Abstract
This work aims to use experimental data from thermal characterization and adsorption/desorption isotherms of two tropicals woods species (Ayous and Tali) to propose an empirical model of thermal conductivity as a function of air relative humidity. A static gravimetric method was used to determine the adsorption isotherms of Tali and Ayous at 30 °C, and 40 °C. The GAB, Henderson and Nelson models were used to predict the isotherms. Exponential models of thermal conductivity and volumetric heat capacity with air relative humidity were proposed. The influence of hysteresis phenomenum was studied on these properties. The reliability of the developed empirical correlation between thermal properties and air relative humidity was evaluated by comparing the experimental and predicted curves. The relative errors were less than 8% for both Ayous and Tali. The correlation coefficients obtained were greater than 99% for both species in adsorption and desorption. There was also an increase in the equilibrium water content of both species with the increase in water activity at constant temperature. The correlation coefficients between GAB model and sorption experimental data were lower than 99% when Ayous was subjected to a temperature of 40 °C in adsorption and Tali to a temperature of 40 °C in desorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of wood construction is a significant challenge and an effective response to reduce the environmental impacts of the building sector. The wood construction sector represents strong potential for annual variation of stocks with its 195 million tons of CO2 sequestered per year [1]. In this context, the development of wood construction constitutes a fundamental stake and an effective answer for reducing environmental impacts related to the building sector.
In central Africa, wood construction has been growing steadily for many years. The size of the market is huge with potential consumers. The demand for housing, which is far above the current offer, is an incentive for developers and entrepreneurs to start building wooden houses. Even if the structures remain simple, the main difficulty encountered is the lack of data to optimize the sizing of the structures [2]. This observation is made both on the hygroscopic and thermal levels.
Wood is a hygroscopic material. The amount of water it contains can have a great influence on its thermophysical properties. Many research topics have been carried out on the influence of the water content on thermal properties of wood. The thermal behavior of five tropical wood species with basal density and water content have been studied. The results showed that the thermal conductivity and effusivity increase when the diffusivity decreases with water content [3]. The thermal conductivity of wood in the longitudinal, tangential and radial directions increase with the water content between 0 and 22% [4]. A linear relationship can be fund between thermal conductivity and moisture content [5]. Some tropical wood species' volumetric heat capacity and thermal conductivity increase linearly with their water content below the fiber saturation point (FSP) [6, 7]. The authors also found that the thermal conductivity of tropical woods is lower than conventional building materials. However, no model that makes it possible to determine the thermal properties of hygroscopic material as wood without going through experimental measurements is presented.
The linear variation of thermal conductivity or volumetric heat capacity with water content encounters some practical difficulties, especially when the material is used in an environment with variable temperature and relative humidity (RH). The relationship between RH of the air and the water content of a material for a given temperature is called the sorption isotherm. It is important to evaluate the sorption behavior of wood for each locality when it is known that the water properties of this material can change significantly with the environment. The hysteresis phenomenon is observed when the amount of adsorbed water is larger when the humidity is decreased (desorption branch) than in the opposite case, if the humidity is increased (adsorption branch) [8]. Therefore, it is important to evaluate the effect of hysteresis on the thermal behavior of wood material.
Several empirical and semi-empirical models are proposed in the literature to describe the sorption isotherms of biological products. Among these, we can mention the GAB model, which is an improvement of the BET model [9,10,11]. Henderson's model is one of the few that includes temperature as a variable. It is widely used because linearization is easy, unlike GAB model. Nelson proposed the Nelson model in 1983, and it is based on the Gibbs free energy to describe the sorption isotherm of cellulosic materials. This model is less used in the literature to estimate the wood isotherms [12,13,14]. No single model can describe the sorption isotherms of all biological products in all RH ranges. Thus, the GAB model is very often recommended for wood sorption isotherms [15,16,17].
The use of the parallel hot wire (PHW) method to estimate simultaneously the water content and thermal properties of Ayous (Triplochiton scleroxylon) and Tali (Erythrophleum ivorense) was developed in a previous work [7]. The water content of these tropical wood species can be modified with relative air humidity. It would be interesting to know the conductivity of these materials for a given air relative humidity. We would then dispense with experimental measurements. The aim of this paper was to complete that study by finding a correlation between wood’s thermal properties and air relative humidity. This work proposes an empirical model of thermal conductivity as a function of air humidity. To reach this goal, the thermal conductivity and volumetric heat capacity were firstly estimated using the PHW method. Secondly, the adsorption and desorption isotherms of the wood studied were determined at two temperature levels using the static gravimetric method. The GAB model, the Henderson model and the Nelson model were used to smooth sorption isotherms and a comparative study was done to determine the best model fitting experimental data points.
2 Presentation of models
2.1 Sorption isotherms models
In the literature, three types of models are used to explain the sorption behavior of a product : the theoretical model, the empirical method and the semi-empirical model. These models establish sorption isotherms for temperatures other than those studied experimentally and avoid time-consuming experiments. The choice of a model depends on the assumptions adopted and on its capacity to describe the experimental points as well as possible. According to Van Der Berg, about 77 sorption isotherm models have been constructed to explain the behavior of food products [18]. Three models were chosen in this study: the GAB model, the Henderson model and the Nelson model. In fact, the GAB model is often recommended for wood sorption isotherms [15,16,17, 19]. It is the model wich better fit tropical wood isotherms [20]. The Henderson model is considered as the one which best fit sorption isotherm of tropical wood for humidities inferior to 50 percent [21]. It is less used in the literature when it comes to estimating wood sorption isotherms. However, it gives better results when the FSP and thermodynamic properties of biological species are studied [12,13,14].
2.1.1 GAB’s model
The GAB model is an improvement of the BET model [9,10,11]. It suppose that the thermodynamic properties of water molecules on primary and secondary layers are identical to those of free water. The following equation gives the GAB model:
The GAB model can be rewritten as a polynomial.
with:
A linear least squares regression method of \(\frac{\mathrm{RH}}{{\mathrm{X}}_{\mathrm{e}}}\) versus \(\mathrm{RH}\) was carried out using matlab code in order to determine the values of coefficient of the quadratic term \(\upgamma\), the linear term coefficient \(\upbeta\) and the constant \(\mathrm{\alpha }.\) Then the GAB parameters were calculated as follows.
where: f = β2 - 4 α γ
2.1.2 Nelson's model
In this work, another model chosen to smooth the sorption isotherms of the studied products is the Nelson model. This model allows easy estimation of FSP and the Gibbs free energy of the studied material. The mathematical model to describe this model is:
\({\mathrm{X}}_{\mathrm{FSP}}\) represents the fiber saturation point for desorption,
\({\Delta \mathrm{G}}_{0}(\mathrm{cal}/\mathrm{g})\) Gibbs's free energy variation per gram of water absorbed at a relative humidity of 0%.
\({\mathrm{W}}_{\mathrm{w}}\) molecular weight of water (18 g.mol-1)
Nelson's equation can be rewritten:
\({\mathrm{A}}_{\mathrm{n}}\) and \({\mathrm{B}}_{\mathrm{n}}\) are the parameters of Nelson’s sorption isotherm model. They are given by:
For a hygroscopic specie and at the same temperature, \({\mathrm{A}}_{\mathrm{n}}\) and \({\mathrm{B}}_{\mathrm{n}}\) are constants. Linear regression allows to deduce the values of \({\mathrm{A}}_{\mathrm{n}}\) and \({\mathrm{B}}_{\mathrm{n}}\). \({\mathrm{X}}_{\mathrm{FSP}}\) and \({\Delta \mathrm{G}}_{0}\) are deduced from the equations.
2.1.3 Henderson's model
The Henderson model is one of few which add temperature as a variable. And it is widely use, because the linearization is easy.
The parameters \({\mathrm{A}}_{\mathrm{h}}\), \({\mathrm{B}}_{\mathrm{h}}\) are constants related to the temperature. The linearization of the Henderson equation leads to:
with: \({\mathrm{a}}_{\mathrm{h}}=\frac{1}{{\mathrm{B}}_{\mathrm{h}}}\);\({\mathrm{b}}_{\mathrm{h}}=-\frac{1}{{\mathrm{B}}_{\mathrm{h}}}{\mathrm{lnA}}_{\mathrm{h}}\)
The coefficients \({\mathrm{a}}_{\mathrm{h}}\) et \({\mathrm{b}}_{\mathrm{h}}\) will be estimated by least squares linear regression.
The comparison of the isotherms obtained by the three models at different temperatures will determine which model is the most suitable for wood species.
The calculation of the average relative error, the correlation coefficient and the standard deviation makes it possible to observe the accuracy with which the different models reproduce the experimental values.
The average relative error between the experimental and modeled curves is calculated with the relation 17.
The correlation coefficient between the experimental and theoretical values is given by the expression.
The simulation of isotherms is acceptable when the correlation coefficient is greater than 0.990 and for P values less than or close to 4% [15].
2.2 Thermal properties models
2.2.1 Thermal conductivity
A theoretical model is proposed to describe the evolution of the conductivity of wood as a function of the air's RH.
Many studies have shown that the thermal conductivity of wood increases linearly with the water content of the material. The water content of two tropical wood species using the parallel hot wire method have been previously studied [7]. Furthermore, the equilibrium water content of wood is a function of the relative humidity of the environment for a fixed temperature. Thus, the experimental results of the adsorption isotherms integrated with the linear model of thermal conductivity enable the analysis of its variation with the RH of the air. The statisical cloud of points obtained permitted to determine an empirical relation between \({\lambda }_{th}\) and \(RH\). The exponential model of the thermal conductivity given by Eq. 20 is the one that best fits the experimental curve.
In order to determine the parameters \({\mathrm{A}}_{\mathrm{th}}\) and \({\mathrm{B}}_{\mathrm{th}}\) and \({\mathrm{C}}_{\mathrm{th}}\) a linear least squares regression code of \({\uplambda }_{\mathrm{th}}\) versus \(\mathrm{RH}\) written in Matlab was used. The values of the coefficients \({\mathrm{A}}_{\mathrm{th}}\) and \({\mathrm{B}}_{\mathrm{th}}\) and \({\mathrm{C}}_{\mathrm{th}}\) that minimise the difference between the theoretical values of thermal conductivity and the experimental results were estimated. The Table 5 present the values of coefficients estimated at 30 °C.
2.2.2 Volumetric heat capacity
The heat capacity reflects the ability of a material to absorb heat and heat up. It is the energy required to raise the temperature of the considered material by one Kelvin. The analysis of the results obtained by Bobda et al. [7] showed that, like the thermal conductivity of the products studied, the estimated volumetric heat capacity is sensitive to moisture. The higher the water content in the material, the more the value of the parameter increases, which weakens its insulating power. To take account of this non-negligible phenomenon, an exponential model is proposed by equation 21. In fact, as for the thermal conductivity, experimental results of the adsorption isotherms integrated with the linear model of volumetric heat capacity enable the analysis of its variation with the RH of the air. Then, it is obtain an experimental points of \({{\rho C}_{p}}_{th}\) versus \(RH\) forming a statistical cloud of points. To determine an empirical relation betwenn \({{\rho C}_{p}}_{th}\) and \(RH,\) the form of statistical cloud of point have guide a choice to an exponential model.
In order to determine the parameters \({\mathrm{A}}_{\mathrm{th}}^{\mathrm{^{\prime}}}\) and \({\mathrm{B}}_{\mathrm{th}}^{\mathrm{^{\prime}}}\) and \({\mathrm{C}}_{\mathrm{th}}^{\mathrm{^{\prime}}}\) a linear least squares regression code of \({{\mathrm{\rho C}}_{\mathrm{p}}}_{\mathrm{th}}\) versus \(\mathrm{RH}\) written in Matlab was used. The values of the coefficients \({\mathrm{A}}_{\mathrm{th}}^{\mathrm{^{\prime}}}\) and \({\mathrm{B}}_{\mathrm{th}}^{\mathrm{^{\prime}}}\) and \({\mathrm{C}}_{\mathrm{th}}^{\mathrm{^{\prime}}}\) that minimise the difference between the theoretical values of volumetric heat capacity and the experimental results were estimated. The Table 5 present the values of coefficients estimated at 30 °C.
3 Materials and methods
3.1 Materials
Two tropical wood species were studied. Ayous (Triplochiton scleroxylon) and Tali (Erythrophleum ivorense). They represent respectively 7% and 31% of the wood production in Cameroon in 2015 [21]. Ayous and Tali belong to the Malvaceae family and the Erythroxylaceae family respectively.
The specimens used for the experiments were obtained in a sawmill in the city of Yaoundé. They were taken from greenwood matrices already sawn in the longitudinal direction. After machining, the wood samples were chosen to have no structural defects (pits, knots, etc.). The size and geometry of the samples were determined according to the equipment available in the laboratory. For the measurement of sorption isotherms, the specimens used were saw into parallelepiped with approximately nominal dimension 20 x 10 x 5 mm3 [22]. About 27 samples per specie were retained for the test. For the measurement of thermal properties, the dimensions of the specimens were 10 x 10 x 2 cm3.
3.2 Equipment and methods
3.2.1 The parallel hot wire (PHW)
As stated earlier in this work, thermal conductivity and volumetric heat capacity are estimated in the transient regime by applying the PHW method [7, 23]. The heating wire placed in the center of the wood sample is made of Nickel-Chromium 80/20 with a diameter of 0.5 mm. The temperature \(\mathrm{T}\left(\mathrm{t}\right)\) at the distance \(\mathrm{d}=5\mathrm{ mm}\) is measured by a sheathed K-type thermocouple with a diameter of 0.5 mm. It is recorded with a time step of 0.1s by a Picolog TC-08. The heating wire was arranged in a parallel direction to the wood fibers to measure the thermal conductivity in the perpendicular direction to the fibers. The heat flow in the wire is imposed by the circulation of a constant electric current \(\mathrm{I}\), produced by a stabilized power supply (Thandar TS3021S). The voltage \(\mathrm{U}\) at the terminals of the wire of length \(\mathrm{L}\) is measured by a voltmeter (Velleman DVM1100). A clamping device is used to hold everything in place while reducing contact resistances.
3.2.2 The static gravimetric method
Although the use of dynamic methods is becoming more widespread, the static gravimetric method based on saturated salt solutions is widely use to obtain sorption isotherm data [19, 22, 24, 25].
In the gravimetric method, the samples are weighed on a scale with a precision of 0.001 g, brand JA 2003N. They are placed in an environment whose RH is controlled by saturated saline solutions and whose temperature is controlled by an oven. The temperature inside the oven is regulated by a thermocouple connected to a thermostat. A thermometer placed inside the chamber confirms the temperature. The containers containing the saline solutions are plastic and non-hygroscopic.
Each RH point is determined by a saline solution, and each weight measurement is made when an equilibrium point is reached. It was assumed that the equilibrium conditions had been attained when three subsequent measurements of the sample mass at intervals of 24 h gave identical results [20, 22]. This last recorded mass is used to calculate the water content of the sample at temperature T and RH. The experiment is repeated for different saline solutions (different humidities) at the same temperature for different wood samples. The different values of water content and RH are used to plot the sorption curve at the temperature T of the experiment. Table 1 shows the RHs at which the measurements were made.
The preparation of the saline solutions is done as follows:
-
Take out the containers, clean them, rinse with distilled water, and dry.
-
Prepare the necessary volume of distilled water.
-
Add the salt in small quantities to the prepared volume of water.
-
Shake the mixture.
-
Repeat the process until the solution is saturated.
The dissolution of some salts may also vary with temperature. It is therefore recommended to dissolve the salts at the maximum temperature for which the manipulation is to be performed so that the solution remains saturated at all different temperatures. It is recommended to wait at least 72 hours after the preparation of the solutions before using them. Indeed, the speed of dissolution varies with the quantity of solute in the solution. Thus, 72 hours is the time necessary to be sure that the solution is really saturated.
In order to obtain the equilibrium of the water content, the following protocol is followed:
-
Place the water-saturated wood samples in the containers containing the saline solutions. Indeed, three samples of each species were made for each environment. This in order to have an average of three contents in equilibrium for each species.
-
Perform successive mass measurements of the samples in a regular time interval.
-
Carry out the operation quickly in order to avoid as much as possible the loss or gain of humidity due to the contact with the environment.
-
When the mass no longer varies, place the wood samples in an oven at a temperature of about 105 °C for at least 48 hours and carry out successive weighing until the variation in mass is no longer significant. This gives the anhydrous mass of the samples.
-
The anhydrous samples are put back in the containers, and the reverse process is carried out in order to obtain the adsorption isotherms.
4 Results and discussion
4.1 Evolution of water content as a function of the air relative humidity
Figure 1 illustrates the theoretical models and the experimental adsorption points for Ayous and Tali samples at 30 and 40 °C, over the considered RH range. A preliminary discussion around the choice of the most efficient model is proposed.
Figure 1a shows the GAB model. The isotherms show a sigmoidal shape. A concave shape of the isotherms that is more pronounced for Ayous than for Tali is observed.
The curves in Fig. 1b show the adsorption isotherms smoothed by the Henderson model. As with the GAB model, the Henderson model predicts the experimental points quite well over the entire humidity range considered.
The curves in Fig. 1c show the smoothing of the experimental results by the Nelson model. It can be seen very clearly that this model is limited when it comes to describing the isotherms of the two studied species at low relative humidity (<12%). Thus, the failure of this model to smooth the experimental points at low relative humidity does not depend on the density of the product.
The experimental results of the adsorption isotherms obtained are typical of biological products. An increase in the water content of the product with the increase of the relative humidity for a given temperature is observed. It can be noted a classical shift of the isotherms towards the right with the increase of the temperature. This shift is due to the differences in partial pressure of water vapor between the wood and the environment, higher before equilibrium and a decrease of the water-wood binding forces. For very high relative humidity, close to 100%, the models have convex shapes. Here, the last water molecules attach themselves to the irregularities of the surface, where capillary condensation and binding energy are too low. The upward bend of sorption isotherm at high RH is related to the softening of hemicellulose and capillary water is insignificant below 99.5 % RH [8]. For intermediate RH (between 25% and 75%), the shape of the isotherms is linear. This shape is explained by the behavior of water molecules that joins to those already fixed in the internal layers. In this case, the binding energy is lower. A concave shape of the isotherms is observed for relative humidity lower than 20%. This can be explained by the fixation of water on the first layer of the surface of the solid matrix. The binding energy is very high, and the water molecules are mobile. These results are in agreement with the general characteristics of isotherms and physical adsorption of wood, which predict that the obtained isotherms are, type II according to the BET classification [15, 20, 22, 26, 27].
Moreover, for the same temperature differences, the adsorption isotherms of Tali are more tightened than those of Ayous. It means Ayous is more sensitive to temperature than Tali. In addition, a slight decrease in the variation of the equilibrium water content is observed for different RH at the same temperature. For example, at 30 °C, the experimental points of Ayous vary between 2.56% and 26.21% and those of Tali between 2.37% and 22.14%. This confirms that Tali is more stable than Ayous. The higher density of Tali may explain these results. This is in agreement with the work of some authors [12, 21].
Tables 2, 3, and 4 illustrate the values of the estimated parameters for each of the models used on the Ayous and Tali samples at 30 °C and 40 °C. The relative errors and correlation coefficients for each model are calculated. The comparison between the correlation coefficients and the relative errors of the GAB, Nelson and Henderson models allows deducing the one that smoothest the experimental points of the obtained isotherms. The simulation of the isotherms is acceptable when the correlation coefficient r is greater than 0.990 and for values of the relative error P less than or close to 4% [15, 28, 29].
The water content of the monolayer for tropical woods should be between 0.02 kg/kg and 0.09 kg/kg [29]. The values of \({\mathrm{X}}_{\mathrm{m}}\) presented in Table 2 are in this interval of values.
Table 2 shows the monolayer water content at saturation (\({\mathrm{X}}_{\mathrm{m}}\)) of GAB in adsorption. These values correspond to the points where the sorption of the multilayer begins to predominate over the sorption of the monolayer. They are located at the minimum of the differential of water content versus relative humidity [30]. For all temperatures, Ayous wood has lower \({\mathrm{X}}_{\mathrm{m}}\) than Tali wood. These results are in agreement with those obtained in other wood studies [15, 19, 31]. This parameter is important for the control of product stability during storage. These values of the water content of the monolayer of the products indicate that the chemical weathering reactions are low, and the stability of both products is satisfactory during storage.
The values of the variation of Gibbs free energy \(\Delta {\mathrm{G}}_{0}\) are higher for Tali than for Ayous. This difference is explained by the fact that the values of fiber saturation point \({\mathrm{X}}_{\mathrm{FSP}}\) obtained for Tali are lower than those obtained for Ayous. For the studied woods, it can be concluded that temperature does not have a strong influence on the Gibbs’ free energy.
From the analysis of Tables 3 and 4, it can be seen that the values predicted by the GAB model are very close to the experimental values. Considering the values of the relative errors of the different models, it is the best suited model to describe the experimental points of the isotherms across the considered range of temperature and relative humidity.
It can be seen that the correlation coefficient for the Henderson model is below 0.990 for Ayous for the two temperatures considered in the study. The correlation coefficient is below this same value (0.990) for Tali when the study is conducted at 40 °C, but remains higher than the one obtained at 40 °C for Ayous wood. Thus, It can be concluding that Henderson’s model is less efficient for smoothening the experimental points of Ayous compared to Tali.”
For the Nelson model, the analysis of Table 4 shows that the correlation coefficient r is below 0.990 for Ayous at any temperature. The correlation coefficient is above this same value (0.990) for Tali wood at all temperatures. Like Henderson's model, Nelson's model would perform less well in smoothing the experimental points for light wood species like Ayous compared to heavy wood species like Tali.
However, for both the Henderson and Nelson models, the results remain satisfactory because the relative errors are all less than 4%. But the GAB model remains the most satisfactory because it has the lowest relative errors and the highest correlation coefficient values.
4.2 Influence of the hysteresis on the sorption behavior
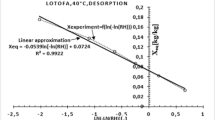
The hygroscopic equilibrium is essentially dependent on the climatic conditions of the environment. Thus, during seasons (rainy and dry), wood gains or loses water to go from a state of equilibrium in one season to another state of relative equilibrium in the other season. To characterize this phenomenon, an experimental campaign was conducted in the laboratory to determine the main desorption curves at 30, and 40 °C from the samples used to obtain the adsorption isotherm of Ayous and Tali wood. The GAB model is used to draw the theoretical curves.
Figures 2 and 3 show the comparison of the main adsorption and desorption paths for these species.
The analysis of Figs. 2 and 3 reveals, first of all, that the hysteresis increase when relative humidity increases. Ayous has higher hysteresis values compared with Tali. This is due to law fraction of small pores in Tali than in Ayous. The adsorption curve underestimates the real value of the water content of the woods. In working conditions, the wood does not undergo such important climatic variations. The amplitude of the expected water content variations will be lower than the integral hysteresis while remaining very significant even after a large number of successive desorption-adsorption cycles. Each species is therefore characterized by its own behavioral pattern.
Theoretical and numerical study of the hysteresis phenomenon seems to be inevitable to properly describe the thermo-hydric behavior of the wood material. On the one hand, to make a correct estimation of the evolution of the thermal parameters according to the relative humidity of the environment.
4.3 Influence of air's relative humidity on the estimated thermal parameters.
The analysis of Figs. 4 and 5 show that the estimated thermal parameters are sensitive to humidity. The higher the water content in the material, the higher the thermal conductivity, weakening its insulating power. A modelization of the thermal conductivity as a function of the relative humidity of the ambient air is carried out to account of this phenomenon. Table 5 presents the parameters of the theoretical model of thermal conductivity and volumetric thermal capacity deduced by linear regression.
Figures 4 and 5 show the evolution of the variation of the thermal conductivity and the thermal capacity of the two studied species with the air humidity obtained from Eqs. 20 and 21, respectively. a good agreement between the experimental results and those given by the model is observed.
An increase of the thermal conductivity with the humidity of the air is observed. This is explained by replacing air molecules with water molecules within the pores of the material. The values of the regression coefficients and relative errors are given in Table 5 for both species.
The reliability of the developed empirical relationship is evaluated by comparing the experimental and predicted curves. The relative errors are less than 1% for both Ayous and Tali. The correlation coefficients found are greater than 99% for both species in adsorption and desorption. The experimental points are well fitted by the models, as indicated by the r and P values.
Figures 4 and 5 show that it is difficult to validate a correlation for both phases of adsorption and desorption. Indeed, because of the pronounced hysteresis phenomenon, it would be interesting to adjust the model so that it takes into account the hysteresis phenomenon.
5 Conclusion
This work aimed to find a relation between thermal conductivity and volumetric heat capacity with air’s relative humidity. The sorption phenomenon of two tropical wood species was used to analyse their hygroscopic properties. The sorption isotherms studied experimentally by the gravimetric method and modelled by the GAB, Nelson and Henderson's models show indeed that the GAB model is the most suitable for the smoothing of sorption isotherms of the two tropical wood species considered in the study. The obtained isotherms are all sigmoidal in shape and are of type II. The smoothing of the experimental results from the three models allows having average relative errors lower than 4% for the two species (0.23 - 1.22) %. The correlation coefficients between GAB model and sorption experimental data are higher than 0.990 for both species except in two cases. This occur when Ayous is subjected to a temperature of 40 °C in adsorption and when Tali is subjected to a temperature of 40 °C in desorption. The existence of differences between the sorption isotherms of the two species can be explained by their chemical composition and internal structure. For example, the water content of the monolayer is higher in Tali wood for all environments. Ayous is more sensitive to temperature than Tali. The modelling effort carried out on the thermal properties as a function of the relative humidity of the ambient air. The parameters of the theoretical model of thermal conductivity and volumetric thermal capacity are deduced by linear regression. The results show an exponentially increase of the thermal properties with the humidity of the air. A good agreement between the experimental results and those given by the model is observed. The models are applicable for these materials where it is not possible to carry out experimental measurements. However, for the case of Ayous, it will be interesting to adjust the model so that it takes into account the hysteresis phenomenon.
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- \({\mathrm{C}}_{\mathrm{m}}\) :
-
Heat of sorption constant of the monolayer dimensionless
- \(\mathrm{K}\) :
-
Heat of sorption factor of the multilayer dimensionless
- P :
-
Average relative error %
- RH:
-
Relative Humidity %
- R:
-
Perfect gases’s constant \({\mathrm{J}.\mathrm{mol}}^{-1}.{\mathrm{K}}^{-1}\)
- \(\mathrm{r}\) :
-
Correlation coefficient dimensionless
- X:
-
Water content \({\mathrm{kg}.\mathrm{kg}}^{-1}\)
- \(\overline{\mathrm{X}}\) :
-
Average water content \({\mathrm{kg}.\mathrm{kg}}^{-1}\)
- \({\mathrm{C}}_{\mathrm{p}}\) :
-
heat capacity \(\mathrm{J}.{\mathrm{kg}}^{-1}{\mathrm{K}}^{-1}\)
- \({\mathrm{\Delta G}}_{0 }\) :
-
Gibbs’s free energy variation per gram of water \({\mathrm{kJ}.\mathrm{kg}}^{-1}\)
- \(\uplambda\)::
-
Thermal conductivity \(\mathrm{W }{\mathrm{m}}^{-1}{\mathrm{K}}^{-1}\)
- \(\uprho\)::
-
Density \({\mathrm{kg}.\mathrm{m}}^{-3}\)
- e:
-
Equilibrium
- g:
-
Gravimetric
- n:
-
Nelson
- h:
-
Henderson
- PHW:
-
Parallel Hot Wire
- th:
-
Theoretical
References
Miner R, Perez-Garcia J (2007) The greenhouse gas and carbon profile of the global forest products industry. For Prod J 57(10):80
Mvondo RRN, Meukam P, Jeong J, De Sousa Meneses D, Nkeng EG (2017) Influence of water content on the mechanical and chemical properties of tropical wood species. Results Phys 7:2096–2103
Ngohe Ekam PS, Meukam P, Menguy G, Girard P (2006) Thermophysical characterisation of tropical wood used as building materials: With respect to the basal density. Constr Build Mater 20:929–938
Kol HS (2009) Thermal and dieletric properties of pine wood in the transverse direction. Biosources 4(4):1663–1669
Sonderegger W, Hering S, Niemz P (2011) Thermal behaviour of Norway spruce and European beech in and between theprincipal anatomical directions. Holzforschung 65:369–375
Mvondo RRN, Damfeu JC, Meukam P, Jannot Y (2019) Influence of moisture content on the thermophysical properties of tropical wood species. Heat Mass Transf 56:1365–1378
Bobda F, Damfeu JC, Mvondo RRN, Meukam P, Jannot Y (2022) Thermal properties measurement of two tropical wood species as a function of their water content using the parallel hot wire method. Constr Build Mater 320
Engelund ET, Thygesen LG, Syensson S (2013) A critical discussion of the physics of wood-water interactions. Wood Sci Technol 47(1):141–161
Anderson RB (1946) Modification of the B.E.T. equation. J Am Chem Soc 68:686–691
De Boer JH (1953) The dynamical character of adsorption. Clarendon Press, Oxford
Guggenheim EA (1966) Application of statistical mechanics. Clarendon Press, Oxford
Simo-Tagne M, Bennamoun L, Léonard A, Rogaume Y (2019) Determination and modeling of the isotherms of adsorption/desorption and thermodynamic properties of obeche and lotofa using nelson’s sorption model. Heat Mass Transf
Derkowski A, Mirski R, Majka J (2015) Determination of sorption isotherms of scots pine (Pinus sylvestris L.) wood strands loaded with melamine-urea-phenol-formaldehyde (MUPF) resin. Wood Res 60(2):201-210
Engelund ET, Klamer M, Venås M (2010) Acquisition of sorption isotherms for modified woods by the use of dynamic vapour sorption instrumentation. Principles and Practice. Paper prepared for the 41st Annual Meeting, Biarritz, France, 9-13 May, IRG/WP10-40518
Simón C, Esteban LG, Paloma DP, Fernández FG, García-Iruela A (2016) Thermodynamic properties of the water sorption isotherms of wood of Limba. Obéché, Radiate Pine and chestnut, Industrial Crops and Products 94:122–131
Bonoma B, Simo T (2005) A contribution to the study of the drying of Ayous (triplochiton scleroxylon) and of Ebony (diospyros ebenum). Phys Chem News 26:52–56
Jannot Y, Kanmogne A, Talla A, Monkam L (2005) Experimental determination and modelling of water desorption isotherms of tropical woods: Afzélia, Ebony, Iroko, Moabi and Obéché. Holz als Roh- und Werkstoff 64:121–124
Van den Berg C (1985) Development of B.E.T. like models for sorption of water on foods; theory and relevance, In Properties of Water in Foods, (eds) D. Simatos & J.L. Multon, Martinus Nijhoff, Dordrecht. 119
Ouertani S, Soufien A, Hassini L, Koubaa A, Belghith A (2014) Moisture sorption isotherms and thermodynamics properties of Jack Pine and palm wood: Comparative study. Ind Crop Prod 56:200–210
Simo T, Zoulalian A, Njomo D, Bonoma B (2011) Modelisation of desorption isotherms and estimation of the thermophysical and thermodynamic properties of tropical woods in Cameroon: the case of Ayous and Ebony woods. Rev Des Energies Renouvelables 14(3):487–500
Nsouandélé JL, Tamba JG, Bonoma B (2018) Desorption isotherms of heavy (AZOBE, EBONY) and light heavyweight tropical woods (IROKO, SAPELLI) of Cameroon. Heat Mass Transf 54:3089–3096
Ouertani S, Soufien A, Hassini L, Koubaa A, Belghith A (2011) Palm wood drying and optimization of the processing parameters. Wood Mat Sci Eng 6(1–2):75–90
Jannot Y, Degiovanni A (2019) An improved model for the parallel hot wire: Application to thermal conductivity measurement of low density insulating materials at high temperature. Int J Therm Sci 142:379–391
Himmel S, Mai C (2015) Effects of acetylation and formalization on the dynamic water vapor sorption behavior of wood. Holzforschung 69:633–643
Popescu CM, Hill CAS, Curling S, Ormondroyd G, Xie Y (2014) The water vapour sorption behaviour of acetylated birch wood: how acetylation affects the sorption isotherm and accesible hydroxcyl content. J Mater Sci 49:2362–2371
Esteban LG, Fernández FG, Casasús AG, de Palacios PD, Gril J (2006) Comparison of the hygroscopic behaviour of 205-year-old and recently cut juvenile wood from Pinus sylvestris L. Annals of Forest Science, Springer Verlag/EDP Sciences. 63(3):309- 317. hal-00883982
Nkolo MYN, Ngamveng JN, Bardet S (2008) Effect of enthalpy–entropy compensation during sorption of water vapour in tropical woods: The case of Bubinga (Guibourtia Tessmanii J. Leonard; G. Pellegriniana J.L.). Thermochim Act 468:1–5
Esteban LG, de Palacios P, García Fernandez F, García-Amorena I (2010) Effects of burial of Quercus spp. Wood aged 5910 + 250 BP on sorption and thermodynamic properties. Int Biodeter Biodegr 64:371–377
Simo T, Rémond R, Rogaume Y, Zoulalian A, Bonoma B (2016) Sorption behaviour of four tropical woods using a dynamic vapor sorption standard analysis system. Maderas Ciencia y tecnología 18(3):403–412
Avramidis S (1997) The basics of sorption. In Proceedings of the International Conference of COST Action E8: Mechanical Performance of Wood and Wood Products, Copenhagen, Denmark
Esteban LG, de Palacios P, Fernandez FG, Guindeo A, Conde M, Baonza V (2008) Sorption and thermodynamic properties of juvenile Pinus sylvestris L. wood after 103 years of submersion. Holzforschung 62:745–751
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bobda, F., Mvondo, R.R.N., Diakhate, M. et al. Thermal properties and water content of two tropical wood species as a function of the air relative humidity.. Heat Mass Transfer 60, 449–461 (2024). https://doi.org/10.1007/s00231-023-03442-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-023-03442-z