humidity level, until thermodynamic equilibrium. According to [30, 31] the non-spontaneous reaction requires input energy from the surrounding environment to the subjected product. Therefore, Gibbs free energy is tied to work necessary to make the sorption sites available to the swelling of the wood structure; the higher the moisture content, the lower the number of available sites [20].
The isokinetic temperature (Tβ) value of Quercus robur and Quercus canariensis is 413 and 356 K, respectively. The harmonic temperatures (Thm) was found to be Thm = 337.78 K (calculated by applying Eq. 9). Based on our results, it is observed that Tβ ≠ Thm for two products. Furthermore, our result shows that Tβ > Thm indicating an enthalpy controlled desorption process. This result indicates the stability of the microstructure of two types of wood during the water desorption reaction in the temperature range from 50 to 80 °C [42]. These observations of enthalpy–entropy compensation are obtained for other woods such as Jack pine and palm wood [11], Douglas fir heartwood [19] and quince [73].
The enthalpy–entropy compensation theory was also applied to model the effect of temperature on moisture desorption. Figure 10 shows the typical evolution of Ln((1/Tβ − 1/T) ln(aw)) with moisture content. A straight line was obtained for the two types of Oak wood. This behaviour confirms that the temperature effect on moisture desorption follows a power law [34, 42]. The values of K1 and K2 parameters of two products were calculated using linear regression and are presented in Table 5. According to [11], the values of K1 and K2 were found to be, respectively, 5506.6 K and 3.69 × 10−9 for water desorption of Jack pine and 5859.38 K and 0.00018 for Palm wood.
Equilibrium moisture data of Oak wood plotted according to Eq. 12
5 Conclusions
The desorption isotherms of Quercus robur and Quercus canariensis wood have determined at 50, 60, 70 and 80 °C and over range of relative humidity by using the static gravimetric method. Based on experimental methods and physical laws, the predictive correlations of essential thermodynamic properties of water desorption isotherms of products, were established and compared. Results from this study led to the following conclusions:
-
The obtained desorption isotherms of Oak woods at various temperature presented sigmoid forms of type II. The equilibrium moisture content of wood decreases with increasing temperature at a given water activity and decreases with decreasing water activity at a constant temperature.
-
From four sorption models tested, the ‘Thermodynamic’ equation was selected to predict the equilibrium moisture content of Oak wood in the whole range of temperature and relative humidity investigated.
-
The isosteric heat and differential entropy of desorption decreases with increasing moisture content in an exponential law.
-
Differential enthalpy versus desorption entropy data satisfies the enthalpy–entropy compensation theory. According to the positive sign of the Gibbs free energy, desorption is a spontaneous process. It requires continuously energy from external environment to maintain the desorption process.
-
As the isokinetic temperature is greater than the harmonic mean temperature, the desorption process is enthalpy-controlled mechanism. Heat was the responsible energy to active the desorption process.
Abbreviations
- A, B and C:
-
Model parameters
- aw :
-
Water activitiy (–)
- Cg :
-
Constant related to the heat of sorption
- cst:
-
Constant
- DM:
-
Dry matter
- K:
-
Constant related to multilayer properties
- Lv :
-
Latent heat of vaporization (J/mol)
- Md :
-
Dry mass (kg)
- Meq :
-
Equilibrium mass (kg)
- n:
-
Total number of isotherms
- q0 :
-
Isosteric heat of sorption of the monolayer (kJ/mol)
- qst :
-
Total isosteric heat of desorption (J/mol)
- Qst,n :
-
Desorption isosteric heat (J/mol)
- R:
-
Perfect gas constant [8.315 J/(mol K)]
- RH:
-
Relative humidity (%)
- T:
-
Temperature (°C)
- Thm :
-
Harmonic mean temperature (K)
- Tβ :
-
Isokinetic temperature (K)
- X0 :
-
Water content of the monolayer [kg water/(kg DM)]
- Xeq cal,i :
-
Equilibrium moisture content calculated
- Xeq :
-
Equilibrium moisture contents [kg water/(kg DM)]
- Xeqi :
-
Experimental value of equilibrium moisture content
- Xfsp :
-
Fiber saturation point [% kg water/(kg DM)]
- nexpdata :
-
Experimental point
- nparam :
-
Parameter number of the particular model
- \(\overline{{{\text{X}}_{\text{eq}} }}\) :
-
Arithmetic average value of the experimental equilibrium moisture content
- \(X_{{eq_{m} }}\) :
-
Monolayer moisture content [kg/(kg DM)]
- ΔS:
-
Desorption entropy (J/mol K)
- Φ :
-
Thermodynamic parameter
References
Ding WD, Koubaa A, Chaala A (2013) Mechanical properties of MMA-hardened poplar wood. Ind Crops Prod 46:304–310
Popescu C-M, Hill CAS, Curling S, Ormondroyd G, Xie Y (2013) The water vapour sorption behavior of acetylated birch wood: how acetylation affects the sorption isotherm and accessible hydroxyl content. J Mater Sci 49:2362–2371
Engelund ET, Thygesen LG, Svensson S, Hill CAS (2013) A critical discussion of the physics of wood–water interactions. Wood Sci Technol 47:141–161
Ouertani S, Azzouz S, Hassini L, Belghith A (2011) Palm wood drying and optimization of the processing parameters. Wood Mater Sci Eng J 6:75–90
Remond R, Passard J, Perre P (2007) The effect of temperature and moisture content on the mechanical behavior of wood: a comprehensive model applied to drying and bending. Eur J Mech A Solids 26:558–572
Payette M, Timothy TW, Drouin P, Koubaa A (2015) Efficacy of microwave irradiation for phytosanitation of wood packing materials. Ind Crops Prod 69:187–196
Aléon D (2004) Traitement phytosanitaire du bois par chauffage à cœur. Bull OEPP/EPPO Bull 34:133–138
Simpson WT (1999) Drying and control of moisture content and dimensional changes. Wood handbook-wood as an engineering material, Forest Products Laboratory, General Technical Report FPL-GTR 113, USDA, Madison
Perré P, Keey BR (2006) Drying of wood: principles and practices. In: Mujumdar AS (ed) Handbook of industrial drying, 3rd edn. Taylor & Francis, London, pp 822–872
Jannot Y, Kanmogne A, Talla A, Monkam L (2006) Experimental determination and modelling of water desorption isotherms of tropical woods: afzelia, ebony, iroko, moabi and obeche. Holz Roh Werkst 64:121–124
Ouertani S, Azzouz S, Hassini L, Koubaa A, Belghith A (2014) Moisture sorption isotherms and thermodynamic properties of Jack pine and Palm wood: comparative study. Ind Crops Prod 56:200–210
Fernandez GF, Esteban LG, Palacios P et al (2014) Sorption and thermodynamic properties of Terminalia superba Engl. & Diels and Triplochiton scleroxylon K. Schum. Through the 15, 35 and 50 °C sorption isotherms. Eur J Wood Prod 72:99–106
Al-Muhtaseb AH, McMinn WAM, Magee TRA (2004) Water sorption isotherms of starch powders: part 1: mathematical description of experimental data. J Food Eng 61:297–307
King CJ (1968) Rates of moisture sorption and desorption in porous dried food stuffs. Food Technol 22:165–171
Young JH (1976) Evaluation of models to describe sorption and desorption equi-librium moisture content isotherms of Virginia type peanuts. Trans ASABE 19:146–150
Labuza TP (1968) Sorption phenomena in foods. Food Technol 22:263–268
Themelin A (1998) Comportement en sorption de produits ligno-cellulosiques. Bois For Trop 2:55–67
Lartigue C (1987) Mécanisme élémentaire mis en jeu lors du séchage du pin maritime. PhD thesis, Université de Bordeaux I
Koumoutsakos A, Avramidis S (1999) Enthalpy–entropy compensation in water sorption by various wood species. Holz Roh Werkst 57:379–382
Nkolo Meze’e YN, Ngamveng JN, Bardet S (2008) Effect of enthalpy–entropy compensation during sorption of water vapour in tropical woods: the case of Bubinga (Guibourtia Tessmanii J. Leonard; G. Pellegriniana J.L.). Thermochim Acta 468:1–5
Tsami E (1991) Net isosteric heat of sorption in dried fruits. J Food Eng 14:327–335
Bahloul N, Boudhrioua N, Kechaou N (2000) Moisture desorption–adsorption isotherms and isosteric heats of sorption of Tunisian olive leaves (Olea europaea L.). Ind Crops Prod 28:162–176
Belghith A, Azzouz S, ElCafsi A (2015) Desorption isotherms and mathematical modelling of thin layer drying kinetics of tomato. Heat Mass Transf 52:407–419
Rawat SPS, Khall DP (1996) Enthalpy–entropy compensation during sorption of water in wood. J Appl Sci 60:787–790
Iglesias HA, Chirife J (1976) Isosteric heats of water vapour sorption on dehydrated foods. Part II: hysteresis and heat of sorption comparison with BET theory. Lebensm Wiss Technol 9:123–127
Mrekeb S, Dubois F, Petit Ch (2009) Modeling of the sorption hysteresis for wood. Wood Sci Technol 43:575–589
Navi P, Heger F (2005) Comportement thermo-hydromécanique du bois: application technologiques et dans les structures, 1st edn. Presses polytechniques et universitaires romandes, Suisse
Kouhila M, Kechaou N, Otmani M, Fliyou M, Lahsasni S (2002) Experimental study of sorption isotherms and drying kinetics of Moroccan Eucalyptus globules. Dry Technol 20:2027–2039
Gabas AL, Menegalli FC, Telis-Romero J (2000) Water sorption enthalpy–entropy compensation based on isotherms of plum skin and pulp. J Food Sci 65:680–684
Oliveira EG, Rosa GS, Moraes MA, Pinto LAA (2009) Moisture sorption characteristics of microalgae sprulina platensis. Braz J Chem Eng 26:1297–1303
Goneli ALD, Correa PC, Oliveira GHH, Botelho FM (2010) Water sorption isotherms and thermodynamic properties of okra seeds. Trans ASABE Am Soc Agric Biol Eng 53:191–197
Avramidis S (1992) Enthalpy–entropy compensation and thermodynamic considerations in sorption phenomena. Wood Sci Technol 26:329–333
Leffler JE (1955) the enthalpy–entropy relationship and its implications for organic chemistry. J Org Chem 20:1202–1231
Aguerre RJ, Suarez C, Viollaz PE (1986) Enthalpy–entropy compensation in sorption phenomena: application to the prediction of the effect of temperature on food isotherms. J Food Sci 51:1547–1549
Ponton S, Dupouey JL, Bréda N, Feuillat F, Bodénès C, Dreyer E (2001) Carbon isotope discrimination and wood anatomy variations in mixed stands of Quercus robur and Quercus petraea. Plant Cell Environ 24:861–868
Mannai Y, Ezzine O, Nouira S, Ben Jamâa ML (2015) First report of the winter moth Operophtera brumata on Quercus canariensis and Q. afares in North West of Tunisia. Tunis J Plant Protect 10:69–73
Labuza TP, Kaanane A, Chen JY (1985) Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J Food Sci 50:385–392
Boudhrioua N, Bahloul N, Kouhila M, Kechaou N (2008) Sorptions isotherms and isosteric heats of sorption of olive leaves (Chemlali variety): experimental and mathematical investigations. Food Bioprod Process 86:67–175
Greenspan L (1977) Humidity fixed point of binary saturated aqueous solutions. J Res Nat Bur Stand A Phys Chem 81:89–96
Arabhosseini A, Huisman W, Müller J (2011) Modeling of desorption of Alfalfa (Medicago sativa) stems and leaves. Ind Crops Prod 34:1550–1555
Vishwakarma RK, Shivhare US, Nanda SK (2011) Moisture adsorption isotherm s of guar (Cyamposis tetragonoloba) grain and guar gum splits. LWT Food Sci Technol 44:969–975
Hassini L, Bettaieb E, Desmorieux H, Sandoval Torres S, Touil A (2015) Desorption isotherms and thermodynamic properties of prickly pear seeds. Ind Crops Prod 67:457–465
Ouertani S, Koubaa A, Azzouz S, Hassini L, Ben Dhib K, Belghith A (2015) Vacuum contact drying kinetics of Jack pine wood and its influence on mechanical properties: industrial applications. Heat Mass Transf 51:1029–1039
American Society for Testing and Materials (1984) Standard test methods for moisture content of wood. ASTM D2016. ASTM, Philadelphia
Hadrich B, Boudhrioua N, Kechaou N (2008) Experimental and mathematical study of desorption isotherms of Tunisian sardine (Sardinella aurita). Food Bioprod Process 86:242–247
Iglesias HA, Chirife J, Ferro Fontan C (1989) On the temperature dependence of isosteric heats of water sorption in dehydrated foods. J Food Sci 54:1620–1631
Madamba PS, Driscoll RH, Buckle KA (1996) Enthalpy–entropy compensation models for sorption and browning of garlic. J Food Eng 28:109–119
Polatoglu B, Vildan Bes A, Kaya M, Aktas N (2011) Moisture adsorption isotherms and thermodynamics properties of sucuk (Turkish dry-fermented sausage). Food Bioprod Process 89:449–456
Ha Villa-Velez, Ha Vaquiro, Bon J, Telis-Romero J (2012) Modelling thermodynamic properties of banana waste by analytical derivation of desorption isotherms. Int J Food Eng 8:1–19
McMinn WA, Al-Muhtaseb AH, Magee TRA (2005) Enthalpy–entropy compensation in sorption phenomena of starch materials. Food Res Int 38:505–510
Sharp K (2000) Entropy–enthalpy compensation: fact or artifact. Protein Sci 10:661–667
Wim W (2014) Hydrostatic pressure and temperature dependence of wood moisture sorption isotherms. Wood Sci Technol 48:483–498
Leffer JE, Grunwald E (1936) Rates and equilibria of organic reactions. Wiley, NewYork
Krug RR, Hunter WG, Greiger RA (1976) Enthalpy–entropy compensation. 1. Some fundamental statistical problems associated with the van’t Hoff and Arrhenius data. J Phys Chem 80:2335–2342
McMinn WAM, Magee TRA (2003) Thermodynamic properties of moisture sorption of potato. J Food Eng 60:157–165
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Eligon AM, Achong A, Saunder R (1992) Moisture adsorption and desorption properties of some tropical woods. J Mater Sci 27:3442–3456
Ishimaru Y, Masato KA, Oshima MK, Iida I (2001) Physical and mechanical properties of wood after moisture conditioning. J Wood Sci 47:185–191
Buckton G (1995) Application of isothermal microcalorimetry in the pharmaceutical science. Thermochim Acta 248:117–129
Moreira R, Chenlo F, Torres MD, Prieto DM (2010) Water adsorption and desorption isotherms of chestnut and wheat flours. Ind Crops Prod 32:252–257
Mazza G, Le Maguer M (1978) Water sorption properties of yellow globe onion (Allium cepa L.). Can Inst Food Sci Technol J 11:189–193
Palipane KB, Driscoll RH (1994) The thin layer drying characteristics of macadamia in-shell nuts and kernels. J Food Eng 23:129–144
Bakour R (2003) Influence de l’espèce et de la provenance de deux principaux chênes français (Quercus robur L.; Quercus petraea Liebl.) sur la structure anatomique et les propriétés physiques du bois de merrain. PhD thesis, ENGREF, Paris
Badel E, Bakour R, Perré P (2006) Investigation of the relationships between anatomical pattern, density and local swelling of oak wood. IAWA J 27:55–71
Siau JF (1984) Transport processes in wood. In: Timell TE (ed). Springer, Berlin, Heidelberg, New York
Vavrčík H, Gryc V, Koňas P (2010) Comparison of wood density in relation to growth rings of English oak and Sessile oak. In Levanic T, Gricar J, Hafner P, Krajnc R, Jagodic S, Gärtner H, Heinrich I, Helle G (eds) TRACE-Tree Rings in Archaeology, Climatology and Ecology, vol 8: proceedings of the DENDROSYMPOSIUM 2009, April 16th–19th 2009, Otočec, Slovenia. GFZ Potsdam, Scientific Technical Report STR 10/05, Potsdam, pp 157–163
Simpson LA, Barton AFM (1991) Determination of the fibre saturation point in whole wood using differential scanning calorimetry. Wood Sci Technol 25:301–308
Machhour H, Mahrouz AI, El Hadrami M, Kouhila M (2012) Sorption isotherms and thermodynamic properties of peppermint tea (Mentha piperita) after thermal and biochemical treatment. J Mater Environ Sci 3:232–247
Quirijns E, Van Boxtel A, Van Loon W, Van Straten G (2005) Sorption isotherms, GAB parameters and isosteric heat of sorption. J Sci Food Agric 85:1805–1814
Talla A (2012) Experimental determination and modeling of the sorption isotherms of kilishi. Brit J Appl Sci Technol 2:379–389
Skaar C (1988) Wood-water relations. In: Timell TE (ed). Springer, Berlin, Heidelberg, New York
Eim VS, Rossello C, Femenia A, Simal S (2011) Moisture sorption isotherms and thermodynamic properties of carrot. Int J Food Ing. doi:10.2202/1556-3758.1804
Noshad M, Shahidi F, Mohebbi M, Mortazavi S (2012) Desorption isotherms and thermodynamic properties of fresh and osmotic–ultrasonic dehydrated quince. J Food Process Preserv 37:1–12
Acknowledgments
The authors acknowledge Mr. Abderrazak Zaaraoui, technician at Laboratoire d’Énergétique et des Transferts Thermique et Massique ‘LETTM’, department of Physics of University of Tunis El Manar for his help in carrying out the experiments. The first author acknowledges the researchers of Laboratoire de Gestion et de Valorisation des Ressources Forestières of Institut Nationale de Recherches en Génie Rural and Mr. Tristan Stein technician at Laboratoire d’Étude et de Recherche sur le Matériau Bois (LERMAB) for their support and helpfulness during wood samples proxy.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Bahar, R., Azzouz, S., Remond, R. et al. Moisture sorption isotherms and thermodynamic properties of Oak wood (Quercus robur and Quercus canariensis): optimization of the processing parameters. Heat Mass Transfer 53, 1541–1552 (2017). https://doi.org/10.1007/s00231-016-1916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-016-1916-0