Abstract
Background
The present study aimed to obtain information enabling optimisation of the clinical effect of mizoribine (MZR) in pediatric patients with kidney disease.
Methods
A total of 105 pediatric patients with kidney disease treated at our institutions were enrolled. Kidney transplant patients were excluded. Population pharmacokinetic analysis of MZR was performed based on serum concentration data. Area under the curve from time zero to infinity (AUC∞) and maximal concentration (C max) were calculated by Bayesian analysis.
Results
In children, the appearance of MZR in the blood tended to be slower and the subsequent rise in blood concentration tended to be more sluggish, compared to healthy adults. Apparent volume of distribution and oral clearance were also higher in children compared to adults. A significant positive correlation was observed between patient age and AUC∞. There were significant differences of AUC∞ and C max by age group. No relationship was observed between the administration method of MZR and serum concentration.
Conclusion
The pharmacokinetics of MZR was different in children compared to adults. To obtain the expected clinical efficacy, the regular MZR dosage schedule (2–3 mg/kg/day) might be insufficient for pediatric patients. In particular, younger patients might require a higher dosage of MZR per unit body weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mizoribine (MZR) is a selective inhibitor of inosine monophosphate dehydrogenase in the pathway responsible for de novo synthesis of guanine nucleotides, resulting in the suppression of T- and B-lymphocyte proliferation [1, 2]. MZR has been used successfully in the treatment of immune-mediated diseases, including transplantation [3], kidney diseases [4–7], and rheumatic diseases [8, 9] in both children and adults. MZR is highly safe compared to other immunosuppressive drugs, but has mild immunosuppressive effects with low doses up to 3 mg/kg/day. Recent clinical reports have indicated that the efficacy of MZR may depend on the peak serum level of the drug [10–15]. Accordingly, a peak serum MZR level of at least 2.5–3.0 μg/ml is now thought to be needed to sustain long-term efficacy in the treatment of patients with glomerular diseases [11, 13]. However, when using the conventional daily low-dose MZR protocol (2–3 mg/kg) in patients with renal disease, the peak blood level of the drug usually remains at around 1.0 μg/ml [10], which may explain the previously reported relatively mild immunosuppressive efficacy of MZR in clinical practice [4, 12]. Furthermore, no correlation exists between the dosage of MZR and the serum concentration, and individual differences are large [3]. This has led to differences in the evaluated clinical efficacy of MZR.
Evaluation of drug efficacy requires pharmacokinetic parameters estimated using several serum samples. However, Bayesian analysis can be used to predict individual pharmacokinetic parameters from a small number of concentration data points [16], allowing pharmacokinetic parameters to be determined from a limited number of samples. Accordingly, an optimal MZR administration schedule for children has been sought. To date, however, population pharmacokinetic (PPK) data have only been available for healthy adults [17].
In the present study, to establish the optimal dosage schedule for obtaining the expected clinical effect of MZR in pediatric patients with kidney disease, PPK analysis of MZR was performed at our institutions, and we evaluated the pharmacokinetic characteristics of MZR.
Patients and methods
A total of 105 pediatric patients with kidney disease being treated at our institutions were targeted, and testing was performed 213 times. Kidney transplant patients were excluded. In all patients, kidney function was maintained (creatinine clearance, ≥90 ml/min/1.73 m2 based on the new Schwartz equation [18]). The study was conducted in remission phase of the patients’ diseases. The patients had no edema and their serum albumin levels were normal. Some subjects had been administered steroids or other immunosuppressants concomitantly with MZR. Background data for the studied patients and details of their diseases are shown in Table 1. In conducting the study, we provided a full explanation of the details in writing to all the patients and their guardians, and asked them to provide written consent, as approved by the ethics committee of each participating institution. The study conformed to the tenets of the 2000 Declaration of Helsinki. The study was approved by the local ethics board, and written informed consent was obtained from the parents of each subject. The ethics committee approval number in Aichi Children’s Health and Medical Center is 200605.
Pharmacokinetic data
MZR [Bredinin®; (AsahiKASEI, Tokyo)] was generally administered once daily or twice daily before meals (before 30 min to just before the start of a meal). In some patients who were given multiple administrations, postprandial administration (from just after to 2 h after the end of a meal), and administration between meals (on empty stomach from 2 h after the end of a meal to 30 min before the start of the next meal) were also tested in this study. The MZR dosage for target patients was 1.0–13.5 mg/kg (40–650 mg) per administration. For serum collection, a blood vessel was secured by inserting an indwelling needle, and serum was sampled just before oral administration and from 1 to 24 h after oral administration, at a total of 1–13 time points. Testing was performed 1–7 times. Collected serum samples were centrifuged (3000 rpm, 15 min), and separated serum samples were stored frozen at −20 °C or below until concentration analysis.
Serum MZR concentrations were determined by Asahi Kasei Pharma by high-performance liquid chromatography (HPLC) according to the method of Hosotsubo et al. [19]. The quantification limit of MZR was 0.05 μg/ml in serum.
Population pharmacokinetic analysis
Using serum MZR concentration data for a total of 984 points in 213 pharmacokinetic trials as a base, PPK parameters and inter-individual variations were estimated using the NONMEM program (Version 5 Level 1.1 [20]). Estimation of PPK parameters was performed using the first-order method based on the assumption that the apparent volume of distribution correlated with oral clearance. Good absorption of MZR from the digestive tract of animals and adult humans has been demonstrated, and in each case, MZR was excreted by the body without being metabolized [21]. A one-compartment model was, therefore, adopted for the present pharmacokinetic analysis. Using a one-compartment model in which first-order absorption was assumed to be the analytical model, ADVAN2 and TRANS2 were selected from NONMEM-PREDPP library subroutines, and the absorption lag time (ALAG), absorption rate constant (KA), apparent volume of distribution (V/F), and oral clearance (CL/F) were calculated (where F denotes bioavailability). We estimated PPK parameters based on the assumption that the apparent volume of distribution correlated with oral clearance. Furthermore, the obtained PPK parameters were assumed to show a normal logarithmic distribution. The equations used for the calculation of the various parameters were as follows.
Absorption lag time in the ith pharmacokinetic trial (ALAG i ):
where θ 1 is the predicted population mean of the absorption lag time (in h), and \( \eta_{{{\text{ALAG}}_{i} }} \) is a random variable distributed normally with a mean of zero and variance of \( \omega_{\text{ALAG}}^{2} \).
Absorption rate constant in the ith pharmacokinetic trial (KA i ):
where θ 2 is the predicted population mean of the absorption rate constant (in h−1), and \( \eta_{{{\text{KA}}_{i} }} \) is a random variable distributed normally with a mean of zero and a variance of \( \omega_{\text{KA}}^{2} \).
Apparent volume of distribution (V/F i ) and oral clearance (CL/F i ) in the ith pharmacokinetic trial:
where WT is the body weight (in kg), and θ 3 · WT is the predicted population mean of the apparent volume of distribution (in l). CLcr is the creatinine clearance (in ml/min), and θ 4 · CLcr · 60/1000 is the predicted population mean of oral clearance (in l/h). Random variables, \( \eta_{{V/F_{i} }} \) and \( \eta_{{CL/F_{i} }} \), are assumed to be distributed normally with means of zero and co-variance of \( \omega_{V/F}^{2} \), ω V/F,CL/F , and \( \omega_{{{\text{CL}}/F}}^{2} \).
Creatinine clearance (CLcr) (in ml/min) was calculated as follows:
where HT is height (in cm), WT is body weight (in kg), BSA is body surface area (in m2), and S cr is the serum creatinine concentration (in mg/dl).
Finally, the jth observed serum concentration in the ith pharmacokinetic trial (C ij ) was assumed to be randomly and normally distributed from the predicted value\( (C_{ij}^{*} ) \):
where ε ij is a random variable that describes intra-individual variability with a mean of zero and a variance of σ 2.
Calculation of parameters in each case was performed using ALAG, KA, V/F and CL/F obtained by Bayesian analysis. In addition, calculation of pharmacokinetic parameters [area under the curve from time zero to infinity (AUC∞), maximal concentration (C max), and maximal serum concentration time (T max)] in each case was performed using the pharmacokinetic analysis software package WinNonlinR Ver 5.2 (Pharsight Corp., Mountain View, CA, USA).
Effects of aging on pharmacokinetic parameters
Patients overall were divided into three age groups: infants and preschool-age children (<6 years old); school-age children (6–11 years old); and adolescents (>11 years old). Classification into age groups was performed with reference to “Guidance for clinical studies of drugs in child population (2000)” (Ministry of Health, Labour and Welfare, Japan). Patients who underwent the test twice or more belonging to more than one age group were counted separately. Differences in AUC∞, C max and T max among age groups were examined on the basis of changes in serum concentration in each patient calculated by Bayesian analysis. Units for MZR dosage were unified to milligrams per kilogram body weight, and all AUC∞ and C max values were corrected based on the dosage received in each case. All T max values were evaluated without any correction.
Effects of MZR administration method on pharmacokinetic parameters
For examination of the effects of meals on pharmacokinetics, patients were divided into three groups according to differences in administration method: preprandial administration; postprandial administration; and inter-meal administration. Effects on AUC∞ and C max were then examined. In the present examination, none of the patients who underwent multiple tests were reassigned to different administration groups.
Statistical analysis
Data obtained are expressed as mean ± standard deviation (SD), and are shown with the coefficient of variation (%), as an index of scattering. To determine correlations, the regression equation and Spearman’s correlation coefficient (rs) were obtained using the statistical analysis software package SPSS Statistics (IBM SPSS, Tokyo, Japan), and the significance of rs was tested by Spearman’s correlation coefficient test. To examine the effects of meals and age differences, significance was tested using Scheffe’s F test. In each test, the significance level was set at 5 %.
Results
Pediatric population pharmacokinetic parameters of MZR
Means and 95 % confidence intervals for each PPK parameter when MZR was administered to pediatric patients, as calculated by NONMEM analysis, are shown in Table 2. Each 95 % confidence interval was obtained as the mean ± 1.96 · standard errors of the mean (SE). Mean values of ALAG and KA were calculated as 0.513 h and 0.793 h−1, respectively. In comparison with the known average values for healthy adults (ALAG, 0.349 h; KA, 0.869 h−1) [17], the appearance of MZR in the blood tended to be slower, and the subsequent rise in blood concentration tended to be more sluggish. Mean values of V/F and CL/F were also calculated to be 1.29 · WT L and 2.79 · CLcr L/h, respectively, and were higher than those of known average values in healthy adults (V/F, 0.834 · WT L; CL/F, 1.93 · CLcr L/h) [17]. Furthermore, based on the ω value of each parameter, significant inter-individual variations of MZR pharmacokinetics were observed in children. Although examples of serum MZR concentration–time curves in individual patients prepared from PPK parameters are not shown, the predictability determined by Bayesian analysis was mostly good.
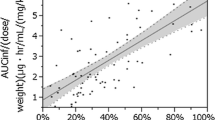
Linearity between patient age and AUC∞
Results are shown in Fig. 1. A significant positive correlation was observed between age and AUC∞ (rs = 0.422, p < 0.0001). Although the data are not shown in the figure, we also examined data by converting MZR dosage units to milligram per square meter of body surface area and milligrams per milliliter total volume of body fluid in patients, and the results were seen to be similar to those obtained with milligrams per kilogram body weight.
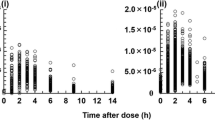
Relationship between patient age and AUC∞, C max and T max
Statistical characteristics for each age group are shown in Table 3. Results for AUC∞ and C max are presented in Fig. 2a, b. Upon comparison of the mean ± SD, each parameter showed significant differences among age groups, and a tendency for an increase with age was observed [AUC∞/dose (μg h/ml)/(mg/kg); 1.52 (<6 years), 2.36 (6–11 years), 3.37 (>11 years), C max/dose (μg/ml)/(mg/kg); 0.30 (<6 years), 0.43 (6–11 years), 0.51 (>11 years)]. On the other hand, mean T max was calculated as 2.510 ± 0.695 h for all groups, almost the same as the known T max for healthy adults (median T max ranges from 2 to 2.5 h for 3, 6, 9 and 12 mg/kg MZR) [21].
Relationship between administration method and AUC∞, C max
Results are shown in Fig. 3a, b. In the present examination, the number of patients with postprandial and inter-meal administration was lower than that of patients with preprandial administration. However, no significant differences were detected among these groups.
Discussion
For accurate evaluation of the efficacy of MZR in pediatric patients, we considered it necessary to clarify the pharmacokinetic parameters specific to children, and conducted a large-scale multicenter PPK analysis. We established details of MZR pharmacokinetics for pediatric groups separated by different parameters, and slower absorption of the drug from the digestive tract in comparison with adult males was demonstrated [17]; higher levels of both V/F and CL/F were clarified. For V/F, a large difference was identified between children and adults. There are two possible reasons for this: lower bioavailability of the drug in children; and/or a larger actual distribution volume (Vd) in children, in comparison to adults. We are planning to clarify whether bioavailability and Vd change with age or remain constant.
Marked individual differences in MZR pharmacokinetics in children are frequently experienced in routine clinical settings. We used AUC∞ as an index reflecting the actual proportion of dose absorbed in the body, and examined the relationship between patient age and AUC∞. Furthermore, to consider cases of similar AUC∞ values even when different amounts of the drug were administered, all AUC∞ values were divided by the oral dose for comparative examination. This correction allowed the bioavailability in each individual to be reflected.
We first examined the correlation between patient age and AUC∞, revealing a significant positive correlation (Fig. 1). This suggests that AUC∞ decreases due to reductions in the oral bioavailability of MZR in younger patients. Furthermore, a tendency for up- and down-scattering of AUC∞ values was seen among individuals in the same age groups. We will examine the possibility that factors other than patient age have an effect on the pharmacokinetics of MZR, particularly its bioavailability.
In a comparison of age-related MZR efficacy (Fig. 2), both AUC∞ and C max per unit dosage of MZR increased with patient age, and the inter-group differences were significant. On the other hand, T max is almost the same as in healthy adults. Measurement of blood concentrations at 2 h (C2) or 3 h (C3) after MZR administration was considered important in the calculation of pharmacokinetic parameters in individual patients. When MZR is used for the treatment of pediatric kidney disease, a strategy for increasing the dosage for single administration becomes necessary.
An experiment using a rat small intestine loop model has demonstrated competitive inhibition of MZR absorption with concomitant use of nucleic acids [22], and possible effects of the time after a meal until administration or the contents of a meal on MZR absorption have been indicated for postprandial administration. Although the comparison was performed in a small number of cases (Fig. 3), no significant differences were detected among the preprandial, postprandial and inter-meal administration groups. We have previously demonstrated that equilibrative nucleoside transporters (ENTs) are largely responsible for absorption of MZR using the rat small intestine [23], and the lack of an effective administration method may be reflected in the fact that ENT plays a major role in MZR absorption in humans.
Recent studies have shown that serum MZR concentrations >2.5–3.0 μg/ml are sufficient for an expectation of clinical efficacy [11, 13]. From the findings in this study, high-dose treatment of MZR should be applied for pediatric patients to obtain the expected clinical effect. The estimated optimal dose to obtain 3.0 μg/ml in each age is 10.00 mg/kg (<6 years), 6.97 mg/kg (6–11 years) and 5.88 mg/kg (>11 years). Whether a significant relationship exists between C max/AUC of MZR and clinical efficacy remains unclear. Further studies with high-dose MZR treatment based on the findings in this study are necessary to clarify the optimal dosage schedule for obtaining expected clinical efficacy.
In this study, some patients had been administered steroids or other immunosuppressants concomitantly with MZR. These drugs might influence the pharmacokinetics of MZR. This is an important limitation of this study. Further studies to clarify the effects of concomitant drugs on the pharmacokinetics of MZR are necessary.
In conclusion, despite the limitations of this study, our findings suggest that high-dose treatment with MZR should be applied for pediatric patients to obtain the expected clinical effect of MZR. The optimal dose of MZR should be considered in each age for pediatric patients.
References
Yokota S. Mizoribine: mode of action and effects in clinical use. Pediatr Int. 2002;44:196–8.
Kawasaki Y. Mizoribine: a new approach in the treatment of renal disease. Clin Dev Immunol. 2009;2009:681482.
Sonda K, Takahashi K, Tanabe K, Funchinoue S, Hayasaka Y, Kawaguchi H, et al. Clinical pharmacokinetic study of mizoribine in renal transplantation patients. Transplant Proc. 1996;28:3643–8.
Yoshioka K, Ohashi Y, Sakai T, Ito H, Yoshikawa N, Nakamura H, et al. A multicenter trial of mizoribine compared with placebo in children with frequently relapsing nephrotic syndrome. Kidney Int. 2000;58:317–24.
Honda M. Nephrotic syndrome and mizoribine in children. Pediatr Int. 2002;44:210–6.
Kawasaki Y, Hosoya M, Suzuki J, Onishi N, Takahashi A, Isome M, et al. Efficacy of multidrug therapy combined with mizoribine in children with diffuse IgA nephropathy in comparison with multidrug therapy without mizoribine and with methylprednisolone pulse therapy. Am J Nephrol. 2004;24:576–81.
Yoshikawa N, Nakanishi K, Ishikura K, Hataya H, Iijima K, Honda M. Combination therapy with mizoribine for severe childhood IgA nephropathy: a pilot study. Pediatr Nephrol. 2008;23:757–63.
Tanaka H, Tsugawa K, Tsuruga K, Suzuki K, Nakahata T, Ito E, et al. Mizoribine for the treatment of lupus nephritis in children and adolescents. Clin Nephrol. 2004;62:412–7.
Yumura W, Suganuma S, Uchida K, Moriyama T, Otsubo S, Takei T, et al. Effects of long-term treatment with mizoribine in patients with proliferative lupus nephritis. Clin Nephrol. 2005;64:28–34.
Tanaka H, Suzuki K, Nakahata T, Tsugawa K, Ito E, Waga S. Mizoribine oral pulse therapy for patients with disease flare of lupus nephritis. Clin Nephrol. 2003;60:390–4.
Tanaka H, Tsugawa K, Suzuki K, Nakahata T, Ito E. Long-term mizoribine intermittent pulse therapy for young patients with flare of lupus nephritis. Pediatr Nephrol. 2006;21:962–6.
Kuroda T, Hirose S, Tanabe N, Sato H, Nakatsue T, Ajiro J, et al. Mizoribine therapy for patients with lupus nephritis: the association between peak mizoribine concentration and clinical efficacy. Mod Rheumatol. 2007;17:206–12.
Tanaka H, Oki E, Tsuruga K, Sato N, Matsukura H, Matsunaga A, et al. Mizoribine treatment of young patients with severe lupus nephritis: a clinicopathologic study by the Tohoku pediatric study group. Nephron Clin. Pract. 2008;110:c73–9.
Ohtomo Y, Fujinaga S, Takada M, Murakami H, Akashi S, Shimizu T, et al. High-dose mizoribine therapy for childhood onset frequently relapsing steroid-dependent nephrotic syndrome with cyclosporin nephrotoxicity. Pediatr Nephrol. 2005;20:1744–9.
Kawasaki Y, Takano K, Isome M, Suzuki J, Suyama K, Kanno H, et al. Efficacy of single dose of oral mizoribine pulse therapy two times per week for frequently relapsing nephrotic syndrome. J Nephrol. 2007;20:52–6.
Mahmood I, Miller R. Comparison of Bayesian approach and a limited sampling model for the estimation of AUC and Cmax: a computer simulation analysis. Int J Clin Pharmacol Ther. 1999;37:439–45.
Honda M, Itoh H, Suzuki T, Hashimoto Y. Population pharmacokinetics of higher-dose mizoribine in healthy male volunteers. Biol Pharm Bull. 2006;29:2460–4.
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37.
Hosotsubo H, Takahara S, Tanaka N. Simplified high-performance liquid chromatographic method for determination of mizoribine in human serum. J Chromatogr. 1988;432:340–5.
Beal S, Boeckmann A, Sheiner L. NONMEM users guides: NONMEM Project Group. San Francisco: University of California; 1992.
Stypinski D, Obaidi M, Combs M, Weber M, Stewart AJ, Ishikawa H. Safety, tolerability and pharmacokinetics of higher-dose mizoribine in healthy male volunteers. Br J Clin Pharmacol. 2007;63:459–68.
Okada M, Suzuki K, Nakashima M, Nakanishi T, Fujioka N. The nucleotide derivatives inosine and inosinic acid inhibit intestinal absorption of mizoribine in rats. Eur J Pharmacol. 2006;531:140–4.
Ishida K, Takaai M, Yotsutani A, Taguchi M, Hashimoto Y. Membrane transport mechanisms of mizoribine in the rat intestine and human epithelial LS180 cells. Biol Pharm Bull. 2009;32:741–5.
Acknowledgments
We thank Asahi Kasei Pharma, Tokyo, for measuring serum MZR concentrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
About this article
Cite this article
Kaneda, H., Shimizu, M., Ohta, K. et al. Population pharmacokinetics of mizoribine in pediatric patients with kidney disease. Clin Exp Nephrol 20, 757–763 (2016). https://doi.org/10.1007/s10157-015-1209-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-015-1209-9