Abstract
Purpose
Cyclosporine A (CyA), a potent immunosuppressive agent used in renal transplantation, has a narrow therapeutic window and a large variability in blood concentrations. This study aimed to develop a population pharmacokinetic (PPK) model of CyA in living-donor renal transplant patients at a single center and identify factors influencing CyA pharmacokinetics (PK).
Methods
A total of 660 points (preoperative) and 4785 points (postoperative) of blood concentration data from 98 patients who underwent renal transplantation were used. Pre- and postoperative CyA model structure and PPK parameters were separately estimated with a non-linear mixed-effect model, and subsequently, covariate analysis of postoperative data were comprehensively estimated, including preoperative PK parameters.
Results
A two-compartment model with first-order absorption and absorption lag time was selected in this study. Aspartate aminotransferase, body surface area (BSA), pretransplant area under the whole blood concentration–time curve/dose, and postoperative days were identified as the covariates on oral clearance. BSA was selected as a covariate of the distribution volume of the central compartment. In addition, diabetes mellitus was selected as a covariate of the first-order absorption rate.
Conclusions
This PPK study used the largest number of blood concentration data among previous reports of living-donor renal transplant patients. Moreover, all patients received the same immunosuppressive regimen in a single center. Therefore, the validity of the selected covariates is reliable with high precision. The developed PPK model and selected covariates provide useful information about factors influencing CyA PK and greatly contributes to the identification of the most suitable dosing regimen for CyA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute allograft rejection is an extremely severe complication of organ transplantation and is associated with a reduction in patient survival rates [1]. Therefore, adequate immunosuppression is one of the most important factors in the management of transplant recipients [2]. Cyclosporine A (CyA), a calcineurin inhibitor, is a potent immunosuppressive agent used in organ transplantation (e.g., heart, liver, and kidney) [1, 3, 4]. However, CyA has a narrow therapeutic window and numerous factors influence its pharmacokinetics (PK), including patient demographics, diet, underlying disease, and concomitant drugs [5, 6]. Thus, adjusting the CyA dosage is difficult. Therefore, patients receiving CyA should be closely monitored for treatment effectiveness; acute graft-versus-host disease onset; and various adverse effects, such as CyA-induced acute renal dysfunction and opportunistic infections [7, 8]. In contrast, daily monitoring of CyA AUC is an impractical approach that requires multiple samplings of at least three to four time points. A number of medical institutions currently routinely monitor the predose (trough, C0) and 2-h postdose (C2) CyA concentrations as predictors for dose adjustment [9, 10]. Population PK (PPK) is a useful and valid approach to estimate individual blood concentration–time profiles, which is a Bayesian estimation from sparse data [11, 12]. Numerous previous studies have developed the available PPK models of CyA [13,14,15,16]; however, these studies reported large inter-individual and inter-facility PK variabilities. Nevertheless, there is currently little consensus regarding the covariates that influence CyA PK, suggesting the influence of yet unidentified factors. This study also considered preoperative PK as a candidate covariate because these data included the patients’ original PK characteristics. If accurately predictable, it will also lead to greater effectiveness and safety of immunosuppressive therapy. The aims of this study were to develop a PPK model of CyA in living-donor renal transplant patients and identify factors influencing CyA PK by comprehensive analysis at a single center.
Patients and methods
Patients and studies
This retrospective study included 660 points (preoperative) and 4785 points (postoperative) of whole blood concentration data from 98 patients with living-donor renal transplantation who were treated with CyA at the University Hospital, Kyoto Prefectural University of Medicine from February 2005 to February 2013. Exclusion criteria were a history of functional gastrointestinal disorders. The characteristics of the study population are presented in Table 1. All patients were treated with the same immunosuppressive therapeutic regimen comprising nifedipine (for renal hypertension), azole antifungal drugs, and lafutidine (to prevent steroid-induced gastric ulceration). The study protocol was approved by the Ethics Committee of Kyoto Prefectural University of Medicine and was conducted in concordance with the ICH Guidelines for Good Clinical Practice [17]. In addition, informed consent was obtained from all patients prior to participation in this study.
Immunosuppressive regimen of CyA
Immunosuppressive therapy was performed according to the following regimen. Initial oral administration of CyA (Neoral®; Novartis Pharmaceuticals, East Hanover, NJ, USA) at a dose of approximately 12 mg/kg/day for 2–5 days before transplantation (preoperative CyA administration). On the day of transplantation, CyA (Sandimmun®; Novartis Pharmaceuticals) was continuously infused at 4 mg/kg/day, and after surgery, CyA (Neoral®) was orally administered twice daily after meals at a dose of approximately 12 mg/kg/day. The CyA dose was adjusted according to the collected daily C0 (target range, 200–300 ng/mL) and weekly AUC0–9 (target range, 5000–6000 ng h/mL) [18]. On the day of transplantation, methylprednisolone was also continuously infused at 500 mg/day. Subsequently, prednisolone was orally coadministered at 50 mg/day until postoperative day 3 (POD3), 40 mg/day (POD4–11), 30 mg/day (POD12–18), 25 mg/day (POD19–25), 20 mg/day (POD26–32), 15 mg/day (POD33–39), and 10 mg/day (from POD40 onward). In addition, azathioprine (1–1.5 mg/kg/day) or mycophenolate mofetil (20–25 mg/kg/day) was added to the regimen on POD21. This immunosuppressive CyA regimen was developed by the Department of Transplantation and Regenerative Surgery, Kyoto Prefectural University of Medicine.
Sample collection and bioanalysis of CyA
PK studies for determining AUC0–9 were performed from the oral administration at weekly intervals pre- and postoperatively. In all PK studies, a series of whole blood samples were collected at 0, 1, 2, 3, 4, 6, and 9 h after administration of CyA. AUC0–9 was calculated using the linear trapezoidal rule up to the trough sampling point. Furthermore, the C0 of CyA was monitored daily until discharge. In this study, the PK of CyA in individual patients was estimated at initiation of oral administration (2–5 days before transplantation). All whole blood CyA concentrations were determined using an antibody-conjugated magnetic immunoassay with the Dimension Xpand® system (Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). The lower limit of detection was >25 ng/mL. The relative standard error (RSE) of inter- and intra-assay reproducibility was 5–10% according to the International Proficiency Testing Scheme (DW Holt, Analytical Services International, Ltd., London, England).
PPK modeling
Pre- and postoperative CyA model structure and PPK parameters were separately estimated with a non-linear mixed-effect model using Phoenix® NLME™ 1.4 software (Certara LP, Princeton, NJ, USA), and PPK parameters were estimated by the first-order conditional estimate-extended least squares method. Several compartment models, including one-, two-, and three-compartment models with first-order absorption, with or without an absorption lag time (T-lag), were evaluated on the basis of likelihood ratio tests and visual assessment of goodness-of-fit (GOF) plots. The results of likelihood ratio tests were statistically evaluated by the chi-square test of the difference in objective function value (OFV; 2 log-likelihood) between the two nested models. A decrease in OFV of >3.84 (P ≤ 0.05, one degree of freedom), 5.99 (P ≤ 0.05, two degrees of freedom), 6.63 (P ≤ 0.01, one degree of freedom), and 9.21 (P ≤ 0.01, two degrees of freedom) was required for confirmation. As a result of the structural model selection, a two-compartment model with first-order absorption and T-lag was the best fit for both pre- and postoperative data (see below). The structural PK parameters were the apparent CyA distribution of the volumes of the central compartment [V1/F (L)] and peripheral compartment [V2/F (L)], first-order absorption rate constant [Ka (h−1)], oral or distribution clearance [CL/F or CLD2/F (L/h)], and T-lag (h). All inter-individual error models were described using the following exponential error model:
where P i is the PK parameter for the ith individual, θ p is the population mean value of the parameters, and η i is a normally distributed inter-individual random effect of mean 0 and variance ω 2. The residual error model was described using a proportional error model as follows:
where C obs,ij and C pred,ij are the jth observed and predicted concentrations of the ith individual, respectively, and ε is a normally distributed residual error with mean 0 and variance σ 2.
Covariate analysis
Covariate analysis of postoperative data was performed using a stepwise forward addition procedure (P ≤ 0.05) followed by a backward elimination procedure (P ≤ 0.01). The following covariates were comprehensively evaluated: nine demographic parameters [including sex (male, 0; female, 1), age, height, body weight (BW), body mass index (BMI), lean body weight (LBW), and body surface area (BSA)], 26 biological tests [including hematocrit, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and serum creatinine (SCr)], underlying diseases [including diabetes mellitus (DM)], and POD. Furthermore, in this study, the estimated individual preoperative PK parameters (structure and secondary parameters) were also evaluated as candidate covariates (Table 2). These preoperative PK parameters were estimated by a post hoc method using the PPK models [12]. LBW and BSA were calculated according to methods reported by Yanaga [19] and Du Bois [20], [LBW (male) = 1.1 × total BW − 0.0128 × BMI; LBW (female) = 1.07 × BW − 0.0148 × BMI; and BSA = BW0.425 × height0.725 × 0.007184]. Some of the biological tests were classified as normal or abnormal (normal, 0; abnormal, 1), according to the clinical examination guidelines for JSLM2015. The cut-off values were <40% (male) or <35% (female) for hematocrit, >35 IU/L for AST, >30 IU/L for ALT, >20 mg/dL for BUN, and >1.0 mg/dL (male) or >0.8 mg/dL (female) for SCr. Continuous covariates were introduced using a power type (Eq. 3) or liner type (Eq. 4), discrete covariates were introduced using a category type (Eq. 5), and POD was introduced using the Michaelis–Menten type (Eq. 6), as follows:
where P i is the PK parameter for the ith individual, θ p is the population mean value of the parameters, θ cov and θ′ cov are coefficients of the covariate, COV i is the covariate value for the ith individual, and COV median is the median value of the covariates in the study population (Tables 1 and 2). All of the selected candidate covariates were evaluated according to the difference in the OFV and graphic inspection of the relationships between estimated individual PK parameters and covariate plots.
Evaluation and validation of the PPK model
All candidate model structures and the accuracy of the PPK parameters were evaluated and validated by the RSE% and η-shrinkage for the estimated PPK parameters, likelihood ratio test, GOF plots, non-parametric bootstrap method, and the prediction accuracy test. The adequacy of the candidate and final PPK model structures were visually evaluated by four GOF plots: population prediction (PRED) vs. observation (OBS), individual prediction (IPRED) vs. OBS, time after dose (TAD) vs. conditional weighted residuals (CWRES), and PRED vs. CWRES. The non-parametric bootstrap method was used to evaluate the precision and robustness of the final PPK model. A total of 1000 replicated datasets were reconstructed by random sampling with replacement from the original dataset. Median values and 95th percentile confidence intervals (95th CI) of estimates obtained from the bootstrap replications were compared with those obtained from the original dataset. The prediction accuracy test of all blood concentrations of CyA was assessed by the percentage mean prediction error (MPE, bias) and the percentage mean absolute prediction error (MAPE, precision), respectively [24]:
where N indicates the total number of observations and X obs,i and X pred,i represent individual observations and the corresponding prediction of all blood concentrations of CyA, respectively. C0 and AUC0–9, as target indicators, were also evaluated by the same prediction accuracy test.
Results
Demographics and patient disposition
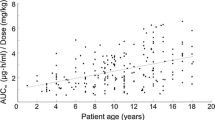
Patient characteristics are summarized in Table 1. Only BUN and SCr showed a marked improvement at POD7 as compared with preoperative data, whereas there was no significant difference in any other patient characteristics (according to the one-way repeated measures analysis of variance followed by the Tukey’s test). Moreover, there was no significant difference in the postoperative characteristics between patients with and without DM (by unpaired t test). A total of 660 points (preoperative) and 4785 points (postoperative) of dose-normalized whole blood concentration data obtained from 98 patients are shown in Fig. 1; both groups exhibited high inter-patient variability. The percentages within the target C0 range and AUC0–9 range of CyA were 60.7% (below the target range, 23.0%; above the target range, 16.2%) and 54.5% (below the target range, 8.50%; above the target range, 36.4%), respectively.
Final PPK model of CyA
The estimated PPK parameters obtained from the final PPK model are summarized in Table 3. As a result of the likelihood ratio tests and visual assessment of GOF plots, the best fit model was a two-compartment model with first-order absorption and T-lag in both pre- and postoperative analysis. The covariate evaluation and estimated covariate parameters obtained from the final PPK model are summarized in Tables 3 and 4. Candidate covariates were comprehensively analyzed. Table 4 shows the final selected covariates. AST, BSA, pre-AUC/dose, and POD were identified by stepwise selection as covariates of CL/F. BSA was selected as a covariate of V1/F. In addition, DM was selected as a covariate of Ka.
Validation of the PPK model
The GOF plots of the final PPK model are shown in Fig. 2. The plots of PRED vs. OBS and IPRED vs. OBS were symmetrically distributed and close to the identity line at Y = X (Fig. 2(i, ii)), indicating good prediction. The plots of TAD vs. CWRES and PRED vs. CWRES showed no trend and were randomly scattered around the identity line at CWRES = 0 (Fig. 2(iii, iv)), indicating the suitability of the error model for this study population. The RSE% and η-shrinkage results for the estimated PPK parameters and bootstrap validation are presented in Table 3. The ranges of RSE% and η-shrinkage for the estimated PPK parameters were 1.51–8.13% and 7.2–35.8%, respectively. All PPK parameters were very close to the median values obtained from the bootstrap replications (n = 1000; success rate, 100%) and fell within the 95th CI, indicating good robustness and accuracy of the final PPK model. The prediction accuracy test results of the basic and final PPK models are shown in Table 5. MPE and MAPE of the final PPK model were both closer to 0% than those of the basic PPK model, also indicating high prediction accuracy of the final PPK model.
Goodness-of-fit plots for a population pharmacokinetic model. i PRED vs. OBS. ii IPRED vs. OBS. iii TAD vs. CWRES. iv PRED vs. CWRES. Solid lines (i, ii) represent Y = X and (iii, iv) zero conditional residuals; dashed lines (iii, iv) represent ±2 SD. CWRES conditional weighted residuals, IPRED individual predictions, OBS observations, PRED population predictions, TAD time after dose
Discussion
In this study, a PPK model of CyA in Japanese patients who underwent living-donor renal transplantation in single center was developed by comprehensive analysis. This two-compartment model with first-order absorption and T-lag was analyzed by the blood concentration data of 5445 points from 98 patients. This population size is the largest reported yet; therefore, these results are highly reliable and precise. The estimated pre- and postoperative population means of CyA PPK parameters were similar to those of previous studies using the same model [13]. On the other hand, there was a significant difference between the estimated individual pre- and postoperative PK parameters of V1/F, T-lag, and V2/F (P ≤ 0.01 by paired t test). Thus, the various treatments during renal transplant have an influence on CyA PK (absorption and distribution).
Orally administered CyA is absorbed from the upper part of the small intestine as a microemulsion [21]. Most of the absorbed CyA is subsequently metabolized by hepatic cytochrome P450 (CYP) and excreted via the biliary route [22]; therefore, DM and AST were statistically significant covariates associated with a decrease in Ka and CL/F, respectively. PK alteration of various drugs has been reported in DM [23, 24]. For example, P-glycoprotein (P-gp) expression in intestinal epithelial cells is involved in intestinal absorption of CyA, a P-gp substrate. In particular, P-gp expression is reportedly induced by type 2 DM [25]. Thus, DM was selected as a covariate of Ka. The CL/F of CyA decreased with increasing AST, reflecting hepatic dysfunction. In many cases, the metabolic capacity of CyA is decreased by hepatic dysfunction; thus, AST was selected as a covariate of CL/F. However, the influence of DM or elevated AST on CyA PK was relatively small as either covariate led to a reduction of approximately 0.8 times. Conversely, we have experienced that DM patients are susceptible to alterations of the CyA absorption phase, including absorption delays and peak blood concentration reductions. Thus, caution is required when treating patients with DM or acute/chronic hepatic injury with CyA.
Anthropometric parameters are correlated with drug distribution and metabolism. Covariate analysis showed that BSA was a highly significant factor influencing CL/F and V1/F. In fact, BSA induced up to 1.5 and 2.7 times difference in CL/F and V1/F, respectively (Table 3). BW is the most widely used marker for dose adjustment of drugs. It reported that BW influences V1/F and/or CL/F of CyA [13,14,15]; however, BW does not adequately reflect other patient characteristics, such as body fluid volume and body fat percentage. Conversely, BSA is highly correlated with several physiological functions [26, 27]. CyA is well distributed in fat tissue [28]. In fact, BSA is better correlated with body fat percentage [29]. Further, BSA is reportedly a significant covariate of CL [30]. Thus, the results of the present and previous studies suggest that BSA is a more important factor of dose adjustment of CyA than BW.
If the inter-individual variability of a drug can be completely explained by any covariate; the individual dosing strategy of a drug would be markedly improved. Preoperative information on the inter-individual variability of CyA PK may be useful for the dosing strategy after renal transplantation. Because numerous known and unknown factors may be involved, it is impractical and difficult to predict the inter-individual variability before renal transplantation. Therefore, in this study, the individual PK parameters of CyA before renal transplantation (preoperative PK parameters) were evaluated as a covariate of the postoperative PPK. Herein, pre-AUC/dose, which well reflects the blood clearance of drugs, was found to be a statistically significant covariate of postoperative CL/F (Table 4). Likewise, due to the above reasons, it is considered that the numerous known covariate factors, such as hematocrit, polymorphisms of CYP3A5, and underlying diseases [15], were not selected as covariates of CL/F.
CyA PK is known to change with the postoperative course. There have been several conflicting studies about the change to CyA PK with POD [14,15,16]. Irtan et al. [16] reported a statistically significant increase in CL/F with POD after renal transplantation. In contrast, Wu et al. [14] suggested a decrease in CL/F by an increase in bioavailability with POD after renal transplantation. However, no final consensus has yet been reached. Our results, which showed increasing CL/F with POD, are more reliable than those of previous studies because of the larger population size. In our comprehensive analysis, CL/F was the most significant covariate of Michaelis–Menten type (Table 4). The estimated values of CL/F and θ12 (maximum effect of CL/F) were 17.1 L/h and 1.47, respectively (Table 3), i.e., the estimated CL/F with sufficient elapsed time approached 25.2 L/h. The estimated value of θ13 (the time required to obtain 50% of θ12) was 0.706 days (Table 3). This result shows that it takes approximately 7 days after renal transplantation for CL/F to recover to >90%. That is, more careful monitoring of CyA blood concentrations should be performed at an early phase after renal transplantation. Herein, BUN and SCr, which are indicators of renal function, showed a Michaelis–Menten type improvement by POD (data not shown). Furthermore, BUN and SCr were significantly improved after POD7 (BUN, 29.3 ± 0.9 mg/dL; SCr, 1.14 ± 0.04 mg/dL) as compared with immediately before renal transplantation (BUN, 53.3 ± 2.5 mg/dL; SCr, 9.46 ± 0.38 mg/dL). These results were in accordance with the recovery period (approximately 7 days) of CL/F in our study. Recovery of renal function reflects renal engraftment. Thus, renal engraftment may be a factor influencing the recovery of CyA CL/F in the post-transplantation period. Conversely, CYP3A inhibitors, such as coadministered prednisolone, have the potential to decrease the CL/F of CyA by competitive antagonism [31, 32]. The estimated CL/F on POD showed a weak correlation with the dose reduction of prednisolone, which may be the reason for the correlation of CL/F with POD. In addition, postoperative feeding and activities of daily living, a factor of alternating CyA PK, improved with time, which might be the reason for the correlation of CL/F with POD. These results suggest that POD is an important factor for dose adjustment of CyA in the post-transplantation period.
To the best of our knowledge, this PPK study used the largest number of blood concentration data among previous reports of living-donor renal transplant patients; moreover, all patients received the same immunosuppressive regimen in a single center. Therefore, the validity of the selected covariates is reliable with high precision. Furthermore, this study was the first to attempt a comprehensive analysis of preoperative PK data. In conclusion, several factors were found to influence CyA PK. AST, BSA, POD, and pre-AUC/dose were influential covariates of CL/F, and BSA and DM were influential covariates of V1/F and Ka, respectively. The developed PPK model and selected covariates provide useful information about factors influencing CyA PK and greatly contributes to identifying the most suitable CyA dosing regimen.
References
Eisen HJ, Hobbs RE, Davis SF et al (2001) Safety, tolerability, and efficacy of cyclosporine microemulsion in heart transplant recipients: a randomized, multicenter, double-blind comparison with the oil-based formulation of cyclosporine—results at 24 months after transplantation. Transplantation 71:70–78
MacDonald A (2001) Improving tolerability of immunosuppressive regimens. Transplantation 72:S105–S112
Kahan BD, Grevel J (1988) Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation 46:631–644
Burke JF Jr, Pirsch JD, Ramos EL, Salomon DR, Stablein DM, Van Buren DH, West JC (1994) Long-term efficacy and safety of cyclosporine in renal-transplant recipients. N Engl J Med 331:358–363. doi:10.1056/NEJM199408113310604
Fahr A (1993) Cyclosporin clinical pharmacokinetics. Clin Pharmacokinet 24:472–495
Wacke R, Rohde B, Engel G, Kundt G, Hehl EM, Bast R, Seiter H, Drewelow B (2000) Comparison of several approaches of therapeutic drug monitoring of cyclosporin A based on individual pharmacokinetics. Eur J Clin Pharmacol 56:43–48
Remuzzi G, Perico N (1995) Cyclosporine-induced renal dysfunction in experimental animals and humans. Kidney Int Suppl 52:S70–S74
Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ (1988) Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med 319:65–70. doi:10.1056/NEJM198807143190201
Billaud EM (2005) C2 versus C0 cyclosporine monitoring: still not the end. Transplantation 80:542
Knight SR, Morris PJ (2007) The clinical benefits of cyclosporine C2-level monitoring: a systematic review. Transplantation 83:1525–1535. doi:10.1097/01.tp.0000268306.41196.2c
Sheiner LB (1984) The population approach to pharmacokinetic data analysis: rationale and standard data analysis methods. Drug Metab Rev 15:153–171. doi:10.3109/03602538409015063
Thomson AH, Whiting B (1992) Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet 22:447–467. doi:10.2165/00003088-199222060-00004
Han K, Pillai VC, Venkataramanan R (2013) Population pharmacokinetics of cyclosporine in transplant recipients. AAPS J 15:901–912. doi:10.1208/s12248-013-9500-8
Wu KH, Cui YM, Guo JF, Zhou Y, Zhai SD, Cui FD, Lu W (2005) Population pharmacokinetics of cyclosporine in clinical renal transplant patients. Drug Metab Dispos 33:1268–1275. doi:10.1124/dmd.105.004358
Chen B, Zhang W, Gu Z, Li J, Zhang Y, Cai W (2011) Population pharmacokinetic study of cyclosporine in Chinese renal transplant recipients. Eur J Clin Pharmacol 67:601–612. doi:10.1007/s00228-010-0959-2
Irtan S, Saint-Marcoux F, Rousseau A, Zhang D, Leroy V, Marquet P, Jacqz-Aigrain E (2007) Population pharmacokinetics and bayesian estimator of cyclosporine in pediatric renal transplant patients. Ther Drug Monit 29:96–102. doi:10.1097/FTD.0b013e3180310f9d
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). http://www.ich.org. Accessed 12 November 2016
Kokuhu T, Fukushima K, Ushigome H, Yoshimura N, Sugioka N (2013) Dose adjustment strategy of cyclosporine A in renal transplant patients: evaluation of anthropometric parameters for dose adjustment and C0 vs. C2 monitoring in Japan, 2001-2010. Int J Med Sci 10:1665–1673. doi:10.7150/ijms.6727 eCollection 2013
Yanaga Y, Awai K, Nakaura T, Oda S, Funama Y, Bae KT, Yamashita Y (2009) Effect of contrast injection protocols with dose adjusted to the estimated lean patient body weight on aortic enhancement at CT angiography. AJR Am J Roentgenol 192:1071–1078. doi:10.2214/AJR.08.1407
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known. Nutrition 5:303–311
Nishi Y (2001) Neoral (Cyclosporin microemulsion preconcentrate): pharmacokinetics, pharmacodynamics and its improved clinical outcome. Nihon Yakurigaku Zasshi 118:107–115
Maurer G (1985) Metab cyclosporine Transplant Proc 17:19–26
Gwilt PR, Nahhas RR, Tracewell WG (1991) The effects of diabetes mellitus on pharmacokinetics and pharmacodynamics in humans. Clin Pharmacokinet 20:477–490. doi:10.2165/00003088-199120060-00004
Lee JH, Yang SH, Oh JM, Lee MG (2010) Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol 62:1–23. doi:10.1211/jpp.62.01.0001
Nawa A, Fujita-Hamabe W, Tokuyama S (2011) Altered intestinal P-glycoprotein expression levels in a monosodium glutamate-induced obese mouse model. Life Sci 89:834–838. doi:10.1016/j.lfs.2011.08.019
Halliday D, Hesp R, Stalley SF, Warwick P, Altman DG, Garrow JS (1979) Resting metabolic rate, weight, surface area and body composition in obese women. Int J Obes 3:1–6
Nawaratne S, Brien JE, Seeman E, Fabiny R, Zalcberg J, Cosolo W, Angus P, Morgan DJ (1998) Relationships among liver and kidney volumes, lean body mass and drug clearance. Br J Clin Pharmacol 46:447–452
Wagner O, Schreier E, Heitz F, Maurer G (1987) Tissue distribution, disposition, and metabolism of cyclosporine in rats. Drug Metab Dispos 15:377–383
Sardinha LB, Silva AM, Minderico CS, Teixeira PJ (2006) Effect of body surface area calculations on body fat estimates in non-obese and obese subjects. Physiol Meas 27:1197–1209. doi:10.1088/0967-3334/27/11/012
Zhou Y, Sheng XY, Xu JY, Bi SS, Lu W, Cui YM (2013) Population pharmacokinetic study of cyclosporine in the hematopoietic stem cell transplant recipients. Int J Clin Pharmacol Ther 51:568–575. doi:10.5414/CP201815
Konishi H, Sumi M, Shibata N, Takada K, Minouchi T, Yamaji A (2004) Influence of intravenous methylprednisolone pulse treatment on the disposition of ciclosporin and hepatic CYP3A activity in rats. J Pharm Pharmacol 56:477–483. doi:10.1211/0022357023114
Press RR, Ploeger BA, den Hartigh J, van der Straaten T, van Pelt H, Danhof M, de Fijter H, Guchelaar HJ (2010) Explaining variability in ciclosporin exposure in adult kidney transplant recipients. Eur J Clin Pharmacol 66:579–590. doi:10.1007/s00228-010-0810-9d
Acknowledgements
This study was supported by Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan (AO).
Author information
Authors and Affiliations
Contributions
AO, HU, Tadashi K, Takatoshi K, KF, NY, and Nobuyuki S equally contributed to the conception, design of the research, and acquisition of the data; AO, MK, AM, HK, AN, Nobuhito S, and KF contributed to the analysis of the data. All the authors critically revised the manuscript, read, and approved the final manuscript and agree to be fully accountable for ensuring the integrity and accuracy of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Okada, A., Ushigome, H., Kanamori, M. et al. Population pharmacokinetics of cyclosporine A in Japanese renal transplant patients: comprehensive analysis in a single center. Eur J Clin Pharmacol 73, 1111–1119 (2017). https://doi.org/10.1007/s00228-017-2279-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2279-2