Abstract
Purpose
Dried blot spot (DBS) analysis of drugs or clinical parameters offers many advantages. We investigated the feasibility of using DBS for analysis of anti-diabetic drugs concomitantly with the estimated creatinine clearance (Clcrea).
Methods
The cross-sectional study involved physicians in an enabling analysis with 70 diabetic patients and community pharmacists in a field investigation with 84 participants. All 154 DBS samples were analyzed for creatinine, metformin, and sitagliptin.
Results
The diabetic patients revealed of a wide range of age (32–88 years), BMI values (19.8–54.7 kg/m2), and extent of polypharmacotherapy (1–21 drugs). A correlation factor to convert capillary blood creatinine from DBS into plasma concentrations was determined. Patients’ Clcrea ranged from 21.6–155.9 mL/min. The results indicated statistically significant correlations (p < 0.05) between the use of two or three particular drug classes (diuretics, NSAIDs, renin-angiotensin system blockers) and a decreased renal function. DBS concentrations of metformin ranged between 0.23–4.99 μg/mL. The estimated elimination half-life (t ½) of metformin was 11.9 h in patients with a ClCrea higher than 60 mL/min and 18.5 h for diabetics with lower ClCrea. Sitagliptin capillary blood concentrations ranged between 11.12–995.6 ng/mL. Calculated t ½ of sitagliptin were 8.4 h and 13.0 h in patients with a ClCrea above and below 60 mL/min, respectively.
Conclusions
DBS allow for the analysis of concentrations of predominantly renally eliminated drugs and community pharmacists can provide a valuable contribution to DBS sampling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research and clinical application of compound analysis from dried blood spots (DBS) has significantly increased over the past years [1, 2]. DBS, collected by fingerpicking and spotting capillary blood on filter paper, which is subsequently dried before analysis by liquid chromatography methods, offer many advantages such as the low blood volumes required, the usually high stability of the dried analytes, and the easy sampling process. Thereby, DBS sampling is attractive, e.g., therapeutic drug monitoring in children and adults. DBS home sampling offers a convenient and potentially cost saving alternative to conventional sampling [2, 3].
If used for therapeutic monitoring for predominantly renally eliminated drugs, the patient’s plasma creatinine concentration allows the estimation of the glomerular filtration rate. The Cockcroft-Gault formula [4] has been suggested to perform best for renal function assessment in older adults [5]. However, to the best of our knowledge, there are no reports about a factor to convert capillary blood levels of creatinine from DBS into the serum/plasma concentrations.
For our investigations, we chose two model drugs that are frequently prescribed in diabetes. Metformin is a first line oral antidiabetic with beneficial effects on morbidity and mortality of type 2 diabetic patients [6]. Accumulation of metformin in blood in case of impaired renal function has been discussed to increase the risk of lactic acidosis [7]. Until recently, metformin use has been contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 60 mL/min [8]. Metformin displays a significant distribution into erythrocytes [9, 10], and thus, it might be useful in monitoring capillary blood concentrations. The dipeptidyl peptidase-IV inhibitor sitagliptin is often combined with metformin. Like metformin it is predominantly cleared renally [11] and shows distribution into blood cells [12]. It is recommended to adapt the sitagliptin dose in patients with renal impairment [13].
Diabetes is a risk factor for kidney diseases [14], but also, various drugs can negatively impact renal function [15]. The combined therapy with diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and inhibitors of the renin-angiotensin system (RAS) has been described as potentially nephrotoxic [16, 17]. Concomitant use of representatives of these highly prescribed three drug classes (“target drugs”) has been termed “triple whammy.” It might induce kidney injury based on glomerular hemodynamic alterations and result in a reduced glomerular filtration rate [18], particularly within the first 30 days of use [19].
The purpose of the present study was to investigate the feasibility of using DBS for concentration monitoring of anti-diabetic drugs in relation to the creatinine clearance (Clcrea) estimated according to Cockcroft and Gault (C&G Clcrea) as a measure of renal function [4]. We aimed at determination of “real-life” by recruiting a highly heterogeneous population of diabetic patients. The study was organized as joined project involving physicians specialized in diabetology and community pharmacists.
Methods
Study design and patients
The cross-sectional study was conducted in two phases. An enabling investigation was performed in cooperation with physicians; the field investigation involved community pharmacies. Patients with type 2 diabetes mellitus receiving a therapy with metformin and/or sitagliptin as fixed dose combination or monotherapy were recruited and enrolled in the study after written informed consent. Patients younger than 18 years and patients with an infectious disease were excluded.
In the enabling investigation, adult patients (n = 70; 34 women, 36 men) were recruited in a medical practice of physicians specialized in diabetology near Würzburg (Bavaria, Germany). Technical assistants collected the capillary blood samples between February 2015 and May 2016 and sent the dried blood spots (DBS) to the Institut für Pharmazie for analysis. Additionally, data determined in a clinical chemistry lab in the course of routine analysis of patients’ plasma were utilized. The study was approved by the ethics committee of the Medical Faculty of the University of Würzburg (reference number 287/14).
In the field study, 84 adult diabetic patients (39 women, 45 men) were recruited in 14 community pharmacies in middle and southern Germany. Pharmacists collected the capillary blood samples between August 2016 and November 2016 and sent the DBS for analysis. The study was approved by the Freiburg Ethics Committee International (FEKI) and registered by ISRCTN (ISRCTN14518136).
Analytical methods
Samples of 40.0 μL capillary blood were spotted on a precut filter paper disc and dried before packing in a plastic bag containing a desiccant. Concentrations of creatinine, metformin, and/or sitagliptin in DBS were determined using a validated liquid chromatography method as detailed previously [12]. The lower limits of quantification (LLOQ) were 0.2 μg/mL (1.55 μM) for metformin, 3 ng/mL (5.73 nM) for sitagliptin, and 0.15 mg/dL (13.26 μM) for creatinine. The validated analytical ranges comprised 0.2–5 μg/mL metformin, 3–500 ng/mL sitagliptin, and 0.15–1.5 mg/dL creatinine.
Determination of a correlation factor of capillary blood to plasma creatinine
The creatinine concentrations of the 70 patients participating in the enabling study were analyzed in DBS and compared with patients’ plasma creatinine concentrations as determined with standard enzymatic methods in a clinical chemistry lab. A conversion factor was established by splitting the patient data set at a ratio of 65%/35% to be used for development and confirmation of a conversion factor F0. The whole data set (n = 70) yielded an optimized conversion factor F.
Data analysis
For all patients, DBS concentrations of creatinine, metformin and/or, sitagliptin were determined. Depending on the time of withdrawal in relation to the last drug intake, the measured concentrations corresponded to mean steady state, peak, or trough concentrations. C&G Clcrea [4] was calculated using the patients’ ideal body weight. For correlation analysis, the Pearson coefficient was determined. The elimination half-lives t ½ for metformin and sitagliptin were calculated after dose normalization to 2000 mg metformin and 100 mg sitagliptin and assuming first-order kinetics. Plotting the normalized concentrations of all patients in relation to the time of the last intake of the medication, the elimination half-life of the population was calculated using the equation t ½ = ln2/ke with ke (eliminations rate constant) being the negative slope of the linear regression line. Mean values were compared using a t test. Statistical significance was defined as p ≤ 0.05.
Results
The average age of all participants (73 female, 81 male) was 67 ± 11.8 years (range, 32–88 years). The mean body mass index was 30.2 ± 5.5 kg/m2 (range 19.8–54.7 kg/m2). Patients concomitantly used 1–21 different drugs with a median drug number of 6 (Table 1). This information was derived from the patients’ medication plans supplemented with interviews by participating physicians (enabling study) and pharmacists (field study). The groups of the enabling investigation (n = 70) and the field study (n = 84) did not differ statistically significantly regarding BMI or number of drugs used. Both groups differed slightly, but statistically significant regarding the age with 64.6 ± 12.1 years in the enabling investigation and 68.9 ± 11.2 years in the field study (t test, p = 0.02).
Correlation between creatinine levels in capillary blood and plasma
The correlation between creatinine concentrations in capillary blood and plasma was determined in the enabling investigation with n = 70 diabetic patients. Capillary blood concentrations in dried blood spots (DBS) and plasma values of the first 45 participants were used as a “training” data set and the ratio of patients’ creatinine concentrations in capillary blood and plasma (cCapillary Blood/cPlasma) was calculated. The mean ratios revealed moderate variability with a relative standard deviation of 11.80%. The resulting initial conversion factor F0 was 0.918 ± 0.108 (mean ± standard deviation).
To evaluate the precision of the conversion factor F0, capillary blood concentrations of additional 25 patients were converted into plasma concentrations using F0. There was no statistical significant difference between the means of calculated and measured plasma creatinine levels (paired t test). The maximal deviation between measured and calculated concentrations was 0.042 mg/dL, corresponding to 5.21%. The median of the differences was − 0.0007 mg/dL.
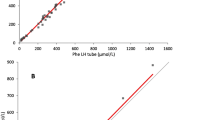
To improve the accuracy of cCapillary Blood/cPlasma, the ratio was calculated based on the data of all 70 study patients. The resulting conversion factor F of 0.916 ± 0.088 displayed a slightly improved relative standard deviation of 9.58%. However, the interquartile range of the differences between calculated versus measured values was clearly smaller for F (− 0.047 to 0.056 mg/dL) compared to F0 (− 0.098 to 0.085 mg/dL) (Electronic supplement Fig. S1). Plotting measured against the calculated concentrations of creatinine in plasma showed a significant linear correlation (r = 0.944; α = 0.05). The Bland-Altman plot revealed that the mean of the deviations of all data was 0.001 mg/dL (Fig. 1; line A) and that 95% of all results were distributed within the mean ± 1.96 standard deviations (0.165 mg/dL and − 0.163 mg/dL) of the differences (Fig. 1; lines B and C).
Bland-Altman plot to compare measured and calculated plasma concentrations of creatinine in the enabling study (n = 70). Line A represents the mean value of the differences of the concentrations, B and C represent the mean ± 1.96 standard deviations of the differences between measured and calculated concentrations. More than 95% of the differences were located within this range
Renal function estimation and the “triple whammy”
The plasma creatinine concentrations of all patients (n = 153) were calculated based on the capillary blood concentration using the conversion factor F = 0.916. Patients’ C&G Clcrea ranged from 21.6–155.9 mL/min (median 67.46 mL/min; Table 2).
The serum creatinine concentrations of patients with a complete medication plan (n = 142) were analyzed. Patients taking two or three target drugs (diuretics, NSAIDs, RAS blockers) had a significantly lower ClCrea compared to patients without target drug prescription (t test, p < 0.05; Fig. 2).
Combined therapy with diuretics, nonsteroidal anti-inflammatory drugs, inhibitors of the renin-angiotensin system (“target drugs”) and estimated creatinine clearance of the diabetic patients. Taking two or three target drugs correlated with a significant lower renal function compared to taking none of the drugs (*p < 0.05)
Metformin
Metformin was prescribed to 139 patients, and DBS concentrations were quantified in all but two patients (n = 137). Based on the prescription history, all patients were assumed to be in the steady state. Metformin concentrations ranged from 0.23 μg/mL to 4.99 μg/mL after daily intake of 500–2250 mg metformin with a median dose of 2000 mg (Table 2). The mean concentration (± SD) was 2.22 ± 1.16 μg/mL.
The interval to the last metformin intake ranged from 30 to 1545 min (0.5–25.75 h). Varying intervals allowed the estimation of the elimination kinetics of metformin after dose normalization to 2000 mg. Metformin then ranged between 0.35 and 7.26 μg/mL with mean levels of 2.69 ± 1.34 μg/mL. The elimination half-life of metformin for patients with ClCrea above 60 mL/min was 12 h (714 min) and 18.5 h (1111 min) for the patients with the lower ClCrea.
Both the interval to the last metformin intake and the estimated ClCrea revealed a weak but significant correlation with the normalized concentration of metformin (Table 3; Pearson correlation; p < 0.05; interval to last intake r = − 0.319, ClCrear = − 0.368). If patients with ClCrea lower and higher 60 mL/min were analyzed separately, the normalized concentration of metformin correlated stronger with the interval to the last metformin intake. The coefficient of correlation was r = − 0.389 (p < 0.05) for patients with higher ClCrea (n = 78) and r = − 0.439 (p < 0.05) for patients with ClCrea lower than 60 mL/min (n = 57). Metformin levels also significantly correlated with the patients’ age (p < 0.05, r = 0.259).
Sitagliptin
Sitagliptin was prescribed to 69 patients and concentrations were quantified in all but two patients (n = 67) whose samples revealed concentrations below the LLOQ of 3 ng/mL or above of the validated range, respectively. The concentrations ranged from 11.12–995.6 ng/mL after daily intake of 25–100 mg metformin (median dose 100 mg). The mean concentration (± SD) was 432.2 ± 268.8 ng/mL. The high variability of capillary blood concentrations was also seen after dose normalization to 100 mg sitagliptin per day, ranging between 11.12 and 1226 ng/mL with mean levels of 458.4 ± 289.1 ng/mL.
The interval to the last sitagliptin intake ranged from 35 to 1545 min (0.6–25.75 h). The elimination half-life of sitagliptin for patients with ClCrea above 60 mL/min was 8.4 h (505 min) and 13 h (779 min) for the patients with the lower ClCrea.
Sitagliptin concentrations showed a significant weak correlation with the ClCrea (Table 3; Pearson correlation; p < 0.05; r = − 0.294) and a stronger correlation with the interval to the last intake (Pearson correlation; p < 0.05; r = − 0.522; n = 64 patients). Sitagliptin capillary blood concentrations and the interval to the last intake correlated stronger for patients with higher ClCrea (n = 40; r = − 0.570, p < 0.05) compared to patients with ClCrea lower than 60 mL/min (n = 24; r = − 0.479, p < 0.05). Sitagliptin levels also significantly correlated with the patients’ hematocrit (p < 0.05, r = 0.396; hematocrit concentrations were available for n = 35 patients).
Discussion
In the present cross-sectional study, we investigated the feasibility of using dried blood spots (DBS) for concentration monitoring of predominantly renally eliminated anti-diabetic drugs in relation to the estimated creatinine clearance (C&G Clcrea).
For calculating plasma creatinine levels based on DBS samples, a conversion factor was established based on the strategy described for the development of the MDRD formula [20]. Therefore, the patient data set was split at a ratio of 65%/35% to be used for development and verification of an initial factor (F0 0.918 ± 0.108) which was optimized using the whole data set, yielding the improved conversion factor (F 0.916 ± 0.088).
There are controversial reports about the deviation of creatinine concentrations determined in plasma and venous or capillary blood samples. Higher mean creatinine concentrations in capillary dried blood spots compared to plasma samples have been communicated [21, 22]. In contrast, others found lower creatinine levels in capillary blood [23] or dried venous blood samples [24] in relation to plasma concentrations which is consistent with the results of the present study.
The patients’ renal function was calculated using the Cockcroft-Gault formula and the patients’ ideal body weight, which has been shown to yield best results [5]. The estimated C&G Clcrea revealed a decrease by 1.26 mL/min per year in the diabetic patients in the current study. This is consistent with a yearly decline of 1.18 mL/min reported for a cohort of 230 subjects with different comorbidities such as hypertension, diabetes mellitus, or impaired fasting glucose [25]. Most of the diabetes patients in the present study also suffered from hypertension as comorbidity.
A reduced glomerular filtration rate due to glomerular hemodynamic alterations has been reported for patients concomitantly taking non-steroidal anti-inflammatory drugs (NSAIDs), diuretics, and inhibitors of the renin-angiotensin system (RAS) (“target drugs”). This association has been termed “triple whammy effect” [16, 18]. Although a polypharmacotherapy can also be the consequence of an impaired renal function, the mechanism of kidney injury caused by a combination of particular drug classes has been analyzed in detail [18]. The number of patients taking no/1/2/3 target drugs in our study (22/57/41/22) was lower than in other clinical investigations (e.g. 135/87/60/19; [16]). However, the correlation of lower Clcrea and number of target drugs was still statistically significant. Though this does not prove a causality the results are consistent with the described “triple whammy effect”.
To the best of our knowledge, capillary blood concentrations for metformin have not been reported. However, it is well-known that metformin significantly distributes into erythrocytes and therapeutic values for plasma and erythrocyte concentrations have been published [9, 10, 13]. In the present study, analysis of metformin in DBS revealed mean capillary blood concentrations of 2.22 ± 1.16 μg/mL (median 2.01 μg/mL) which approximate published values if concentrations suggested for plasma (0.5 ± 0.4 μg/mL) and erythrocytes (0.8 ± 0.4 μg/mL) were combined [9]. Metformin concentrations determined under “real-life” use of the drug were reported as 2.7 ± 7.3 μg/mL in plasma and 2.0 ± 4.4 μg/mL in erythrocytes, and values up to 5 μg/mL in plasma were assessed as “slightly to moderately elevated” [10]. This would cover the range of DBS concentrations (0.23 and 4.99 μg/mL) in the present study, allowing for the conclusion that most of the patients were within the therapeutic range.
The negative correlation between Clcrea and plasma metformin concentrations was known [26, 27] and also observed in our study. Although pharmacokinetic parameters depend on the eGFR (corresponding to the Clcrea) according to a continuous function, we chose dichotomous categories with a threshold of 60 mL/min. This value has been meeting the National Kidney Foundation’s definition of a chronic kidney disease and until recently metformin use has been contraindicated in patients with an eGFR below this threshold [8]. Based on the estimated Clcrea and the elimination calculated using population pharmacokinetic methods, a prolonged metformin half-life of 18.5 h was determined in patients with Clcrea below 60 mL/min. In contrast, patients with higher Clcrea revealed a t ½ of about 12 h. These elimination half-lives are considerably longer than the reported 5–6 h [6, 28], but consistent with the published t ½ of 11–14 h [29]. In the present study the elimination was determined based on capillary blood, not on plasma samples. Due to the distribution of metformin into erythrocytes serving as an additional compartment, a longer t ½ in capillary blood as compared to plasma should be expected.
We are not aware of any published full or capillary blood concentrations of sitagliptin. Peak plasma concentrations of sitagliptin in the steady state levels were reported to be 383 ng/mL (941 nM) after 100 mg [30] and 167–174 ng/mL after 50 mg doses [29]. Sitagliptin also distributes into erythrocytes [12], and thus, mean DBS concentrations of 432.2 ± 268.8 ng/mL detected in the present study are consistent with the documented range. In contrast to published data, sitagliptin concentrations in the present study displayed a pronounced variability; they ranged between 11.1 and 995.6 ng/mL.
Compared to metformin, sitagliptin values showed a weaker negative correlation with Clcrea. Yet, the calculated t ½ of sitagliptin was clearly longer in patients with Clcrea below 60 mL/min (13 h) compared to patients with higher Clcrea (8.4 h). This is consistent with reported plasma half-lives between 10 and 14 h [11, 29, 30]. Apparently, the distribution of sitagliptin into erythrocytes does not significantly influence its t ½.
A limitation of this study is that only a single DBS sample was collected per patient, and thus, the individual pharmacokinetics could not be determined. However, after demonstrating the general feasibility of the approach, this can be realized in future studies. Another limitation was that we did not compare the capillary blood concentrations of metformin and sitagliptin with simultaneously obtained venous blood levels of the same patients. Lastly, we had to trust the patients’ medication plans being complete and up-to-date for prescribed and OTC medications.
To summarize, for the first time, we report DBS concentrations of metformin and sitagliptin in relation to the patients’ Clcrea. The diabetic patient population was highly heterogeneous including patients of a wide range of age, BMIs, and varying extent of polypharmacotherapy, so that the results can be regarded as “real-life” data. The fact that the determined drug concentration ranges are consistent with data published for plasma and the detail that we uncovered are indications for the “triple whammy” effect in the study population suggest that DBS can be used for concentration monitoring of predominantly renally eliminated drugs.
In the field study part, DBS samples were collected in community pharmacies. There are sparse reports about such an approach [31]. The present study confirms that community pharmacists can provide a valuable contribution to DBS sampling.
References
Enderle Y, Foerster K, Burhenne J (2016) Clinical feasibility of dried blood spots: analytics, validation, and applications. J Pharm Biomed Anal 130:231–243. https://doi.org/10.1016/j.jpba.2016.06.026
Wilhelm AJ, den Burger JC, Swart EL (2014) Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet 53(11):961–973. https://doi.org/10.1007/s40262-014-0177-7
Martial LC, Aarnoutse RE, Schreuder MF, Henriet SS, Bruggemann RJ, Joore MA (2016) Cost evaluation of dried blood spot home sampling as compared to conventional sampling for therapeutic drug monitoring in children. PLoS One 11(12):e0167433. https://doi.org/10.1371/journal.pone.0167433
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Drenth-van Maanen AC, Jansen PA, Proost JH, Egberts TC, van Zuilen AD, van der Stap D, van Marum RJ (2013) Renal function assessment in older adults. Br J Clin Pharmacol 76(4):616–623. https://doi.org/10.1111/bcp.12199
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50(2):81–98. https://doi.org/10.2165/11534750-000000000-00000
Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK (2014) Metformin in patients with type 2 diabetes and kidney disease: a systematic review. Jama 312(24):2668–2675. https://doi.org/10.1001/jama.2014.15298
DeFronzo R, Fleming GA, Chen K, Bicsak TA (2016) Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 65(2):20–29. https://doi.org/10.1016/j.metabol.2015.10.014
Lalau JD, Lacroix C (2003) Measurement of metformin concentration in erythrocytes: clinical implications. Diabetes Obes Metab 5(2):93–98
Lalau JD, Lemaire-Hurtel AS, Lacroix C (2011) Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Investig 31(6):435–438
Scheen AJ (2010) Pharmacokinetic and pharmacodynamic evaluation of sitagliptin plus metformin. Expert Opin Drug Metab Toxicol 6(10):1265–1276. https://doi.org/10.1517/17425255.2010.513699
Scherf-Clavel M, Högger P (2015) Analysis of metformin, sitagliptin and creatinine in human dried blood spots. J Chromatogr B Anal Technol Biomed Life Sci 997:218–228. https://doi.org/10.1016/j.jchromb.2015.06.014
Scheen AJ (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30(5):359–371
Piccoli GB, Grassi G, Cabiddu G, Nazha M, Roggero S, Capizzi I, De Pascale A, Priola AM, Di Vico C, Maxia S, Loi V, Asunis AM, Pani A, Veltri A (2015) Diabetic kidney disease: a syndrome rather than a single disease. Rev Diabet Stud 12(1–2):87–109. https://doi.org/10.1900/RDS.2015.12.87
Pazhayattil GS, Shirali AC (2014) Drug-induced impairment of renal function. Int J Nephrol Renov Dis 7:457–468. https://doi.org/10.2147/IJNRD.S39747
Loboz KK, Shenfield GM (2005) Drug combinations and impaired renal function -- the 'triple whammy'. Br J Clin Pharmacol 59(2):239–243. https://doi.org/10.1111/j.0306-5251.2004.2188.x
Onuigbo MA (2013) The nephrotoxic "triple whammy" of combining diuretics, ACE inhibitors, and NSAIDs. BMJ 346:f678. https://doi.org/10.1136/bmj.f678
Prieto-Garcia L, Pericacho M, Sancho-Martinez SM, Sanchez A, Martinez-Salgado C, Lopez-Novoa JM, Lopez-Hernandez FJ (2016) Mechanisms of triple whammy acute kidney injury. Pharmacol Ther 167:132–145. https://doi.org/10.1016/j.pharmthera.2016.07.011
Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S (2013) Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ 346:e8525. https://doi.org/10.1136/bmj.e8525
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130(6):461–470
den Burger JC, Wilhelm AJ, Chahbouni AC, Vos RM, Sinjewel A, Swart EL (2015) Haematocrit corrected analysis of creatinine in dried blood spots through potassium measurement. Anal Bioanal Chem 407(2):621–627. https://doi.org/10.1007/s00216-014-8291-9
Koop DR, Bleyle LA, Munar M, Cherala G, Al-Uzri A (2013) Analysis of tacrolimus and creatinine from a single dried blood spot using liquid chromatography tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 926:54–61
Keevil BG (2011) The analysis of dried blood spot samples using liquid chromatography tandem mass spectrometry. Clin Biochem 44(1):110–118
Koster RA, Greijdanus B, Alffenaar JW, Touw DJ (2015) Dried blood spot analysis of creatinine with LC-MS/MS in addition to immunosuppressants analysis. Anal Bioanal Chem 407(6):1585–1594. https://doi.org/10.1007/s00216-014-8415-2
Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, Krause I (2014) A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 27(6):635–641. https://doi.org/10.1007/s40620-014-0077-9
Bardin C, Nobecourt E, Larger E, Chast F, Treluyer JM, Urien S (2012) Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol 68(6):961–968
Duong JK, Kroonen M, Kumar SS, Heerspink HL, Kirkpatrick CM, Graham GG, Williams KM, Day RO (2017) A dosing algorithm for metformin based on the relationships between exposure and renal clearance of metformin in patients with varying degrees of kidney function. Eur J Clin Pharmacol 73(8):981–990. https://doi.org/10.1007/s00228-017-2251-1
Dissanayake AM, Wheldon MC, Hood CJ (2018) Pharmacokinetics of metformin in patients with chronic kidney disease stage 4 and metformin-naive type 2 diabetes. Pharmacol Res Perspect 6(5):e00424. https://doi.org/10.1002/prp2.424
Chen X, Zhao Q, Zhang J, Liu T, Jiang J, Hu P (2016) An open-label, multiple-dose study to assess the pharmacokinetics and tolerability of sitagliptin/metformin fixed-dose combination (FDC) tablet in healthy Chinese adult subjects. Int J Clin Pharmacol Ther 54(9):705–711. https://doi.org/10.5414/CP202646
Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA (2006) Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther 28(1):55–72. https://doi.org/10.1016/j.clinthera.2006.01.015
Radley A, Melville K, Tait J, Stephens B, Evans JMM, Dillon JF (2017) A quasi-experimental evaluation of dried blood spot testing through community pharmacies in the Tayside region of Scotland. Frontline Gastroenterol 8(3):221–228. https://doi.org/10.1136/flgastro-2016-100776
Acknowledgements
The authors would like to thank the patients and all 14 engaged community pharmacies for their participation in this study. The excellent technical assistance of Sabrina Rösel (office of Drs. Albert and Zieher) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Patients were recruited and enrolled in the study after written informed consent. The study was approved by the ethics committee of the Medical Faculty of the University of Würzburg (reference number 287/14) and the Freiburg Ethics Committee International (FEKI) and registered by ISRCTN (ISRCTN14518136).
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
(DOCX 283 kb)
Rights and permissions
About this article
Cite this article
Scherf-Clavel, M., Albert, E., Zieher, S. et al. Dried blood spot testing for estimation of renal function and analysis of metformin and sitagliptin concentrations in diabetic patients: a cross-sectional study. Eur J Clin Pharmacol 75, 809–816 (2019). https://doi.org/10.1007/s00228-019-02637-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02637-w