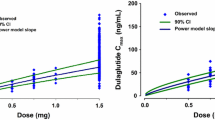
Abstract
Purpose
The objective of this study was to develop a population pharmacokinetic model and investigate the effect of several demographic covariates on metformin pharmacokinetics in patients with type 2 diabetes mellitus, over a wide range of weights.
Methods
A total of 105 patients received different metformin regimens, and pharmacokinetic sampling included a minimum of two concentrations per patient. Plasma determination of metformin was assayed by high performance liquid chromatography. Population pharmacokinetics was modelled using a nonlinear mixed effects model program (Monolix version 3.1 s).
Results
An open one-compartment model adequately described metformin data. Lean body weight was a better size descriptor than actual body weight or ideal body weight for clearance (CL/F) and volume (V/F) parameters. CL/F was negatively related to age and serum creatinine (SCr). The estimation of specific coefficients for these effects gave better results than the use of renal function descriptors (Cockroft or MDRD). A dose effect in the relative bioavailability was demonstrated.
Conclusion
The pharmacokinetics of metformin was influenced by lean body weight on an allometric basis and was related to markers of renal function, age, and serum creatinine in this population of 105 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metformin hydrochloride alone or in combination is the first line therapy for the management of type 2 diabetes mellitus. Metformin is an insulin-sensitizing agent, which is effective in combination with sulfonylureas, insulin, thiazolidinediones, and incretin mimetics [1].
Obesity is a worldwide problem with major health consequences, especially in patients with diabetes mellitus. However, little is known about the influence of obesity on the drug exposure profile, resulting in few clear dosing guidelines for the obese. Physiologic changes in obesity can alter both the volume of distribution and clearance of many drugs. Drug distribution into tissues is affected by body composition, regional blood flow, and drug lipophilicity [2, 3]. Obesity increases both fat and lean masses; however, the percentage of fat tissue increases more than does the lean mass, affecting the apparent volume of distribution of drugs according to their lipid solubility. Nevertheless, predictability based on lipid solubility is difficult, and selection of the optimum size descriptor for dose calculation is drug-specific and must be founded on specific prospective studies [3, 4]. The current dosing of metformin was determined empirically. Garber et al. [5] demonstrated that the antihyperglycemic activity of metformin was generally dose-dependent. Individualization of the dosage of metformin based on pharmacokinetic variability has also recently been suggested [6]. Nevertheless, few studies have been done to identify patient characteristics that could influence metformin pharmacokinetics. Regarding obese patients with type 2 diabetes mellitus, it is necessary to have information about size descriptors in these patients to optimize drug safety and to insure optimal dosage of metformin.
The objectives of this study were to develop a population pharmacokinetic model for metformin in patients with type 2 diabetes mellitus over a wide range of body weights and to evaluate more specifically different size descriptors.
Methods
Study design
Adult patients with type 2 diabetes mellitus were hospitalized in the diabetology unit for teaching purposes and received metformin hydrochloride (Glucophage® or generic drugs) or metformin embonate (Stagid®) orally for at least 1 month so they were at pharmacokinetic steady state. Metformin was prescribed either alone or combined with oral antidiabetes drugs and/or insulin. Dosage regimens ranged from 500 to 3,000 mg/day (1,000 mg /8 h) according to biological and clinical settings. None of the patients showed recent alteration of renal function, so they could also be considered to be in a steady state concerning renal function. Obese and nonobese patients were included to evaluate a wide range of body size descriptors. Ethics committee approval and patient consent are not compulsory in France in order to use therapeutic drug monitoring data, and thus they were not collected.
Two or three blood samples per patient were drawn, including morning trough levels just before intake and 1, 2.5, 4, or 6 h after drug intake. Precise time elapsed between administration and sampling time, dosage regimen, age, body weight (BW), height (HT), and serum creatinine levels were recorded. Whole blood was collected in heparinized tubes and centrifuged within 30 min at 4,000 × g for 15 min. Plasma was transferred into glass tubes and stored at −20°C until analysis. Regarding size descriptors, body mass index (BMI), ideal body weight (IBW), and lean body weight (LBW) were calculated using the following equations:
\( {\text{IBW}} = {45}.{4} + 0.{89 } \times { }\left( {{\text{HT}}--{152}.{4}} \right) + {4}.{5 }\left( {\text{if male}} \right) \) [7]
\( {\text{LBW}} = {9279} \times {\text{BW}}/\left[ {{668}0 + \left( {{216} \times {\text{BMI}}} \right)} \right]{\text{ for females}} \) [8]
\( {\text{LBW}} = {9279} \times {\text{BW}}/\left[ {{878}0 + \left( {{244} \times {\text{BMI}}} \right)} \right]{\text{ for males}} \) [8], where BW is body weight (in kg) and HT is height (in cm).
Creatinine clearance (CLCR) was predicted with the Cockroft equation:
CLCR was standardized to 70 kg and calculated as CLCR × 70/BW.
Glomerular filtration rate normalized for body surface area was estimated with the MDRD equation:
Metformin dosage was expressed in metformin base from embonate or hydrochloride salts.
Bioassay
Concentrations of metformin base in plasma were determined using a modified version of a validated high-pressure liquid chromatography (HPLC) assay with UV absorbance as initially described by Amini et al. [9]. Liquid-liquid extraction of metformin was performed as follows: 200 μL of plasma sample was buffered by adding 200 μL of sodium hydroxide 8 M and spiked with 40 μL phenylbiguanide (internal standard). Then 2.6 mL of a mixture of 1-butanol/n-hexane 50/50 was added, and the mixture was shaken for 2 min. After being centrifugated, 200 μL of acetic acid 1% was added to the upper organic layer. Mixture was vortex-mixed and centrifuged. The upper organic layer was discarded, and 50 μL of the aqueous layer was then injected onto a Spherisorb® S5W column (250 × 4.6 mm ID, 5 μm) maintained at 30°C. Flow rate was set at 1 mL/min, and compounds were detected at 234 nm on a Dyonex Ultimate 3000 variable-wavelength detector. Retention times for metformin and phenylbiguanide were 7.0 and 5.8 min, respectively. Lower limit of quantification was 15 ng/mL. Based on quality control samples, intraday and between-day precision and accuracy were less than 10% over the entire range of quantification. The mean absolute recoveries for metformin and internal standard were 94 and 91%, respectively.
Population pharmacokinetic modelling
Data were analyzed using the nonlinear mixed effect modelling software program Monolix version 3.1 s [10] (http://wfn.software.monolix.org). Parameters were estimated by computing the maximum likelihood estimator of the parameters without any approximation of the model (no linearization) using the stochastic approximation expectation maximization (SAEM) algorithm combined with a MCMC (Markov Chain Monte Carlo) procedure. The number of MCMC chains was fixed to 10 for all estimations. A constant error model was used to describe the residual variability, and the between-subject variabilities (BSV) were ascribed to an exponential error model. Parameter shrinkage was calculated as [1 − sd(eta)/omega], where sd(eta) and omega are the standard deviation of individual eta parameters and the population model estimate of the BSV, respectively. The likelihood ratio test (LRT) including the log-likelihood, the Akaike information criterion (AIC), and the Bayesian information criterion (BIC) were used to test different hypotheses regarding the final model, covariate effect on pharmacokinetic parameter(s), residual variability model (proportional versus proportional plus additive error model), and structure of the variance-covariance matrix for the BSV parameters. The normalized prediction distribution error (NPDE) metrics and the visual predictive check (VPC) were used as the main diagnostic tools to evaluate the final model. The mean (m) and variance (v) of the NPDE must be 0 and 1, respectively, with a normal distribution. Diagnostic graphics and other statistics were obtained using the R program [11].
Results
From the 105 patients investigated, 317 time-plasma concentrations were available for analysis. Patient characteristics are summarized in Table 1. Distribution of BW and BMI among the 105 patients is depicted in Fig. 1.
An open one-compartment model adequately described metformin data. BSV was estimated for all structural parameters with a significant covariance term between the clearance (CL/F) and central volume of distribution (Vc/F). Residual variability was described by a constant error model. At this step, CL/F was 27.5 L/h [relative standard error (rse) 6%], and the corresponding BSV was 0.53 (rse 7%).
No covariate effect was observed on the absorption rate constant (ka). The main covariate effects were size effects, BW, LBW, and IBW. Finally LBW was the best size descriptor for clearance and volume parameters. Table 2 summarizes the model building steps for these size effects. The pharmacokinetic parameters were allometrically normalized for LBW to a 60 kg individual as follows:
where i denotes the ith individual. The PWR exponents were 3/4 and 1 for the clearance and volume terms, respectively. Inclusion of LBW covariate decreased the BIC criterion by 9 units and improved the predictive performance of the model.
As summarized in Table 2, CL/F was also negatively related to age and serum creatinine (SCr). Also the relative bioavailability was influenced by the given dose as follows, FREL = 1 × (dose/780)−0.26.
The final population model included LBW as covariate on metformin volume; LBW, age, and SCr as covariates on metformin clearance; and a dose effect on FREL. The effects of these covariates decreased the BIC criterion by 48 units and significantly improved the predictive performance of the model (Fig. 2). The linear function of CL/F versus LBM [(LBM/60)1] as described by McLeay et al. [12] did not improve the model, i.e., the AIC/BIC criteria (273/299) were similar. An alternative model described by Mould et al. [13] was also investigated. In this model, CL is thought to be separated into non-renal and renal components, as
where RF is a parameter estimating the renal function relative to a standard CLR,std of 70 mL/min. Although all the population parameters were well estimated, the AIC/BIC increased to 333/357, so this model was not retained. Also, the use of LBW in this formula did not improve the fit (AIC/BIC 369/390). Thus, regarding V/F and CL/F, the final model was as follows:
Observed metformin concentrations (in mg/L) and population predicted concentrations. Improvement of the predictive performance from the covariate-free model (left) to the final model including the effects of LBW, age, SCr, and dose effect (right). Black solid line is the identity line, red line actual regression line, blue curve spline function
Table 3 summarizes the final population pharmacokinetic estimates. Inclusion of these covariates decreased BSV on metformin clearance from 0.55 to 0.39 (rse 4%). Most of the parameters were well estimated with low relative standard errors. The empirical Bayesian estimate shrinkages were generally low, except for ka.
As shown in Fig. 3, the NPDE values are randomly distributed in time and concentration.The mean and variance of these metrics must be 0 and 1, respectively, with a normal distribution: m = −0.007 (p = 0.923), v = 1.04 (p = 0.676) ,and Shapiro-Wilk test normality test, p = 0.373. All the criteria were fullfilled. The results of the visual predictive check (VPC), based upon 200 simulations of the final model, are depicted in Fig. 4. Because patients did not receive the same dosage, observed and predicted concentrations were normalized to 663 mg every 8 h or 780 mg every 12 h. For all groups, the observed concentrations were centered about the model-predicted median, and the proportion of observations out of the model-predicted 5th and 95th percentile curves was not significantly different from 10%.
Diagnostic plots for the final population pharmacokinetic model—normalized prediction distribution error (NPDE) metrics. Normal quantile-quantile plot for NPDE (top left), distribution (top right), and NPDE metrics versus time (bottom left) or versus predictive (bottom right). The mean (m) and variance (v) of these metrics must be 0 and 1, respectively, with a normal distribution: m = −0.007 (p = 0.923), v = 1.04 (p = 0.676), and Shapiro-Wilk test normality test, p = 0.373
Metformin concentration versus time—visual predictive checks for the final population model. The solid and dashed lines denote the median and 5th and 95th percentiles of 200 Monte Carlo simulated concentrations from the final model. Top Administration every 8 h (THO8), dose normalized to 663 mg. Bottom Administration every 12 h, dose normalized to 780 mg (THO12)
Discussion
The pharmacokinetics of plasma metformin in patients with type 2 diabetes mellitus was satisfactorily described by an open one-compartmental model with linear elimination. The metformin CL/F estimate, 56 L/h/60 kg LBW was slightly higher than previously reported estimates in adult patients, 26.5–42.4 L/h [6, 14–17]. Hong et al. reported a higher metformin CL/F, 79.2 L/h [18].
In these patients, representing a wide range of BW and BMI values, the effect of size descriptors on pharmacokinetic parameters could be accurately evaluated. The best descriptor was LBW for volume, very close to IBW, as expected from the hydrophilic nature of metformin, which is completely ionized at plasma pH under physiological conditions [19]. LBW was estimated using the LBW equation from Janmahasatian [8]. One key advantage of this LBW equation is that the estimate never declines as BW increases, and this appears to be the more appropriate method of calculating LBW in obese and nonobese individuals [8, 20]. Indeed, the BW was a poor size descriptor because it included a great proportion of fat mass, in which metformin is not expected to distribute. Pharmacokinetic studies in obesity show that the behavior of molecules with weak or moderate lipophilicity is generally rather unpredictable, as these drugs are distributed mainly in lean tissues. The dosage of these drugs should be based on other body size indexes that exclude the excess of fat mass, for example, LBW or the ideal body weight (IBW). However, some of these drugs (e.g., antibacterials and some anticancer drugs) are partly distributed in adipose tissues, and their dosage is based on IBW plus a percentage of the excess body weight as a correction factor [3, 21, 22]. IBW and LBW have often been considered as the best descriptors for weak lipophilic drugs. In our study, IBW and LBW are the most relevant size descriptors for volume with only one point different on AIC and BIC. As a dosing scalar, IBW is not an optimum metric and was developed initially for purposes unrelated to pharmacokinetics. LBW is a potentially useful predictor of the pharmacokinetic behavior of drugs that are highly water soluble [8, 20, 23] and was the best size descriptor in our model.
In the case of a chronic dosing regimen, CL/F is the main pharmacokinetic parameter. Different renal function descriptors were tested as covariates on CL/F [e.g., SCr, CLCR/BW (CLCR normalized for weight), and MDRD index]. CLCR was normalized for weight to remove the effect of body size from the predicted creatinine clearance, thereby separating renal function from body size effects on the clearance of metformin [13, 20]. Finally, combining different size and renal function descriptors gave better results than the use of each covariate taken alone, and the best model for CL/F included age, SCr, and LBW as covariates. The design of the study did not allow us to distinguish different allometric exponents for this LBW effect. The 0.75 allometric exponent was ultimately retained on the basis of a very small difference in the criterion values. The effects of age and SCr on CL/F are related to the renal metformin elimination as previously reported [24]. The estimation of specific coefficients for these effects gave better results than the use of renal function descriptors, i.e., CLCR/BW or MDRD index. Regarding the renal function, our results are consistent with earlier studies. In a study by Tucker et al. [16], a correlation was found between renal clearance of metformin and creatinine or creatinine clearance. Thus adjusting dosage to renal function could be satisfactory even in elderly patients [25]. However, most of these studies did not include a large number of obese patients. LBW was the best size descriptor for metformin CL/F, which seems plausible as the major drug clearing organ, kidney for metformin, is associated with LBW.
There was a great between-subject variability in metformin pharmacokinetics, which was probably related to bioavailability issues. Metformin has an absolute oral bioavailability of 40–60%, and absorption is estimated to be complete within 6 h of administration with an active and saturable absorption process [16, 26]. Our results confirmed a “dose effect” in the relative bioavailability: FREL = 1 for a 780 mg dose (expressed in metformin base) and FREL = 1.17 for a 390 mg dose (+17%) [27]. It has also been suggested that genetic factors might contribute to the variability in metformin pharmacokinetics because the organic cation transporter OCT1 variants are associated with increased renal elimination of metformin [28].
In conclusion, in a large population of obese patients with type 2 diabetes mellitus, metformin apparent clearance and volume of distribution were influenced by the lean body weight on an allometric basis, and clearance was also related to markers of renal function: age and serum creatinine. Relative bioavailability was influenced by dose. The model developed here suggests a dose individualization using these covariates. These results should contribute to define dosage guidelines in diabetic obese patients.
References
Nathan DM, Buse JB, Davidson MB et al (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 32:193–203
Hanley MJ, Abernethy DR, Greenblatt DJ (2010) Effect of obesity on the pharmacokinetics of drugs in human. Clin Pharmacokinet 49:71–87
Cheymol G (2000) Effects of obesity on pharmacokinetics. Clin Pharmacokinet 39:215–231
Sparreboom A, Wolff AC, Mathijssen RHJ et al (2010) Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol 25:4707–4713
Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL (1997) Efficacy of metformin in type II diabetes: results of a double-blind, placebo controlled, dose response trial. Am J Med 103:491–497
Graham GG, Punt J, Arora M et al (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50:81–98
Devine D (1974) Case study number 25 gentamicin therapy. Drug Intell Clin Pharm 8:650–655
Janmahasatian S, Dufull SB, Ash S et al (2005) Quantification of lean bodyweight. Clin Pharmacokinet 44:1051–1065
Amini H, Ahmadiani A, Gazerani P (2005) Determination of metformin in human plasma by high-performance liquid chromatography. J Chrom B 824:319–322
Kuhn E, Lavielle M (2005) Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Anal 49:1020–1030
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
McLeay S, Morrish G, Kirkpatrick C et al (2009) Encouraging the move towards predictive population models for the obese using propofol as a motivating example. Pharm Res 26:1626–1634
Mould D, Holford N, Schellens J et al (2002) Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther 71:334–348
Sirtori CR, Franceschini G, Galli-Kienle M et al (1978) Disposition of metformin (N, N-dimethylbiguanide) in man. Clin Pharmacol Ther 24:683–693
Pentikaïnen PJ, Neuvonen PJ, Pentillä A (1979) Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol 16:195–202
Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF (1981) Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol 12:235–246
Scheen AJ (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30:359–371
Hong Y, Rohatagi S, Habtemariam B, Walker J, Schwartz SL, Mager DE (2008) Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus. J Clin Pharmacol 48:696–707
Schäfer G (1983) Biguanides: a review of history, pharmacodynamics and therapy. Diab Metab 9:148–163
Han PY, Duffull SB, Kirkpatrick CM, Green B (2007) Dosing in obesity: a simple solution to a big problem. Clin Pharmacol Ther 82:505–508
Erstadt BL (2004) Dosing of medications in morbidly obese patients in intensive care unit setting. Intensive Care Med 30:18–32
Traynor AM, Nafziger AN, Bertino JS (1995) Aminoglycoside dosing weight correction factors for patients of various body sizes. Antimicrob Agents Chemother 39:545–548
Green B, Duffull SB (2004) What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58:119–133
Sambol NC, Chiang J, Lin ET et al (1995) Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol 35:1094–1102
Lalau JD, Vermersch A, Hary L, Andrejak M, Isnard F, Quichaud J (1990) Type 2 diabetes in the elderly: an assessment of metformin. Int J Clin Pharmacol Ther Toxico 28:329–332
Karim A, Slater M, Bradford D et al (2007) Oral antidiabetic drugs: bioavailability assessment of fixed-dose combination tablets of pioglitazone and metformin. Effect of body weight, gender, and race on systematic exposures of each drug. J Clin Pharmacol 47:37–47
Sambol NC, Brookes LG, Chiang J et al (1996) Food intake and dosage level, but not tablet vs solution dosage form, affect the absorption of metformin HCl in man. Br J Clin Pharmacol 42:510–512
Tzvetkov MV, Vormfelde SV, Balen B et al (2009) The effects of genetic polymorphisms in the organic cation transporters OCT1, OCT2 and OCT3 on the renal clearance of metformin. Clin Pharmacol Ther 86:299–306
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bardin, C., Nobecourt, E., Larger, E. et al. Population pharmacokinetics of metformin in obese and non-obese patients with type 2 diabetes mellitus. Eur J Clin Pharmacol 68, 961–968 (2012). https://doi.org/10.1007/s00228-011-1207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1207-0