Abstract
The water vapour sorption isotherms and sorption kinetics of birch (Betula pendula L) acetylated to different levels have been determined using a dynamic vapour sorption (DVS) apparatus. A DVS instrument was also used to determine the accessible hydroxyl content in the wood samples using deuterium exchange. The results are reported in terms of the reduced equilibrium moisture content (EMCR), in which the moisture content per unit mass of wood substance is used for the calculation. As the level of acetylation of the wood samples increased there was a corresponding reduction in EMCR of the wood samples, which was accompanied by a decrease in hysteresis in the same order. The sorption kinetics were also determined using the DVS and analysed using the parallel exponential kinetics model, in which the sorption kinetics curve is composed of two processes (labelled fast and slow). Using this analysis, it is possible to calculate two pseudo-isotherms associated with the two processes. The sorption isotherm is a composite of the sorption isotherms associated with the fast process water and the slow process water and there are significant differences in behaviour between the two. It is suggested in this paper that the fast process is related to diffusion limited kinetics, whereas the slow process is a relaxation-limited phenomenon. The reduction in accessible OH content due to acetylation was well correlated with the weight gain due to acetylation, although the relationship did not exactly correspond with that theoretically determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood is an important natural resource, which has many technical advantages, such as: high specific stiffness and strength, low processing energy, high toughness, renewability, aesthetic properties, etc. However, despite these positive benefits, wood exhibits some deleterious properties, such as: dimensional instability in a changing moisture environment and susceptibility to fungal decay. Chemical modification (where chemical moieties are covalently bonded to the wood cell wall polymers) is one method to improve the dimensional stability, decay resistance, and water sorption of wood. Different chemical modifications [1–6] can be applied to change the properties of wood [6]. As a result, the hydroxyl groups in the cell wall are substituted partially and the cell wall of the wood is bulked with the bonded chemicals [7]. The substitution of the hydroxyl groups reduces the number of primary sorption sites (which are generally assumed to be the OH groups); while the bulking reduces the volume in the wood cell wall which is available to water molecules. Of the various potential chemical modifications, acetylation using acetic anhydride is the most studied and is also produced commercially. It is thought that acetylation reduces the equilibrium moisture content (EMC) of the modified wood due to bulking and/or OH blocking, although the role of the OH groups (if any) is not clear. Analysis of the sorption isotherm of acetylated wood requires some caution. Chemical modification leads to an increase in the weight of the wood sample due to the addition of non-polar acetyl groups, which means that for a given mass of water in a given mass of wood substance, a lower EMC is measured due to the extra mass of the bonded acetyl groups. It is a common practice to report the EMC of acetylated wood, using the standard method of calculation (mass of water divided by mass of substrate). However, it is also essential to calculate a ‘reduced’ EMC (labelled EMCR) which takes account of the additional mass of the bonded acetyl and thereby reports the moisture content in terms of wood substance only. This allows for a better understanding of the mechanisms by which acetylation affects the sorption behaviour (bulking of the cell wall by bonded acetyl, or OH blocking, or a combination of the two) [8]. In a study intended to determine the mechanism by which acetylation reduces the moisture sorption of wood, Papadopoulos and Hill [9] modified wood samples with a homologous series of linear chain anhydrides. In this way at comparable WPGs, it was possible to have a range of levels of hydroxyl substitution. Subsequently, Hill re-evaluated this data to remove the effect of the weight of the bonded acyl groups [8]. The conclusion from both these studies was that the reduction in EMC was solely due to bulking of the cell wall by the covalently bonded groups and that degree of hydroxyl substitution was not a factor. Until now, the OH content of acetylated wood has always been calculated and there has been no study where the level of accessible hydroxyl groups has been experimentally determined. The research reported herein was conducted to make such a measurement and furthermore to see if there might be any correlation between hydroxyl accessibility and EMC.
The gravimetric method [9, 10] is a suitable way to determine the EMC by conditioning of wood samples over different saturated salt solutions to reach a certain RH, and continues to be a popular approach, even though in the last few years the dynamic vapour sorption (DVS) technique has been increasingly used [7, 11, 12]. The DVS technique gives highly reproducible data and is able to supply precise sorption isotherms over a wide RH range. Additionally, the DVS technique allows for the determination of sorption kinetics.
The sorption kinetics data of wood are often interpreted in terms of Fickian diffusion [13, 14]. A more detailed description is given by Engelund and co-workers [15] in their review paper. More recently, the parallel exponential kinetic (PEK) model has been introduced to the wood science literature by Hill and co-workers [16–20]. For small wood samples, the PEK model gives exceptionally accurate and reproducible fits of the sorption kinetic data [16–20]. This model has a double exponential form, as described in the equation below:
where MC—is the moisture content at time t of the sample exposed to a constant relative humidity (RH%), MC0—is the moisture content of the sample at the time 0.
The equation contains two exponential terms: \( \left[ {1 - \exp \left( { - \frac{t}{{t_{1} }}} \right)} \right] \)and \( \left[ {1 - \exp \left( { - \frac{t}{{t_{2} }}} \right)} \right] \), representing fast and slow kinetic processes, taking place simultaneously, with the characteristic times t 1 and t 2 respectively. The moisture contents, MC1 and MC2, are defined at infinite time being also associated with the fast and slow processes. A summation of MC0, MC1 and MC2 gives the EMC.
The water sorption/desorption properties of chemically modified wood and the accessibility of the primary sorption sites evaluated by hydrogen–deuterium exchange can be determined by the DVS technique in separate experiments. It is well known that the chemical modification of wood reduces the number of accessible hydroxyl groups in the wood cell wall by substitution, although this has never been measured directly. This study reports the evaluation of the equilibrium moisture content of the unmodified and chemically modified (acetylated) birch wood samples and an investigation of the potential influence of the hydroxyl group accessibility on the sorption properties.
Experimental
Materials
Birch wood (Betula pendula L) samples of dimension 20 × 20 × 5 mm3 (radial × tangential × longitudinal) were cut and the faces lightly sanded to remove loose adhering fibres. The samples were labelled using a pencil, placed in a Soxhlet apparatus and subjected to solvent extraction for 24 h using a mixture of toluene:methanol:acetone (4:1:1, by volume). The samples were air dried in a fume hood for 6 and then placed in an oven set at 105 °C for 24 h. After drying, the samples were removed from the oven in batches of five and transferred to a vacuum desiccator containing silica gel to cool to ambient temperature. The samples were then weighed on a four figure balance. A flanged reaction flask of 5 l capacity with a two neck lid was charged with 2 l of acetic anhydride (Aldrich, 99 %+) to one neck was fitted to a condenser and the other (used for periodically adding wood samples) was stoppered. The flask was suspended in an oil bath set at 110 °C and the system allowed to reach equilibrium. Meanwhile, batches of labelled wood samples were vacuum impregnated at ambient temperature with acetic anhydride in a vacuum desiccator attached to a diaphragm pump. Acetic anhydride impregnated samples were then periodically added to the reaction flask (in batches of five) to allow for a distribution of levels of acetylation. At the end of the reaction period, the still hot acetic anhydride was decanted from the flask, the flask placed in an ice bath and acetone added to quench the reaction. Once the flask and contents had cooled, the top of the flask was removed and the samples were placed in a Soxhlet apparatus. The samples were then extracted with a mixture of toluene:ethanol:acetone (2:1:1, by volume) for 24 h, with one change of solvent. At the end of the extraction period, the samples were removed and allowed to air dry in a fume cupboard for 6 h. Subsequently, the samples were then transferred to an oven set at 105 °C for 24 h and then weighed as before to determine the weight gain due to acetylation.
Weight percent gain (WPG) of the wood samples due to acetylation was determined according to the following equation:
where W 0 is the oven dry weight of the sample before modification and W f is the oven dry weight of the sample after modification.
The different values of the WPG for the acetylated birch wood samples are listed in Table 1.
For all the DVS tests, small microtomed slices (approximately 100 μm thick) of wood samples in the transverse direction relative to the fibres were used.
Method: dynamic water vapour sorption
Analyses of the wood samples were performed using a DVS apparatus (DVS Intrinsic, Surface Measurement Systems Ltd., London, UK). The wood samples were placed on the sample holder which was connected to a microbalance by a hanging wire. This is located in a thermostatically controlled chamber through which there was a constant flow of dry nitrogen gas and into which is precisely mixed another flow of nitrogen containing water vapour. For the deuterium exchange experiment, D2O (Aldrich 99 %+) was used instead of water.
The sorption/desorption RH was changed in steps of 5 % RH from 0 to 95 % RH and then again in reverse to 0 % RH. The temperature was constant (25 °C) during the entire experiment. The instrument maintained the sample at a constant RH until the rate change in mass (dm/dt) was less than 0.002 % per minute over a 10 min period. Data on mass change was acquired every 20 s. The running time, target RH, actual RH and sample weight were recorded throughout the isotherm run.
By plotting percentage mass gain/loss against time, with time zero corresponding to the point, at which a RH step change occurs, and the kinetic curves were obtained. The sorption/desorption curves were fitted with the Exponential Association function in Origin 8.5 software (OriginLab Corporation, Northampton, MA, USA). Because the RH for each step (i.e. from 0 to 5 %) does not take place instantly, there being a finite time during which the RH is moving from one stable value to the next, during this period, the moisture content (MC) of the sample is not initially moving towards a static equilibrium point. Therefore, the first three data points were eliminated from the fit. This work was performed under the same experimental conditions as reported previously [11, 17–20]. The data was recorded as EMC which is calculated on the basis of the sample mass, and also as the reduced equilibrium moisture content (EMCR) which is calculated on the basis of the mass of the wood substance only
For the deuterium exchange experiment, the DVS reservoir was filled with D2O and the instrument programed to conduct six sorption cycles (0–90 % RH), with the same sorption parameters as before. In practice, the actual upper RH achieved by the DVS apparatus was stable at 84 %. The final dry mass of the sample after the sixth sorption cycle was used to calculate the accessible hydroxyl content. The process uses a minimum of six sorption/desorption cycles to ensure that full exchange of the accessible OH groups has occurred, as determined by a stable and positive mass gain due to exchange of the protons by deuterons. In fact, the sample mass became stable after the second cycle, showing that full exchange of the accessible OH content had occurred. In order to calculate the accessible OH content, it is necessary to make a correction for the added mass due to the bonded acetyl groups, since it is necessary to know the change in accessible hydroxyl content on a dry wood mass basis and the same correction factor was therefore applied as for the calculation of EMCR. Accessible OH content in mol/g of dry wood mass is determined directly from the mass increase of the sample.
where M ex is the dry mass of the sample after the deuterium exchange process, M or is the original dry mass of the wood sample prior to the exchange process and M red is the reduced mass of the sample (i.e. the mass of wood material).
The theoretical OH content (mol/g of dry wood mass) was determined by dividing the mass change due to acetylation by 42 (MW of acetyl group—1) and subtracting this value from the average accessible OH content of the unmodified wood sample, as determined from deuterium exchange.
Results and discussion
Dynamic water vapour sorption
Sorption isotherms for the acetylated wood samples reported as EMC and EMCR against RH are shown in Fig. 1. It can be seen that as the level of acetylation increases, there is a commensurate reduction in EMC when calculated based on the weight of the whole sample (wood mass plus weight of bonded acetyl) versus RH. Nonetheless, when the effect of the mass of bonded acetyl is removed, there is still a real reduction in the EMCR, which has to be due to other physical effects.
The sorption isotherms of the reference (R) compared with the acetylated wood samples and the hysteresis (obtained by subtracting the adsorption from desorption isotherm loop for EMC) are presented in Figs. 2 and 3, respectively (black square symbols—R). The isotherm curves for adsorption and desorption show the typical sigmoidal shape commonly observed for wood.
When compared to the reference birch wood sorption curves, the increase of the WPG reduces the EMCR of the samples from 23.2 % for R to 20.5 % for Ac1, 18.8 % for Ac2, 15.7 % for Ac3 and 12.4 % for Ac4 at a RH of 95 % (see Fig. 1). Since, it is EMCR that is being plotted, this represents a real reduction in moisture content and not an artefact due to increased sample mass.
This large decrease of the reduced EMC has been attributed to the presence of the covalently bonded acetyl groups in the nanopores in the cell wall matrix [6, 9]. Since the acetyl groups occupy space within the cell wall, this is consequently not available to the sorbed water molecules and hence a reduction in moisture content is observed (i.e. a bulking mechanism). This has been shown in the previous publications, where it was found that the reduction in EMCR was solely a function of WPG, when the wood was modified with a range of different chain length anhydrides [6, 9].
Sorption hysteresis of acetylated wood
All reference and acetylated birch wood samples exhibit the hysteresis phenomenon common for wood (Fig. 3), but with a reduction in hysteresis as the level of acetylation is increased. The change in hysteresis is quite regular, although the sample Ac2 is somewhat anomalous. However, given the relatively small differences between the Ac2 and the other samples, no significance is attributed to this.
Sorption kinetic analysis using the parallel exponential (PEK) model
The difference between sorption and desorption cycles may be reflected in the kinetic processes taking place as the sample attains equilibrium. The PEK model has been used extensively in studies of the sorption kinetics behaviour with different wood samples [12, 16, 17]. As seen before, the PEK model gave an excellent fit to the experimental values, independent of the relative humidity range.
An example of sorption kinetics curve with the PEK fit to the experimental data measured at the 10–15 % RH step for reference birch wood sample is given in Fig. 4. The open symbols represent the experimental data, while the line represents the PEK calculated values. The fitted curve is composed from the fast and slow processes associated with the moisture contents, MC1 and MC2 according to Eq. 1.
Two types of assignments have previously been proposed in the literature for these two processes. The fast kinetic process was associated to the moisture sorption at the sites of most accessible internal surfaces and amorphous regions [21] or to the monolayer formation or strongly bounded water to the polar groups within the cell wall (so called non-freezing water) [22]. At the same time, the slow kinetic process was associated with the water sorption into the inner surfaces and crystalline regions [21] or to the multilayer or weakly bound water in the substrate or in the larger water clusters (so called freezing water) [22]. However, there is no evidence to show that sorbed water can be assigned to these two states in wood [15].
It was recently demonstrated that there is no evidence supporting the idea of assigning the two processes to specific sites or water types [23, 24], although it is not yet conclusively known what these two processes represent. The most recent work in this area has suggested that the fast one can be attributed to a diffusion process according to the linear driving force mass transfer model, whereas the slow process is linked to relaxation-limited kinetics [20]. It has also been recently reported that the contribution from the slow process disappears in samples when the isotherm is determined above the glass transition temperature of the material [25]. Hence, the most likely interpretation is one where the slow process is attributed to relaxation limited diffusion and the fast process to the linear driving force mass transfer model. The response times for the fast and slow process for sorption onto and desorption from unmodified and acetylated (WPG = 16 %) woods are shown in Fig. 5.
In most cases, the response times for the acetylated wood sample are faster, except for the upper and lower regions of the hygroscopic range under adsorption conditions. Interpretation of this data requires caution. A number of variables affect the response times (mass of sample, RH step, MC step, temperature) apart from physical or chemical composition of the sample, and it is essential that comparisons of the characteristic times are not influenced by these variables. In the case of the acetylated wood samples, the MC step change is not the same between the unmodified and acetylated wood samples, when identical RH steps are used. By visual inspection of the kinetic curves, it is observed that the acetylated wood sample does respond faster than the unmodified wood, but this is simply due to the lower MC change.
By cumulatively adding the MC1 or MC2 values for adsorption and desorption cycles from the PEK fitting, it is possible to create pseudo-isotherms associated with the fast and slow processes and consequently to determine what is the contribution of these two processes to the hysteresis effect. The pseudo-isotherm curves for the reference and acetylated birch wood samples are presented in Fig. 6. The sorption isotherm is a classic IUPAC type II isotherm, whilst the sorption pseudo-isotherms for the fast process of IUPAC type I and for the slow process are of type III [22, 26]. This may be supporting evidence that the fast process can be associated with the diffusion of water molecules in the cell wall, whereas the slow process is linked to relaxation processes associated with cell wall expansion or contraction. As the WPG increases, both the adsorption and desorption curves for the fast process change in shape, the ones of the Ac4 sample being almost linear, whereas the other samples exhibit an upturn in the curve at the highest part of the RH range. According to van der Wel and Adan [26], a linear isotherm implies that the interactions between sorbate molecules and the polymer molecules of the substrate are insignificant, whereas deviations from linearity indicate interactions between sorbate and sorbent, such as dissolution, plasticisation, etc.
The non-acetylated wood sample shows a much greater allocation of water associated with the fast process in the adsorption region below 50 % RH, after which the slow adsorption process begins to dominate (Fig. 6—R). At 95 % RH, this sample displays a moisture content associated with the fast process of 10.5 and 13.8 % with the slow process.
Modification of wood by acetylation reduced the values of the cumulative MC with the increase of the WPG (Fig. 6—Ac1–Ac4), but with a much larger influence on the moisture content associated with the slow process. The mass gain associated with the fast adsorption process is higher than the one for slow adsorption process in the region below 70–75 % RH for the samples Ac1–Ac3, while for the Ac4 sample the fast process dominates up to the 90 % RH region.
There is a minor difference between fast process for the R and treated sample, with a low WPG (Ac1), while the slow process is more affected. With a further increase of the WPG, the fast process starts to be affected, too; the values being lower than those of the reference (non-acetylated) sample. The slow processes for Ac2–Ac4 samples are strongly affected by the acetylation process.
The fast and slow sorption curves for the untreated (R) and low WPG acetylated (Ac1) birch wood samples exhibit closed pseudo-isotherm loops; whereas, the Ac2–Ac4 acetylated birch wood samples show open fast pseudo-isotherm loops. The open pseudo-isotherm loop of the fast process is accompanied by a crossover in the slow process pseudo-isotherm, where the desorption EMCR value is higher than the adsorption at the upper end of the hygroscopic range. This crossover was referred to as ‘extra water’ by Kohler et al. [21] who assigned the water to different sites leading to the conclusion that water was transferred from ‘fast sites’ to ‘slow sites’.
According to a recent study [20], the fast sorption process is associated with the physical diffusion of water molecules in the cell wall, and the slow process is linked to relaxation processes associated with the cell wall matrix. Therefore, it can be considered that after acetylation, the matrix becomes stiffer and the relaxation process is less significant with an increasing influence of the diffusion process, with more limited penetrant–polymer interaction.
The hysteresis for both fast and slow sorption isotherms were calculated and are presented in Fig. 7.
All samples exhibited different behaviour for the hysteresis associated with the fast and slow kinetic processes. As can be seen, the hysteresis obtained from the experimental data is mostly influenced by the slow process; the curve associated with the fast process did not affect the hysteresis of the sorption curves at low values of the RH, this starting to contribute over 30 % RH. At the same time, a decrease of the hysteresis for both fast and slow processes can be observed as the WPG increases. An interesting observation is that at low RH (up to about 15 %) the hysteresis associated with the fast process has negative values for three of the samples.
Accessibility of hydroxyl groups
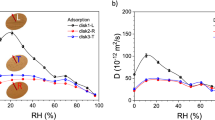
The accessibility of the hydroxyl groups of untreated and acetylated birch wood samples is shown in Fig. 8. For the reference (non-acetylated) birch wood sample, the value for the accessible OH groups is about 8.1 mmol/g. The chemical modification, as was expected, decreased the number of accessible OH groups with increasing values of WPG. However, the relationship between the OH accessibility does not follow that predicted theoretically (shown by the upper line). The reason for this is not known, but may point to a loss of thermally labile cell wall substances as the reaction proceeds. Work is underway to understand this relationship further. This will involve the saponification of bonded ester in the wood and measurement of the released acetyl using HPLC.
When plotting the EMCR values at 95 % RH for the reference and acetylated birch wood samples as a function of the accessible hydroxyl groups an approximately linear correlation is obtained (Fig. 9a). A similar relationship is found if WPG is plotted against EMCR (Fig. 9b). Based on this evidence alone, it is not possible to determine if OH substitution or bulking, or a combination thereof is responsible for the reduction in the EMCR.
Conclusions
Acetylation results in a reduction in both the equilibrium moisture content and the reduced equilibrium moisture content of the birch wood samples. Using a PEK analysis of the sorption kinetics, it is possible to produce two pseudo-isotherms associated with the fast and slow kinetic processes. The pseudo-isotherm of the fast process has a shape which can be attributed to an IUPAC Type I isotherm, whereas that associated with the slow process is more akin to a Type III. It is suggested that the fast process is linked to a diffusion process in which there is no or limited sorbate–polymer interaction, whereas the slow process is linked to relaxation-limited kinetics. Evaluation of the accessible hydroxyl content using deuterium exchange has shown that there is a reduction in this property as the level of acetylation is increased, although the relationship does not follow that theoretically predicted. Based upon this study it is not possible to determine the mechanism responsible for the reduction in the equilibrium moisture content of acetylated wood. This requires modification with two different anhydrides, whereby different levels of OH substitution can be achieved at comparable levels of bulking.
References
Li JZ, Furuno T, Katoh S, Uehara T (2000) Chemical modification of wood without solvents or catalysts. J Wood Sci 46(3):215–221. doi:10.1007/BF00776452
Larsson Brelid P, Simonson R, Bergman O, Nilsson T (2000) Resistance of acetylated wood to biological degradation. Holz Roh Werkst 58:331–337. doi:10.1007/s001070050439
Papadopoulos AN, Hill CAS (2002) The biological effectiveness of wood modified with linear chain carboxylic acid anhydrides against Coniophora puteana. Holz als Roh- und Werkstoff 60:329–332. doi:10.1007/s00107-002-0327-8
Hill CAS, Papadopoulos AN, Payne D (2004) Chemical modification employed as a means of probing the cell-wall micropore of pine sapwood. Wood Sci Technol 37(6):475–488. doi:10.1007/s00226-003-0193-5
Ozmen N, Çetin NS, Tingaut P, Sèbe G (2006) A new route for the functionalisation of wood through transesterification reactions. Eur Polym J 42:1617–1624. doi:10.1016/j.eurpolymj.2006.01.020
Hill CAS (2006) Wood modification: chemical, thermal and other processes. Wiley, Chichester
Xie Y, Hill CAS, Xiao Z, Mai C, Militz H (2011) Dynamic water vapour sorption properties of wood treated with glutaraldehyde. Wood Sci Technol 45:49–61. doi:10.1007/s00226-010-0311-0
Hill CAS (2008) The reduction in the fibre saturation point of wood due to chemical modification using anhydride reagents: a reappraisal. Holzforschung 62:423–428. doi:10.1515/HF.2008.078
Papadopoulos AN, Hill CAS (2003) The sorption of water vapour by anhydride modified softwood. Wood Sci Technol 37:221–231. doi:10.1007/s00226-003-0192-6
Mantanis GI, Papadopoulos AN (2010) The sorption of water vapour of wood treated with a nanotechnology compound. Wood Sci Technol 44:515–522. doi:10.1007/s00226-010-0326-6
Jalaludin Z, Hill CAS, Samsi HW, Husain H, Xie Y (2010) Analysis of water vapour sorption of oleo-thermal modified wood of Acacia mangium and Endospermum malaccense by a parallel exponential kinetics model and according to the Hailwood–Horrobin model. Holzforschung 64:763–770. doi:10.1515/HF.2010.100
Xie Y, Hill CAS, Xiao Z, Jalaludin Z, Militz H, Mai C (2010) Water vapor sorption kinetics of wood modified with glutaraldehyde. J Appl Polym Sci 117:1674–1682. doi:10.1002/app.32054
Salin JG (2010) Problems and solutions in wood drying modelling: history and future. Wood Mat Sci Eng 5:123–134. doi:10.1080/17480272.2010.498056
Pfriem A, Zauer M, Wagenfuhr A (2010) Alteration of the unsteady sorption behaviour of maple (Acer pseudoplatanus L.) and spruce (Picea abies (L.) Karst.) due to thermal modification. Holzforschung 64:235–241. doi:10.1515/HF.2010.029
Engelund ET, Thygesen LG, Svensson S, Hill CAS (2013) A critical discussion of the physics of wood-water interactions. Wood Sci Technol 47:141–161. doi:10.1007/s00226-012-0514-7
Hill CAS, Norton AJ, Newman G (2010) The water vapour sorption properties of Sitka spruce determined using a dynamic vapour sorption apparatus. Wood Sci Technol 44:497–514. doi:10.1007/s00226-010-0305-y
Hill CAS, Ramsay J, Keating BK, Laine K, Rautkari L, Hughes M, Constant B (2012) The water vapour sorption properties of thermally modified and densified wood. J Mater Sci 47(7):3191–3197. doi:10.1007/s10853-011-6154-8
Hill CAS, Keating BA, Jalaludin Z, Mahrdt E (2012) A rheological description of the water vapour sorption kinetics behaviour of wood invoking a model using a canonical assembly of Kelvin–Voigt elements and a possible link with sorption hysteresis. Holzforschung 66(1):35–47. doi:10.1515/HF.2011.115
Sharratt V, Hill CAS, Zaihan J, Kint DPR (2010) Photodegradation and weathering effects on timber surface moisture profiles as studied using dynamic vapour sorption. Polym Degrad Stab 95:2659–2662. doi:10.1016/j.polymdegradstab.2010.07.011
Popescu C-M, Hill CAS (2013) The water vapour adsorption–desorption behaviour of naturally aged Tilia cordata Mill. wood. Polym Degrad Stab 98(9):1804–1813. doi:10.1016/j.polymdegradstab.2013.05.021
Okubayashi S, Griesser UJ, Bechtold T (2004) A kinetic study of moisture sorption and desorption of lyocellfibers. Carbohyd Polym 58:293–299. doi:10.1016/j.carbpol.2004.07.004
Kohler R, Renate D, Bernhard A, Rainer A (2003) A numeric model for the kinetics of water vapour sorption on cellulosic reinforcement fibers. Compos Interfaces 10:255–257. doi:10.1163/156855403765826900
Hill CAS, Norton A, Newman G (2010) Analysis of the water vapour sorption behaviour of Sitka spruce [Picea sitchensis (Bongard) Carr.] based on the parallel exponential kinetics model. Holzforschung 64:469–473. doi:10.1515/HF.2010.059
Xie Y, Hill CAS, Jalaludin Z, Sun D (2011) The water vapour sorption behaviour of three celluloses: analysis using parallel exponential kinetics and interpretation using the Kelvin–Voigt viscoelastic model. Cellulose 18:517–530. doi:10.1007/s10570-011-9512-4
Keating B, Hill C, Sun D, English R, Davies P, McCue C (2013) The water vapor sorption behavior of a galactomannan cellulose nanocomposite film analyzed using parallel exponential kinetics and the Kelvin–Voigt viscoelastic model. J Appl Polym Sci 129(4):2352–2359. doi:10.1002/app.39132
van der Wel GK, Adan OCG (1999) Moisture in organic coatings: a review. Prog Org Coat 37:1–14. doi:10.1016/S0300-9440(99)00058-2
Acknowledgements
This study was performed in the framework of the COST Action FP1006. Carmen-Mihaela Popescu wishes to thank the Royal Society of Edinburgh for funding the visiting fellowship to FPRI, Edinburgh Napier University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popescu, CM., Hill, C.A.S., Curling, S. et al. The water vapour sorption behaviour of acetylated birch wood: how acetylation affects the sorption isotherm and accessible hydroxyl content. J Mater Sci 49, 2362–2371 (2014). https://doi.org/10.1007/s10853-013-7937-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-013-7937-x