Abstract
Flexibility in foraging strategy is an important mechanism by which seabirds cope with spatiotemporal heterogeneity in food availability and the variable energetic constraints of their annual life cycle. Foraging strategy flexibility was investigated in the grey-faced petrel Pterodroma gouldi breeding on Ihumoana Island (36°53′S, 174°26′E) using stable isotope analyses. Intra- and inter-annual variations in stable isotope values, isotopic niches and diet inferred from isotope mixing models were studied by analysing δ15N and δ13C in adult wing feathers and blood, chick down and body feathers, and muscle from spontaneously regurgitated prey, collected during 2013 and 2014 breeding seasons. Grey-faced petrels exhibited variations in stable isotopes, isotopic niches and diet more markedly throughout their annual life cycle than between years. A trophic segregation occurred between adults and chicks presumably from adults feeding inshore and chicks being fed more oceanic prey of higher trophic level. Stable-isotope mixing models revealed that adult diet during the breeding season could consist mainly of ram’s horn squids Spirula spirula and chick diet of crustaceans, fish and other cephalopods being secondary prey throughout the breeding season. Adult male and female isotopic niches slightly differed. Finally, isotopic niche in adults during non-breeding was similar to that during breeding, suggesting non-breeding foraging areas located off the eastern Australian coast, around the limit between the Tasman and Coral seas. Our results demonstrated plasticity in the foraging strategy of grey-faced petrels in response to the changing nutritional demands of their annual cycle and to changes in oceanographic conditions likely driven by El Niño Southern Oscillation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Energetic demands vary throughout the annual life cycle in animals and they are particularly high during reproduction because adults must balance their own energy requirements with those of their offspring (Stearns 1992; Bryant 1997; Whittow 2001; Sibly et al. 2012). This is especially the case for long-lived animals which have the potential to reproduce for many years. Striking examples of these long-lived animals are seabirds. In addition, population dynamics of these marine predators are generally driven by the abundance and availability of their prey (bottom-up control, Frederiksen et al. 2006) which are highly dynamic in marine systems. Flexibility in foraging strategy is thus an important mechanism by which seabirds cope with spatiotemporal heterogeneity in food availability (extrinsic factors) and the variable energetic constraints of their annual life cycle (intrinsic factors) (e.g. Kato et al. 2003; Shaffer et al. 2003; Tremblay and Cherel 2003; Peery et al. 2009; Zimmer et al. 2011; Ramos et al. 2015; Leal et al. 2017; Thabet et al. 2019). These energetic constraints are particularly high during breeding stages and moult. Optimal foraging theory predicts that animals adopt foraging strategies that provide the most energy for the lowest cost, maximizing net energy gain and thus fitness (MacArthur and Pianka 1966; Schoener 1971). In accordance with this theory, seabirds can adjust their foraging strategies in response to extrinsic and intrinsic factors. They can thus switch their diet to prey that are more accessible (more abundant and/or closer to their nesting grounds), temporally available or more energetic/nutrient-rich (Quillfeldt 2002; Navarro et al. 2009; Machovsky-Capuska et al. 2018). They can also forage at more or less distant and/or large areas (Leal et al. 2017; Ramos et al. 2018; Cerveira et al. 2020), and/or utilise different foraging habitats (Quillfeldt et al. 2005; Navarro et al. 2007; Cherel et al. 2014a; Geary et al. 2020).
During the breeding period, seabirds need to return to colonies regularly to take incubation shifts or feed their chicks, thus behaving as central place foragers (Orians and Pearson 1979). This adds further constraints to seabirds during the breeding period as their foraging ranges, and likely access to habitats and food resources, are reduced in comparison with the period outside reproduction when they may move continually through vast oceanic areas searching for food. In addition, breeding seabirds have to adapt their foraging strategies according to their breeding duties that vary throughout the stages of the breeding cycle, to the needs of their offspring and to spatiotemporal variation in food availability. This allows them to maximize foraging efficiency and reproductive output (Shaffer et al. 2003; Navarro et al. 2007; Cherel et al. 2014a; Chiaradia et al. 2016; Dehnhard et al. 2016; Leal et al. 2017; Booth et al. 2018). Moreover, to meet the high energetic demands of chick provisioning, some seabird species, or populations of a given species, particularly in Procellariiformes, are known to employ a dual-foraging strategy alternating between frequent, short, chick-provisioning foraging trips punctuated by long self-maintenance trips (Weimerskirch et al. 1994; Ropert-Coudert et al. 2004; Congdon et al. 2005; Steen et al. 2007; Magalhães et al. 2008; Shoji et al. 2015). During the longer trips, seabirds generally target more productive but more distant areas, such as inherently richer deep oceanic waters, seasonally stable sea fronts, marginal sea ice zones or continental shelf edges (Catard et al. 2000; Magalhães et al. 2008; Jakubas et al. 2012; Shoji et al. 2015). This results in the use of different foraging areas by adults for self-feeding and for chick-provisioning as well as in segregated trophic niches between adults and chicks (Catard et al. 2000; Alonso et al. 2012). However, trophic niche segregation between parents and offspring can also be explained by adults provisioning offspring with higher-quality food (prey of higher trophic levels) to facilitate their growth (e.g. Hodum and Hobson 2000; Cherel et al. 2008; González-Medina et al. 2017). Finally, seabird foraging strategies can also differ between sexes (e.g. Forero et al. 2005; Bearhop et al. 2006; Miller et al. 2018; Zango et al. 2020). While this may reflect sexual habitat or dietary specialization or avoidance of competition in sexually dimorphic species, this may result from sexually differing reproductive roles, parental investment, nutritional requirements or from risk partitioning in monomorphic or dimorphic species (Phillips et al. 2011; 2017). Sex-specific foraging strategies of seabirds tend to be more common in species with marked sexual size dimorphism and to be more likely during the pre-laying and later breeding stages than during the non-breeding period.
Stable isotope analysis of various tissues allows investigation of seabird foraging strategy flexibility over different time periods and at different time scales (e.g. whole blood is expected to retain information on diet and foraging habitat used for a period from 3 to 4 weeks previous and feathers for the period when they were grown), since different body tissues incorporate the isotopic values of resources at different rates (Hobson 1993; Hobson and Bond 2012; Cherel et al. 2014a; Thabet et al. 2019). Stable-nitrogen and stable-carbon isotope ratios (δ15N and δ13C, respectively) allow the determination of seabird isotopic niches (i.e. the δ-space they use, defined by an area with isotopic values as coordinates; Newsome et al. 2007). Hence, δ15N reflects seabird trophic position/diet, while δ13C reflects seabird spatial foraging distribution (Hobson et al. 1994; Kelly 2000), mostly in relation to coastal–offshore, benthic–surface and/or latitudinal gradients (Jaeger et al. 2010; Paiva et al. 2010; Roscales et al. 2011).
Using stable isotope analyses, this study investigates foraging strategy flexibility in the grey-faced petrel Pterodroma gouldi, a highly pelagic Procellariiform seabird endemic to the northern half of North Island, New Zealand (Greene et al. 2015; Wood et al. 2017). With hundreds of thousands of breeding pairs, the grey-faced petrel is one of the few burrowing seabird species remaining on the main island and in high abundance. As such, it has a vital role replenishing terrestrial areas with marine resources transported from the ocean and feeding grounds. Because of the tight link between grey-faced petrels and the health of marine ecosystems, New Zealand Department of Conservation uses the species as an indicator species for long-term monitoring of changes in the marine environment (Monks et al. 2013; Russell et al. 2017). An understanding of grey-faced petrel trophic ecology and foraging strategy can help identify causes for population declines and provide information about the changes in food web structure or in prey abundance and diversity across its foraging areas. El Niño Southern Oscillation (ENSO) affects New Zealand weather through changes in air pressure, sea temperature, and wind direction. The impact on oceanographic conditions can affect grey-faced petrel prey distribution and abundance. Over two consecutive years with different Southern Oscillation Indices (SOIs: a measure of ENSO strength), we analysed δ15N and δ13C in adult blood collected at two different breeding stages to characterise incubation and chick rearing. Over the same years, we analysed δ15N and δ13C in chick down and body feathers to characterise two distinct periods of the chick rearing stage, as well as in adult wing feathers to characterise the non-breeding period. Finally, we used δ15N and δ13C values of muscle from spontaneously regurgitated prey to infer petrel diet by stable isotope mixing modelling. We thus examined variations in grey-faced petrel foraging areas and diet throughout their annual life cycle, over consecutive years with different ENSO oceanographic conditions, between adults and chicks and between sexes, using stable isotope analyses. We specifically tested if (1) stable isotope values vary during annual cycle stages and over years, resulting from diet and foraging habitat segregation over time, (2) adults and chicks exhibit different stable isotope values, suggesting that adults have a different diet or habitat when feeding themselves vs their chicks, and (3) adult males and females have different stable isotope values, exploiting different prey or foraging habitats.
We expected to find significant intra- and inter-annual variations in grey-faced petrel stable isotope values, as found in other seabird species including Procellariiformes (e.g. Navarro et al. 2007; Alonso et al. 2012; Cherel et al. 2014a; Thabet et al. 2019), due to changes in nutritional and energy demands throughout the annual life cycle and to potential variation in food availability related to ENSO. We also hypothesized that, in accordance with the dual-foraging strategy which is common in Procellariiformes (Weimerskirch et al. 1994; Catard et al. 2000; Congdon et al. 2005; Magalhães et al. 2008; Shoji et al. 2015), adults feed their chick with more coastal prey than those they feed on for themselves. In addition, adult males and females could exhibit different stable isotope values from different foraging strategies, as observed in some other Procellariiformes with various levels of sexual size dimorphism (Forero et al. 2005; Quillfeldt et al. 2008b; Pinet et al. 2012; Hedd et al. 2014; Jaeger et al. 2014; Danckwerts et al. 2016; Paiva et al. 2018; Zango et al. 2020). Finally, because petrels generally spend their non-breeding period in areas several thousand kilometres away from their foraging areas during the breeding season (e.g. Rayner et al. 2012; Priddel et al. 2014; Ramos et al. 2017), we expected different stable isotope values in grey-faced petrels between these two periods.
Materials and methods
Study site and species
We studied grey-faced petrel on Ihumoana, a 1.7 ha island connected to the mainland at low tide and situated at the northern end of Te Henga (Bethells Beach) on the North Island of New Zealand (36°53′S, 174°26′E). Ihumoana hosts a grey-faced petrel breeding colony of approximately 120 pairs, that has been monitored (burrow occupancy, breeding success, adult and chick ringing) for over 20 years (G.A. Taylor unpubl data). This study was conducted during the breeding seasons of 2013 and 2014 which exhibited different SOIs (June–December: n = 7, 0.46 ± 0.46 in 2013 and − 0.50 ± 0.37 in 2014, paired t test, t6 = 7.4, P < 0.001; www.ncdc.noaa.gov/teleconnections/enso/indicators/soi/).
The grey-faced petrel is an austral winter breeder that nests in underground burrows. Potential breeders return to the colony to re-establish pair bonds in April. Females lay a single-egg clutch between late June and late July (Imber 1976). Chicks hatch from mid-August to early-September (26 August ± 8 days in 2013, n = 26), after both parents share egg incubation for an average of 55 days. Both parents contribute to chick provisioning during an average of 118 days of chick rearing. On Ihumoana, chicks fledge in December-early January (19 December ± 10 days in 2013, n = 23).
While at sea, adults of the grey-faced petrel forage widely in the southwest Pacific Ocean and the Tasman Sea between 20° and 50°S, feeding over deep pelagic water beyond the continental shelf (Marchant and Higgins 1990; MacLeod et al. 2008). They feed mainly on cephalopods, fish and crustaceans (Imber 1973), that they catch by surface seizing or dipping by night (Harper 1987). Grey-faced petrels thus undertake shallow dives infrequently as their average diving depth is 1.64 ± 0.18 m (average maximal depth: 2.41 ± 0.30 m; Dunphy et al. 2015). The most common prey during the breeding season is the ram’s horn squid Spirula spirula (small Spirulidae squid) but Histioteuthidae and Cranchiidae cephalopods dominate in terms of mass ingested (Imber 1973). Fish (mainly Myctophidae, the lantern-fishes, and Gonostomatidae) and crustaceans (Decapoda: Caridean prawns, Mysidacea: Lophogastridae, Euphausiacea: Euphausiidae, and Amphipoda: Hyperiidea) are secondary prey. Grey-faced petrels mainly feed on deep-sea prey which undertake diel vertical migration (vertical migration toward the surface at night and in deeper waters during the day). Only a few of their prey are available in daylight at the sea surface. Scavenging of fish and cephalopods supplements their diet.
Sample collection
We captured breeding adults and chicks by hand in burrow nesting chambers by reaching through the entrance for short burrows or, for longer burrows, through access holes, or on the ground close to burrow entrances when adults came back to feed the chick. Feather isotopic values retain information on diet and at-sea foraging habitat used when the feather was grown (Hobson and Clark 1992; Bearhop et al. 2002). We clipped 2–3 cm of the fourth primary covert from breeding adults (n = 60 in 2013 and n = 27 in 2014). The fourth primary covert is most likely moulted at the same time as the fourth primary (i.e. during the non-breeding period; Grecian et al. 2016). In order to represent the non-breeding period this feather is thus a safer alternative to primaries, whose collection can impair the birds’ flying ability (Jaeger et al. 2009). We sampled blood from breeding adults attending burrows during incubation (August; n = 26 in 2013, n = 20 in 2014) and chick rearing (November; n = 16 in 2013 only). We collected a maximum of 0.4 mL of blood from the tarsal vein. Blood samples were drawn into heparinised 0.5-mL disposable syringes through a 29-gauge needle. We dispensed the blood into two different small plastic vials, one containing 70% ethanol and the other one absolute ethanol. Blood samples stored in 70% ethanol were used for stable isotope analyses (see Hobson et al. 1997) while those stored in absolute ethanol were used for molecular analyses. By using the same brand and batch of ethanol for all sampling groups, we expected to minimize ethanol storage effects on stable isotope values (Bugoni et al. 2008). Whole blood isotopic value is expected to retain information on diet and at-sea foraging habitat used from 3 to 4 weeks prior to the sample collection (Bearhop et al. 2002). Therefore, blood that we collected in August and in November reflects nutrients ingested during incubation and mid-chick rearing, respectively. Adults were uniquely ringed but not systematically repeatedly sampled: some adults were thus sampled only once, while others were sampled several times (mean number of samples per individual ± SD: 1.8 ± 0.8, n = 85, range: 1–5). We collected secondary down (mesoptiles; Warham 1996) and 2–4 body (breast) feathers from the same chicks within a year (second half of November; n = 23 in 2013 and n = 22 in 2014). Secondary down reflects nutrients ingested subsequent to hatching during early chick rearing (Danckwerts et al. 2016) while chick body feathers reflect their period of growth during late chick rearing. Sexing of adults was previously performed molecularly or by cloacal inspection during the laying period, or the sex was deduced when the mate was reliably sexed. We stored all samples at room temperature (20–25 °C) once back in the laboratory.
Procellariiformes have a well described regurgitation response to handling or harassment by potential predators (Carey 2009). We thus collected grey-faced petrel prey items opportunistically whenever individuals regurgitated during handling. Regurgitations were stored frozen once back in the laboratory before a basic analysis isolating prey flesh for stable isotope analysis (SIA) and identifying prey types. Only a few regurgitations contained prey flesh usable for SIA (i.e. from an identifiable prey type and of sufficient size to be analysed). These prey samples for SIA were then stored in 70% ethanol for a few weeks until analysed. We acknowledge the fact that preservation in ethanol increases the prey muscle δ15N and δ13C values by about 0.7 ‰ (Kaehler and Pakhomov 2001; Ruiz-Cooley et al. 2011). Nevertheless, lipid extraction eliminates the effect of ethanol (Ruiz-Cooley et al. 2011) and trophic enrichment is in the range of 2–4 ‰ for δ15N (Cherel et al. 2005b; Williams et al. 2007). The stable isotope values in prey flesh samples were thus used to discuss the likely contributions of each prey type to the seasonal and annual differences in stable isotopic values of grey-faced petrels. We determined the occurrence of the main prey types (i.e. fish, cephalopods and crustaceans) in regurgitations by the presence of hard remains (fish vertebrae and/or otoliths, cephalopod beaks and/or internal shell, crustacean exoskeleton). We then estimated each prey type frequency of occurrence as the percentage of regurgitations containing individual prey types. The minimum number of individuals of each prey type present in each regurgitation was determined from the largest number of similar sized/shaped paired structures or from the number of unpaired remains. We then calculated the numerical frequency of each prey type as the percentage of the total number of individual prey items represented by each prey type. We also calculated the relative percentage occurrence as the percentage of the number of individual prey items represented by each prey type in each regurgitation (Danckwerts et al. 2016).
Stable isotope analysis
Isotopic analyses were performed at the Cornell Isotope Laboratory at Cornell University (http://www.cobsil.com). Prior to SIA, down and feathers were cleaned of surface contaminants using successive rinses in a 2:1 chloroform/ether solution, air-dried and then ground to fine powder in a freezer mill operating at liquid nitrogen temperature. Ethanol was removed from prey samples by successive rinsing in distilled water and freeze-drying. Prey tissues were lipid extracted using a Soxhlet apparatus with chloroform solvent and then dried at 60 °C for 24 h to remove any residual solvent. They were then ground to fine powder before SIA. Whole blood generally has low lipid content and does not require lipid extraction (Cherel et al. 2005c). Blood samples were dried under a fume hood at 50 °C, for approximately 40 h, and then ground into powder.
Stable-carbon and nitrogen isotope assays were performed on 1.1 ± 0.1 mg, n = 265, subsamples of homogenized materials by loading into tin cups and combusting at 1800 °C in a Robo-Prep elemental analyser. Resultant CO2 and N2 gases were then analysed using a Thermo Delta V isotope ratio mass spectrometer interfaced to a NC2500 elemental analyser with every 10 unknowns separated by laboratory standards routinely calibrated against international reference materials provided by the International Atomic Energy Association. Stable isotope abundances were expressed in δ notation as the deviation from standards (atmospheric N2 (air) for 15N and Vienna Pee Dee Belemnite for 13C) in parts per thousand (‰). Replicate measurements of laboratory standards showed the analytical precision (± 1 SD) equalled ± 0.12 ‰ and ± 0.13 ‰ for stable nitrogen and carbon isotope measurements, respectively.
Data analysis
We compared grey-faced petrel diet composition between years by using randomisation tests (1000 randomisations) for percent frequencies (frequency of occurrence and numerical frequency; Manly 1997; Bonnaud et al. 2007) and Mann–Whitney U tests for relative percentage occurrence. We grouped the prey types into taxonomically and isotopically similar clusters we thus defined as main prey groups. We used a multivariate analysis of variance (MANOVA) with Wilk’s lambda statistics using type III sums of squares to simultaneously compare δ15N and δ13C values between years and among grey-faced petrel main prey groups. Then, we compared δ15N and δ13C values between years and among prey groups using univariate factorial analyses of variance (ANOVAs) followed by post-hoc Bonferroni tests to identify significant differences between prey groups.
In order to compare grey-faced petrel blood and feather isotopic ratios and, consequently, investigate the isotopic niche at different periods of the annual cycle, we needed to take into account the tissue-dependent metabolic routing and enrichment factors (Podlesak and McWilliams 2006; Quillfeldt et al. 2008a). We applied the linear regression equations of Cherel et al. (2014b) to correct blood to feather δ15N and δ13C values. Although feathers and whole blood have generally low lipid content and despite prey delipidation, some samples (n = 4 feather, 18 down, 7 blood and 3 prey samples) exhibited C:N mass ratios > 3.5, i.e. δ13C values biased by lipid content (Post et al. 2007). Lipid-associated biases were reduced by mathematically normalising the δ13C values of these samples using the equation for aquatic animals given by Post et al. (2007). We used a MANOVA with Wilk’s lambda statistics to simultaneously compare δ15N and δ13C values in adults between years, sexes and among non-breeding period and different breeding stages (incubation and mid-chick rearing), thus testing the effect of year, sex, annual cycle stage and their interactions on stable isotope values. We also included individual identity in this MANOVA to account for repeated observations. Because adult blood during chick rearing was collected only in 2013, we performed a second MANOVA including only data relevant to 2013 chick rearing (adult blood during chick rearing, chick down and body feathers) to test the effect of age (adults vs chicks) on stable isotope values. Upon significant results, we then compared δ15N and δ13C values between years, sexes, adults vs chicks and among annual cycle stages (non-breeding, incubation and mid-chick rearing) using univariate factorial ANOVAs followed by post-hoc Bonferroni tests to identify significant differences between groups. Because chick down and body feathers were collected from the same individuals within each year, we performed repeated measures ANOVAs on chick δ15N and δ13C values to test the effects of annual cycle stage (early vs late chick rearing, repeated measures), year and their interaction on stable isotope values. We also used post-hoc Bonferroni tests to identify significant differences between groups when there were more than two comparisons.
We used the SIBER package (Stable Isotope Bayesian Ellipses in R; Jackson et al. 2011) to determine grey-faced petrel isotopic niche width and its intra- and inter-annual variation. We estimated isotopic niches during the non-breeding period and the various breeding stages in each year by using the standard ellipse area after small sample size correction (SEAc). SEAc, which is an estimated ellipse encompassing 40% of the data regardless of sample size, facilitated visualization and characterization of isotopic niches, allowing the measurement of the size (area, ‰2) and the overlap of the isotopic niches between years and annual cycle stages (Jackson et al. 2011; Parnell et al. 2013). We also calculated the total area (TA) occupied as the area of convex hull that incorporated all individuals, which represents a measure of niche width and reflects the isotopic diversity of each group (Layman et al. 2007; Jackson et al. 2011). For statistical comparison, we calculated Bayesian standard ellipse areas (SEAb) with 95% credible limits of each group using Markov chain Monte Carlo simulation with 104 iterations (Jackson et al. 2011). We computed density plots showing the confidence intervals of SEAb to quantify isotopic niche width and measure dietary similarity among groups. This method calculates the probability that the proportion of posterior samples of SEAb differed among groups, allowing a direct probabilistic interpretation of the differences in SEAb.
Mixing models can be used to estimate the relative proportion of different dietary sources. We used Bayesian multisource stable isotope mixing models (SIAR: Stable Isotope Analyses in R; Parnell et al. 2010) to estimate ranges of probable contributions of each prey (or group of prey) to the diet of grey-faced petrels during the various breeding stages in each year. We used the main prey groups that we earlier identified as taxonomically and isotopically distinct. We included in the models a non-informative Dirichlet prior distribution, with zero concentration dependencies, and default SIAR MCMC estimation (iterations = 5 × 105, burning = 5 × 104, thinning = 15). We applied to the mixing models an isotopic mean ± SD diet-feather discrimination factor of 3.42 ± 0.35 ‰ for δ15N and 1.38 ± 0.48 ‰ for δ13C (mean values from studies on seabirds and lipid-extracted marine prey muscle, n = 12: Becker et al. 2007; Ramos et al. 2009) and an isotopic diet-blood discrimination factor of 2.55 ± 0.39 ‰ for δ15N and 0.11 ± 0.62 ‰ for δ13C (mean values from studies on seabirds and lipid-extracted marine prey muscle, n = 7: Bearhop et al. 2002; Cherel et al. 2005b; Williams et al. 2007; Paiva et al. 2010; Dehnhard et al. 2011; Chiaradia et al. 2014). We then assessed proportion densities for each prey group and displayed them with 50%, 75% and 95% credibility intervals in figures. This method allows a direct probabilistic interpretation of the differences in proportions.
Mann–Whitney tests, MANOVAs and ANOVAs, randomisation tests, and SIBER and SIAR analyses were performed using Statistica 6.0 or R software, version 3.5.0 (R Development Core Team 2018). Presented values are means ± SD, and statistical significance was assumed at P < 0.05.
Results
Prey types and stable isotope ratios
A total of 307 individual prey items were identified in 62 spontaneous regurgitations of grey-faced petrels mainly obtained from chicks (93.5%). Cephalopods were the most common prey type in the diet as they were present in more than 84% of the regurgitations analysed (frequency of occurrence) and their items accounted for more than two thirds of all prey items found in all regurgitations (numerical frequency) and on average for more than 50% of all prey items found in a regurgitation (relative percentage of occurrence; Table 1). Typical internal shell remains of ram’s horn squids Spirula spirula were found in particularly high abundance. Their frequency of occurrence was 58.1% in 2013 and 68.4% in 2014 while their relative percentage occurrence was 24.6 ± 30.0%, n = 43, and 25.8 ± 26.6%, n = 19, respectively, and their numerical frequency was 19.4% and 22.5%, respectively. Hard parts from crustaceans were also common (frequency of occurrence > 72%) whereas their abundance was moderate (20–30% of items). Fish were the least common (frequency of occurrence ~ 50%) and least abundant (11–16% of items) prey. Grey-faced petrel diet composition (mainly of chicks) did not differ significantly between 2013 and 2014 (frequency of occurrence: observation = 0.004, P = 0.831 and all observed percentage differences < 0.070, P > 0.396; relative percentage occurrence: all adjusted Z < 1.3 and all P > 0.209; numerical frequency: observation = 0.013, P = 0.498 and all observed percentage differences < 0.047, P > 0.193).
Cephalopods could be separated into two groups according to stable isotope values (Table 2): cephalopod samples of group 1 (cephalopods 1), including ram’s horn squid (typical internal shell found inside mantles of some samples and in all the regurgitations from which these samples were collected), exhibited low δ15N and relatively high δ13C values; while cephalopod samples of group 2 (cephalopods 2) exhibited high δ15N and lower δ13C values. We aggregated the grey-faced petrel prey types into groups of similar taxa and similar isotopic values (Fig. 1): group I (hereafter named fish or FI) included the five fish samples, group II (hereafter named cephalopods 1 or CE1) included the 10 cephalopods 1 samples, group III (hereafter named cephalopods 2 or CE2) included the five cephalopods 2 samples), and group IV (hereafter named crustaceans or CR) included the three crustacean samples.
Biplot of isotopic values of delipidated flesh of grey-faced petrel Pterodroma gouldi prey types obtained from spontaneous regurgitations in 2013 and 2014 at Ihumoana, New Zealand. Circles: cephalopods; diamonds: fish; triangles: crustaceans; filled shapes: 2013; open shapes: 2014. Food items of similar taxa and with similar isotopic values were clustered into groups of food sources: FI fish (brown), CE1 cephalopods 1 (purple), CE2 cephalopods 2 (pink), CR crustaceans (orange)
MANOVA revealed that stable isotope values varied significantly among the four main prey groups (Wilk’s lambda, F6,30 = 27.8, P < 0.001) but did not differ significantly between 2013 and 2014 (Wilk’s lambda, F2,15 = 1.6, P = 0.226) nor vary in relation to the interaction between prey groups and years (Wilk’s lambda, F4,30 = 1.2, P = 0.322). Thus, values from the two years were combined in isotopic mixing models. δ15N values varied significantly among the four groups (ANOVA, F3,16 = 42.4, P < 0.001, Fig. 2), all post-hoc Bonferroni comparisons being significant (all P < 0.002) but between CE2 and FI (P = 1.000) and between CE1 and CR (P = 0.248). Similarly, δ13C values varied significantly among the four groups (ANOVA, F3,16 = 22.3, P < 0.001) and all post-hoc Bonferroni comparisons were significant (all P < 0.007), except for between CE2 and FI (P = 0.059) and between CE2 and CR (P = 0.980).
Isotopic values (mean ± SD) of adult and chick grey-faced petrels Pterodroma gouldi during various annual cycle stages in 2013 and 2014 and their main prey types (delipidated) at Ihumoana, New Zealand. Petrel blood isotope values were corrected and prey muscle isotope ratios were adjusted by adding assumed diet-tissue (feathers) discrimination factors (see “Materials and methods”). Squares: non-breeding (adults, primary 4 covert); circles: incubation (adults, blood); black cross: mid-chick rearing (adults, blood); triangles: early chick rearing (chicks, down); diamonds: late chick rearing (chicks, breast feathers); filled symbols: 2013; open symbols: 2014; FI fish, brown diamond; CE1 cephalopods 1, purple circle; CE2 cephalopods 2, pink circle; CR crustaceans, orange triangle
Petrel stable isotope ratios
The δ13C and δ15N values of grey-faced petrels ranged from − 19.5 ± 0.4 ‰ in chick down in 2014 to − 17.3 ± 0.2 ‰ in adult feathers in 2014, and from 13.5 ± 0.3 ‰ in adult blood during chick rearing in 2013, to 15.5 ± 0.6 ‰ in chick down in 2014, respectively (Table 3). Blood values reported hereafter were corrected using linear regressions (Cherel et al. 2014b) to standardise blood and feather values.
MANOVA revealed that stable isotope values in adults differed significantly between years (Wilk’s lambda, F2,55 = 10.5, P < 0.001) and between males and females (Wilk’s lambda, F2,55 = 7.5, P = 0.001), and varied significantly among non-breeding period and the various breeding stages (Wilk’s lambda, F4,110 = 27.8, P < 0.001). It also revealed a significant effect of individual identity (Wilk’s lambda, F166,110 = 1.4, P = 0.023), of the interaction between year and sex (Wilk’s lambda, F2,55 = 3.3, P = 0.044) and of the interaction between annual cycle stage and sex (Wilk’s lambda, F4,110 = 3.6, P = 0.008). Interactions between year and annual cycle stage, and between year, annual cycle stage and sex did not significantly affect stable isotope values (Wilk’s lambda, F2,55 < 0.4, P > 0.688). ANOVAs revealed significant differences in both δ13C and δ15N values between years and between males and females (Table 4). Both δ13C and δ15N values also varied among non-breeding period and the various breeding stages (Table 4, Fig. 2). Finally, ANOVAs showed significant interactions between annual cycle stage and sex, and between year and sex on δ13C values (Table 4). The MANOVA on 2013 chick rearing data revealed that stable isotope values differed significantly between adults and chicks (Wilk’s lambda, F2,145 = 217.3, P < 0.001). Univariate ANOVAs on these data showed significant differences in both δ13C and δ15N values (Table 4, Fig. 2). Repeated measures ANOVAs on chick data showed significant effects of year, breeding stage (early vs late chick rearing) and their interaction on δ13C values and of the interaction of year and breeding stage on δ15N values.
Petrel isotopic niche
Grey-faced petrel isotopic niches were well separated between annual cycle stages within years, but relatively fixed across years for any given annual cycle stage (Fig. 3). Overlap across years for a given annual cycle stage was 0.0% for early chick rearing, 10.5% for incubation, 34.1% for late chick rearing and 44.3% for non-breeding. Within a given year, the overlap was the highest between incubation and non-breeding (10.8% in 2013 and 5.2% in 2014), and between early and late chick rearing in 2013 (5.7%). There was no overlap between all other annual cycle stages within a year.
Isotopic niche occupancy of adult and chick grey-faced petrels Pterodroma gouldi during various annual cycle stages in 2013 and 2014 at Ihumoana, New Zealand. The biplot depicts δ13C and δ15N isotope values. We applied the linear regression equations of Cherel et al. (2014b) to correct blood to feather δ15N and δ13C values. Ellipses represent the isotopic niche width (SEAc) of 40% of typical individuals within the group based on bivariate normal distribution. Blue squares: non-breeding (adults, primary 4 covert); red circles: incubation (adults, blood); black crosses: mid-chick rearing (adults, blood); cyan triangles: early chick rearing (chicks, down); green diamonds: late chick rearing (chicks, breast feathers)
Isotopic niche was the narrowest during incubation (Table 5, Fig. 4). Isotopic niche width did not vary significantly among all other annual cycle stages.
Density plot depicting the mean Bayesian standard ellipse areas (black dot) and their confidence intervals for adult and chick grey-faced petrels Pterodroma gouldi during various annual cycle stages in 2013 and 2014 at Ihumoana, New Zealand. Shaded boxes represent the 50%, 75% and 95% intervals from dark grey to light grey. NBR non-breeding, INC incubation, MCR mid-chick rearing, ECR early chick rearing, LCR late chick rearing. Different letters indicate significant differences in SEAb between groups
Diet changes inferred by isotopic mixing models
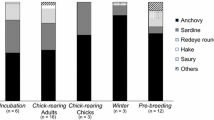
SIAR mixing models based on grey-faced petrel and prey isotope values during the breeding period indicated that cephalopods 1 were the largest component in the diet of the sampled breeding adult grey-faced petrels (mean proportional contributions: 46.3–58.8%), while crustaceans were the largest component in the diet of chicks (49.4–67.0%; Fig. 5). This pattern was consistent across breeding stages and years. Cephalopods 2 and fish consistently constituted secondary prey groups across breeding stages and years, contributing from 5.3 to 28.4% of grey-faced petrel diet.
Proportion of estimated prey groups in the diet of adult and chick grey-faced petrels Pterodroma gouldi during various breeding stages in 2013 and 2014 at Ihumoana, New Zealand. Estimates are from SIAR mixing models, and shaded box plots represent the 50%, 75% and 95% credibility intervals from dark grey to light grey. INC incubation, MCR mid-chick rearing, ECR early chick rearing, LCR late chick rearing. For each prey group, different letters indicate significant differences in contribution proportion in the diet between breeding stages
Discussion
Understanding how marine top predators cope with spatiotemporal heterogeneity in food availability and the variable energetic constraints of their annual life cycle is essential to evaluate their ability to withstand current and future global changes. It also informs the usefulness of marine top predators as indicators of changes in food web structure or in prey abundance and diversity across their foraging areas. The analysis of stable isotope values in the blood and feathers of grey-faced petrels highlighted seasonal (i.e. non-breeding, incubation and early, late and mid-chick rearing) and annual variations in carbon and nitrogen stable isotopes, isotopic niches and diet of the species. Our analyses also showed a trophic segregation between adults and chicks and between males and females. Finally, grey-faced petrel isotopic niches were more similar between the breeding and non-breeding periods in adults than between breeding adults and their chicks. These results demonstrate grey-faced petrel foraging strategy plasticity in response to the changing nutritional demands of their annual cycle stages and potentially to changes in oceanographic conditions.
Seasonal variation in isotopic niche
Grey-faced petrel isotopic niches overlapped little within years as the overlap among non-breeding, incubation, early, mid- and late chick rearing stages averaged 1.4 ± 3.1%, n = 16. Isotopic niche width was narrower during incubation in both the two years studied.
Carbon isotope ratio enrichment is higher in inshore than in offshore food chains (Cherel and Hobson 2007; Bond and Jones 2009) and in benthic than in surface species (Hobson et al. 1994; France 1995). Lower δ13C values in adult grey-faced petrels during the non-breeding period compared to those during the breeding season (differences: 0.3 ‰) could, thus, indicate a change in foraging habitat, in particular the use of more pelagic foraging areas during the non-breeding period. This is consistent with the fact that, outside the breeding season, grey-faced petrels are not central place foragers and can move freely through vast oceanic areas, far from coasts. In contrast, lower δ13C values in chicks during early chick rearing rather than during late chick rearing in 2014 (difference: 1.2 ‰) are more surprising. This suggests that chicks were fed with more pelagic prey during the earlier breeding stage. Yet, Procellariiform chicks are generally fed more frequently during early chick rearing than during late chick rearing since they are gradually fed less towards fledging (Warham 1990). Higher feeding frequency during early chick rearing is not likely to allow adults to travel for longer trips, farther from the nesting site than during late chick rearing. Enrichment in the carbon isotope ratio can also be due to consuming more non-epi-pelagic prey, in particular meso-pelagic and demersal species (Hobson et al. 1994; Navarro et al. 2009; Thabet et al. 2019). However, δ15N values and isotope mixing models do not support this hypothesis for grey-faced petrels since δ15N values in adults were not higher during the breeding season than during the non-breeding period or those in chicks were not higher during late chick rearing than during early chick rearing in 2014, and neither adult nor chick diet composition differed between the two periods or breeding stages, respectively. Higher δ13C values in incubating adult grey-faced petrels and in chicks during late chick rearing in 2014 could also be an artefact due to adult fasting during incubation and chick starvation in late chick rearing, as fasting and nutritional restriction can result in a δ13C increase (Hertz et al. 2015). However, studies of several seabird species have shown no or contrary effects of fasting and starvation on δ13C values (Cherel et al. 2005a; Williams et al. 2007; Sears et al. 2009) and nutritional stress would probably not explain higher adult δ13C values during mid-chick rearing nor the absence of significant difference in chick δ13C values between early and late chick rearing in 2013. Finally, differences in adult δ13C values between the non-breeding and the breeding periods could also be due to differences in baseline δ13C values in the areas used for feeding by adult grey-faced petrels during these two periods (see later).
The nitrogen isotope ratio increases with increasing trophic level (Kelly 2000; Bearhop et al. 2004) and with the depth of marine organism habitat in some particular areas (Choy et al. 2015). Adult grey-faced petrel δ15N values varied among annual cycle stages and were lower during mid-chick rearing (differences: 0.7–0.8 ‰) than during incubation and non-breeding. Thus, grey-faced petrels may have consumed prey richer in protein and energy or prey from more complex food webs (Shaffer et al. 2003; Navarro et al. 2007; Thabet et al. 2019) during incubation and perhaps also during the non-breeding period. However, our mixing models failed to detect a significant variation in adult diet among breeding stages, although the contribution of cephalopods 2 and fish could have been lower and the contribution of cephalopods 1 could have been higher during mid-chick rearing. In contrast, repetitive periods of fasting during incubation could increase δ15N values (Cherel et al. 2005a; Hertz et al. 2015; Doi et al. 2017). Indeed, during incubation, both members of pairs alternately incubate the eggs for shifts averaging 17 days over a total of ca. 55 days (Imber 1976). These long fasting shifts deplete incubating bird lipid reserves and the birds begin catabolising muscular proteins for energy, thus consuming their own lipid and protein reserves during their stay in their burrow (Cherel et al. 2005a; Hertz et al. 2015; Doi et al. 2017). This increases the δ15N values of their blood (by ca. 0.5 ‰; Hertz et al. 2015; Doi et al. 2017) and raises their apparent trophic level. However, such physiological and metabolic factors cannot explain δ15N values in adults during the non-breeding period. For this period, it is more likely that baseline δ15N values are different in foraging areas used by grey-faced petrels.
Isotopic niche segregation between sexes and between adults and chicks
Male and female grey-faced petrels had slightly different isotopic niches throughout the annual cycle (overall difference in δ13C: 0.1 ‰, in δ15N: 0.2 ‰). Lower δ13C values found in males were mainly due to the lowest values of males during the non-breeding period. Males exhibited higher overall δ15N values throughout the annual cycle but the difference between sexes was not significant for any individual annual cycle stage. Significant isotopic differences between sexes are more common in seabirds during the pre-laying or breeding than the non-breeding period (Phillips et al. 2011). This presumably reflects greater between sex partitioning of resources when foraging ranges are more constrained and competition is greater. In medium-sized Procellariiformes, sexual differences in foraging strategy could also be the result of differences in reproductive roles (burrow defence and maintenance by males) and/or energetic constraints (egg development in females, first post-laying long fasting bout in males) during the breeding period (Pinet et al. 2012; Hedd et al. 2014; Yamamoto et al. 2015; Danckwerts et al. 2016). Yet, isotopic differences between sexes were more marked during the non-breeding period in grey-faced petrels. This suggests that males and females could use different marine habitats and/or areas and potentially feed on prey of different trophic levels during non-breeding. There is also a relationship between seabird sexual size dimorphism and their sexual segregation in diet or distribution, with size-dimorphic taxa more commonly exhibiting sex differences in δ15N than δ13C (Phillips et al. 2011). This is more consistent with size-mediated competitive exclusion or dietary specialisation than just habitat specialisation. Higher δ15N values in males have been observed in Procellariiformes with various levels of sexual size dimorphism (Quillfeldt et al. 2008b; Paiva et al. 2018). Grey-faced petrels are slightly sexually dimorphic with males being larger than females in all measurements and sexual size dimorphism being the greatest for bill depth (Bourgeois et al. 2017). Males could thus catch larger prey of higher trophic level.
Adult and chick grey-faced petrel isotopic niches were clearly segregated during chick rearing (no isotopic niche overlap), with chicks showing lower δ13C (difference: 1.4 ‰) and higher δ15N (difference: 1.0 ‰) values than adults. These differences in stable isotope values may reflect the use of a dual-foraging strategy, which was also suggested by an adult burrow attendance study of the species (authors’ unpubl data). The δ13C values found in grey-faced petrel chicks and adults suggest that adults feed in more inshore areas for themselves and chicks are fed more on oceanic prey caught offshore by adults. This contradicts the pattern generally accepted for seabirds that use a dual-foraging strategy, which finds that adults alternate short chick-provisioning trips of 1–4 days with longer self-maintenance trips during which they generally target more productive but more distant areas (Catard et al. 2000; Magalhães et al. 2008; Jakubas et al. 2012; Shoji et al. 2015). However, the inference from stable isotope analyses that chicks consume more oceanic prey than their parents has been commonly observed in Procellariiformes (e.g. Alonso et al. 2012; Danckwerts et al. 2016; Leal et al. 2017; Thabet et al. 2019). On the other hand, metabolic and physiological factors can affect isotopic discrimination and lead to differences in isotope values between chicks and adults (Sears et al. 2009). In particular, Procellariiformes are able to convert fresh prey into concentrated stomach oil (Warham 1977). Adults feed their chicks this oil which is highly energetic but poor in protein and depleted in δ13C (Warham 1977, 1996; Thompson et al. 2000). This may thus explain the low δ13C values measured in grey-faced petrel chicks, but not the δ15N difference. However, adults may also provide whole prey to their chicks in order to supply the protein required for their growth (Warham 1977, 1996). These whole prey often had higher trophic levels than prey used for self-provisioning as suggested by seabird chicks having higher δ15N values (by 1–2‰) than adults (e.g. Hodum and Hobson 2000; Cherel et al. 2008; Forero et al. 2005; Richoux et al. 2010; González-Medina et al. 2017). Stable isotope mixing models revealed that a group of cephalopods was the main diet component of breeding adult grey-faced petrels. This group of cephalopods was characterised by low δ15N values. Because the typical internal shell of the ram’s horn squid was found inside mantles of this cephalopod group and in all the regurgitations from which flesh samples of this cephalopod group were collected, we assume that ram’s horn squids constituted this group of cephalopod prey. This assumption is supported by the low trophic position of this squid in comparison with other cephalopods (Ohkouchi et al. 2013). In contrast, crustaceans were the main diet component of grey-faced petrel chicks and they exhibited higher δ15N values than ram’s horn squids. This difference in diet between adults and chicks could thus explain the difference in δ15N values in their tissues, and potentially the difference in δ13C values as well, particularly if chicks were fed with oil produced from crustaceans as suggested by the orange coloured carotenoid rich oil found in grey-faced petrel regurgitations (Warham 1977, 1996).
Stable isotope values in wing feathers
Because petrels generally spend their non-breeding period in foraging areas several thousand kilometres away from their foraging areas during the breeding period (e.g. Rayner et al. 2012; Priddel et al. 2014; Ramos et al. 2017), we expected different stable isotope values between the two periods in grey-faced petrels. Yet, our study revealed values more similar between these two periods than between breeding adults and chicks. Most grey-faced petrels from Ihumoana disperse to the western Tasman Sea and to the south-western Coral Sea, off the eastern coast of Australia, during the non-breeding period (GLS data; G.A. Taylor unpubl data). During the breeding period, they forage in the eastern and central Tasman Sea, several hundred kilometres west of New Zealand (from more than 50 km and up to about 1500 km from the coast, GPS data; K. Bourgeois unpubl data). The two areas are not as distant as in some other petrel species (e.g. Rayner et al. 2012; Priddel et al. 2014; Ramos et al. 2017) and the similarities of δ15N and δ13C values in grey-faced petrel tissues reflecting the two periods could be coherent with marine isoscapes in the region (Graham and Bury 2019). Indeed, the area off the eastern coast of Australia (between 0 and 500 km from the coast), particularly around the limit between the Tasman and the Coral seas (i.e. the parallel of 30°S), exhibits slightly more depleted δ13C values than the central and eastern Tasman Sea, while grey-faced petrel tissues reflecting the non-breeding period exhibited depleted δ13C values compared to tissues reflecting the breeding period. This area also exhibits slightly depleted δ15N values suggesting that grey-faced petrels could feed on higher trophic level prey during the non-breeding period, since δ15N values in their tissues reflecting the two periods were similar. However, stable isotope values were obtained from blood for the breeding period and from the fourth primary covert feather for the non-breeding period. We thus cannot exclude that the use of linear regressions to correct blood δ15N and δ13C values in order to compare grey-faced petrel blood and feather isotopic ratios could have biased our results.
Annual variation in isotopic niche
Grey-faced petrel isotopic niches overlapped substantially between years for the various annual cycle stages as the inter-annual overlap of isotopic niches during each annual cycle stage averaged 22.2 ± 20.5%, n = 4. However, isotopic niches did not overlap between 2013 and 2014 during early chick rearing. Isotopic niche width did not exhibit a clear difference between the two years studied as it was narrower in 2014 during incubation and wider the same year during early chick rearing. Adult grey-faced petrels exhibited lower δ13C and δ15N values in 2013. In chicks, δ13C values were lower in 2014 and δ15N values in 2013 but these differences were significant only for the early chick rearing period. During years of unfavourable oceanographic conditions, seabirds generally engage in longer trips, resulting in larger home ranges and foraging areas, and they exhibit a wider isotopic niche (Kowalczyk et al. 2014; Ramos et al. 2015, 2018; Cerveira et al. 2020). In contrast, during years of apparent good oceanographic conditions, they have a smaller isotopic niche suggesting a higher specialization, potentially related to the greater abundance of their main food items (Leal et al. 2017; Ramos et al. 2018). The values of δ13C and δ15N measured in adult grey-faced petrels and the corresponding isotopic niche widths suggest that oceanographic conditions in 2013 could have been less favourable than in 2014. During the 2013 breeding period, the Southern Oscillation Index leant towards La Niña while during the 2014 breeding period it leant towards El Niño. A La Niña phase means warmer air temperatures around New Zealand, more north-easterly winds and more rain in north-eastern parts of the North Island. During La Niña like conditions, positive sea surface temperature anomalies are located in the west Pacific. In the 2013 La Niña conditions, sea surface temperatures were higher and this likely affected grey-faced petrel prey distribution and/or abundance. Overall, grey-faced petrel breeding success (excluding on-land causes of breeding failure such as egg and chick predation) and chick growth on Ihumoana did not differ between 2013 and 2014 (authors’ unpubl data). This suggests that their foraging strategy plasticity allows them to buffer the effects of inter-annual environmental differences on these two reproductive parameters, at least to a certain point for the moderate differences over the two years of our study, as has been observed in other seabird species (Dehnhard et al. 2016). However, long-term studies relating isotopic niche, if possible combined with tracking data, with demographic parameters would help evaluate the role of oceanographic conditions in the demography of this species and ultimately the impact of global changes on its populations. Indeed, although the grey-faced petrel is overall not threatened, it is only abundant at a few sites and in very small declining populations at most other sites (small island and mainland colonies; Greene et al. 2015). Causes for decline are interactive; introduced mammal predation on land and changes in oceanographic conditions at sea. Independently of predation, marked spatiotemporal variation in chick development and breeding success has been found, with high chick mortality due to starvation some years at some colonies (authors’ unpubl data). This spatial variation is hypothesised to arise from different marine foraging strategies, either through foraging area location or trophic levels fed upon, while temporal variation is more probably related to variation in oceanographic conditions and in prey distribution and abundance. Grey-faced petrels are thus considered a priority monitoring species in New Zealand because their population status is highly correlated to marine ecosystem health (Monks et al. 2013; Russell et al. 2017). The spatiotemporal monitoring of grey-faced petrel trophic ecology could be informative about the changes in food web structure or in prey abundance and diversity across the Tasman Sea and the southwest Pacific Ocean and serve as a valuable bio-indicator of prey populations that could themselves be affected by the combined effects of overfishing and climate change.
References
Alonso H, Granadeiro JP, Paiva VH, Dias AS, Ramos JA, Catry P (2012) Parent-offspring dietary segregation of Cory’s shearwaters breeding in contrasting environments. Mar Biol 159:1197–1207
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164
Becker BH, Newman SH, Inglis S, Beissinger SR (2007) Diet-feather stable isotope (δ15N and δ13C) fractionation in common murres and other seabirds. Condor 109:451–456
Bond AL, Jones IL (2009) A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar Ornithol 37:183–188
Bonnaud E, Bourgeois K, Vidal E, Kayser Y, Tranchant Y, Legrand J (2007) Feeding ecology of a feral cat population on a small Mediterranean island. J Mammal 88:1074–1081
Booth JM, Steinfurth A, Fusi M, Cuthbert RJ, McQuaid CD (2018) Foraging plasticity of breeding northern rockhopper penguins, Eudyptes moseleyi, in response to changing energy requirements. Polar Biol 41:1815–1826
Bourgeois K, Dromzée S, Welch JR, Russell JC (2017) Sex and geographic variation in grey-faced petrel (Pterodroma gouldi) morphometrics. Waterbirds 40:144–153
Bryant DM (1997) Energy expenditure in wild birds. Proc Nutr Soc 56:1025–1039
Bugoni L, McGill RAR, Furness RW (2008) Effects of preservation methods on stable isotope signatures in bird tissues. Rapid Commun Mass Spectrom 22:2457–2462
Carey MJ (2009) The effects of investigator disturbance on procellariiform seabirds: a review. N Z J Zool 36:367–377
Catard A, Weimerskirch H, Cherel Y (2000) Exploitation of distant Antarctic waters and close shelf-break waters by white-chinned petrels rearing chicks. Mar Ecol Prog Ser 194:249–261
Cerveira LR, Ramos JA, Rodrigues I, Almeida N, Araújo PM, dos Santos I, Vieira C, Pereira JM, Ceia FR, Geraldes P, Melo T, Paiva VH (2020) Inter-annual changes in oceanic conditions drives spatial and trophic consistency of a tropical marine predator. Mar Environ Res 162:105165
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287
Cherel Y, Hobson KA, Bailleul F, Groscolas R (2005a) Nutrition, physiology, and stable isotopes: new information from fasting and molting penguins. Ecology 86:2881–2888
Cherel Y, Hobson KA, Hassani S (2005b) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115
Cherel Y, Hobson KA, Weimerskirch H (2005c) Using stable isotopes to study resource acquisition and allocation in procellariiform seabirds. Oecologia 145:533–540
Cherel Y, Le Corre M, Jaquemet S, Ménard F, Richard P, Weimerskirch H (2008) Resource partitioning within a tropical seabird community: new information from stable isotopes. Mar Ecol Prog Ser 366:281–291
Cherel Y, Connan M, Jaeger A, Richard P (2014a) Seabird year-round and historical feeding ecology: blood and feather δ13C and δ15N values document foraging plasticity of small sympatric petrels. Mar Ecol Prog Ser 505:267–280
Cherel Y, Jacquemet S, Maglio A, Jaeger A (2014b) Differences in δ13C and δ15N values between feathers and blood of seabird chicks: implications for non-invasive isotopic investigations. Mar Biol 161:229–237
Chiaradia A, Forero MG, McInnes JC, Ramírez F (2014) Searching for the true diet of marine predators: incorporating Bayesian priors into stable isotope mixing models. PLoS ONE 9:e92665
Chiaradia A, Ramírez F, Forero MG, Hobson KA (2016) Stable isotope (δ13C, δ15N) combined with conventional dietary approaches reveal plasticity in central-place foraging behavior of little penguins Eudyptula minor. Front Ecol Evol 3:154
Choy CA, Popp BN, Hannides CCS, Drazen JC (2015) Trophic structure and food resources of epipelagic and mesopelagic fishes in the North Pacific Subtropical Gyre ecosystem inferred from nitrogen isotopic compositions. Limnol Oceanogr 60:1156–1171
Congdon BC, Krockenberger AK, Smithers BV (2005) Dual-foraging and coordinated provisioning in a tropical Procellariiform, the wedge-tailed shearwater. Mar Ecol Prog Ser 301:293–301
Danckwerts DK, McQuaid CD, Connan M, Smale MJ, Le Corre M, Humeau L, Kaehler S, Juhasz CC, Orlowski S, Tourmetz J, Jaquemet S (2016) Intra-annual variation in the foraging ecology of the endangered endemic Barau’s petrel (Pterodroma baraui) from Réunion Island, south-western Indian Ocean: insights from a multifaceted approach. Mar Biol 163:18
Dehnhard N, Voigt CC, Poisbleau M, Demongin L, Quillfeldt P (2011) Stable isotopes in southern rockhopper penguins: foraging areas and sexual differences in the non-breeding period. Polar Biol 34:1763–1773
Dehnhard N, Ludynia K, Masello JF, Voigt CC, McGill RA, Quillfeldt P (2016) Plasticity in foraging behaviour and diet buffers effects of inter-annual environmental differences on chick growth and survival in Southern Rockhopper Penguins Eudyptes chrysocome chrysocome. Polar Biol 39:1627–1641
Doi H, Akamatsu F, González AL (2017) Starvation effects on nitrogen and carbon stable isotopes of animals: an insight from meta-analysis of fasting experiments. R Soc Open Sci 4:170633
Dunphy BJ, Taylor GA, Landers TJ, Sagar RL, Chilvers BL, Ranjard L, Rayner MJ (2015) Comparative seabird diving physiology: first measures of haematological parameters and oxygen stores in three New Zealand Procellariiformes. Mar Ecol Prog Ser 523:187–198
Forero MG, González-Solís J, Hobson KA, Doncazar JA, Bertellotti M, Blanco G, Bortolotti GR (2005) Stable isotopes reveal trophic segregation by sex and age in the southern giant petrel in two different food webs. Mar Ecol Prog Ser 296:107–113
France RL (1995) Carbon-13 enrichment in benthic compared to planktonic algae: foodweb implications. Mar Ecol Prog Ser 124:307–312
Frederiksen M, Edwards M, Richardson AJ, Hallyday NC, Wanless S (2006) From plankton to top predators: bottom-up control of a marine food web across four trophic levels. J Anim Ecol 75:1259–1268
Geary B, Leberg PL, Purcell KM, Walter ST, Karubian J (2020) Breeding brown pelicans improve foraging performance as energetic needs rise. Sci Rep 10:1686
González-Medina E, Castillo-Guerrero JA, Herzka SZ, Fernández G (2017) Flexibility in food resource allocation in parents and selectivity for offspring: variations in δ15N and δ13C values during breeding of the blue-footed booby. Mar Biol 164:38
Graham B, Bury S (2019) Marine isoscapes for trophic and animal movement studies in the southwest Pacific Ocean. New Zealand Aquatic Environment and Biodiversity report no. 218. Fisheries New Zealand, Wellington
Grecian WJ, Taylor GA, Loh G, McGill RAR, Miskelly CM, Phillips RA, Thompson DR, Furness RW (2016) Contrasting migratory responses of two closely related seabirds to long-term climate change. Mar Ecol Prog Ser 559:231–242
Greene BS, Taylor GA, Earl R (2015) Distribution, population status and trends of grey faced petrel (Pterodroma macroptera gouldi) in northern North Island, New Zealand. Notornis 62:143–161
Harper PC (1987) Feeding behaviour and other notes on 20 species of Procellariiformes at sea. Notornis 34:169–192
Hedd A, Montevecchi WA, Phillips RA, Fifield DA (2014) Seasonal sexual segregation by monomorphic sooty shearwaters Puffinus griseus reflects different reproductive roles during the pre-laying period. PLoS ONE 9:e85572
Hertz E, Trudel M, Cox MK, Mazumder A (2015) Effects of fasting and nutritional restriction on the isotopic ratios of nitrogen and carbon: a meta-analysis. Ecol Evol 5:4829–4839
Hobson KA (1993) Trophic relationships among high Arctic seabirds: insights from tissue-dependent stable-isotope models. Mar Ecol Prog Ser 95:7–18
Hobson KA, Bond AL (2012) Extending an indicator: year-round information on seabird trophic ecology from multiple-tissue stable-isotope analyses. Mar Ecol Prog Ser 461:233–243
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes. I. Turnover of 13C in tissues. Condor 94:181–188
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798
Hobson KA, Gibbs HL, Gloutney ML (1997) Preservation of blood and tissue samples for stable-carbon and stable nitrogen isotope analysis. Can J Zool 75:1720–1723
Hodum PJ, Hobson KA (2000) Trophic relationships among Antarctic fulmarine petrels: insights into dietary overlap and chick provisioning strategies inferred from stable isotope (δ15N and δ13C) analyses. Mar Ecol Prog Ser 198:273–281
Imber MJ (1973) The food of grey-faced petrels (Pterodroma macroptera gouldi (hutton)), with special reference to diurnal vertical migration of their prey. J Anim Ecol 42:645–662
Imber MJ (1976) Breeding biology of the grey-faced petrel Pterodroma macroptera gouldi. Ibis 118:51–64
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Jaeger A, Blanchard P, Richard P, Cherel Y (2009) Using carbon and nitrogen isotopic values of body feathers to infer inter- and intra-individual variations of seabird feeding ecology during moult. Mar Biol 156:1233–1240
Jaeger A, Lecomte V, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460
Jaeger A, Goutte A, Lecomte VJ, Richard P, Chastel O, Barbraud C, Weimerskirch H, Cherel Y (2014) Age, sex, and breeding status shape a complex foraging pattern in an extremely long-lived seabird. Ecology 95:2324–2333
Jakubas D, Iliszko L, Wojczulanis-Jakubas K, Stempniewicz L (2012) Foraging by little auks in the distant marginal sea ice zone during the chick-rearing period. Polar Biol 35:73–81
Kaehler S, Pakhomov EA (2001) Effects of storage and preservation on the δ13C and δ15N signatures of selected marine organisms. Mar Ecol Prog Ser 219:299–304
Kato A, Watanuki Y, Naito Y (2003) Annual and seasonal changes in foraging site and diving behavior in adélie penguins. Polar Biol 26:389–395
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Kowalczyk ND, Chiaradia A, Preston TJ, Reina RD (2014) Linking dietary shifts and reproductive failure in seabirds: a stable isotope approach. Funct Ecol 28:755–765
Layman CA, Arrington DA, Montana CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48
Leal GR, Furness RW, McGill RAR, Santos RA, Bugoni L (2017) Feeding and foraging ecology of Trindade petrels Pterodroma arminjoniana during the breeding period in the South Atlantic Ocean. Mar Biol 164:211
MacArthur RH, Pianka ER (1966) On the optimal use of a patchy environment. Am Nat 100:603–610
Machovsky-Capuska GE, Miller MGR, Silva FRO, Amiot C, Stockin KA, Senior AM, Schuckard R, Melville D, Raubenheimer D (2018) The nutritional nexus: linking niche, habitat variability and prey composition in a generalist marine predator. J Anim Ecol 87:1286–1298
MacLeod CJ, Adams J, Lyver P (2008) At-sea distribution of satellite-tracked grey-faced petrels, Pterodroma macroptera gouldi, captured on the Ruamaahua (Aldermen) islands, New Zealand. Pap Proc R Soc Tasman 142:73–88
Magalhães MC, Santos RS, Hamer KC (2008) Dual-foraging of Cory’s shearwater in the Azores: feeding locations, behaviour at sea and implication for food provisioning of chicks. Mar Ecol Prog Ser 359:283–293
Manly BFJ (1997) Randomization, bootstrap and Monte Carlo methods in biology, 2nd edn. Chapman and Hall, Boca Raton
Marchant S, Higgins PJ (1990) Handbook of Australian, New Zealand and Antarctic birds, vol 1. Ratites to ducks. Part A, Ratites to petrels. Part B, Australian Pelican to ducks. Oxford University Press, Melbourne
Miller MGR, Silva FRO, Machovsky-Capuska GE, Congdon BC (2018) Sexual segregation in tropical seabirds: drivers of sex-specific foraging in the Brown booby Sula leucogaster. J Ornithol 159:425–437
Monks JM, O’Donnell CFJ, Wright EF (2013) Selection of potential indicator species for measuring and reporting on trends in widespread native taxa in New Zealand. DOC research and development series 338. Department of Conservation, Wellington
Navarro J, González-Solís J, Viscor G (2007) Nutritional and feeding ecology in Cory’s shearwater Calonectris diomedea during breeding. Mar Ecol Prog Ser 351:261–271
Navarro J, Louzao M, Igual JM, Oro D, Delgado A, Arcos JM, Genovart M, Hobson KA, Forero MG (2009) Seasonal changes in the diet of a critically endangered seabird and the importance of trawling discards. Mar Biol 156:2571–2578
Newsome SD, del Rio CM, Bearhop S, Phillips DL (2007) A niche for isotopic ecology. Front Ecol Eviron 5:429–436
Ohkouchi N, Tsuda R, Chikaraishi Y, Tanabe K (2013) A preliminary estimate of the trophic position of the deep-water ram’s horn squid Spirula spirula based on the nitrogen isotopic composition of amino acids. Mar Biol 160:773–779
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. The Ohio State University Press, Columbus, pp 154–177
Paiva VH, Xavier J, Geraldes P, Ramírez I, Garthe S, Ramos JA (2010) Foraging ecology of Cory’s shearwaters in different oceanic environments of the North Atlantic. Mar Ecol Prog Ser 410:257–268
Paiva VH, Ramos JA, Nava C, Neves V, Bried J, Magalhaes M (2018) Inter-sexual habitat and isotopic niche segregation of the endangered Monteiro’s storm-petrel during breeding. Zoology 126:29–35
Parnell AC, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672
Parnell AC, Phillips DL, Bearhop S, Semmens BX, Ward EJ, Moore JW, Jackson AL, Grey J, Kelly DJ, Inger R (2013) Bayesian stable isotope mixing models. Environmetrics 24:387–399
Peery MZ, Newman SH, Storlazzi CD, Beissinger SR (2009) Meeting reproductive demands in a dynamic upwelling system: foraging strategies of a pursuit-diving seabird, the marbled murrelet. Condor 111:120–134
Phillips RA, McGill RAR, Dawson DA, Bearhop S (2011) Sexual segregation in distribution, diet and trophic level of seabirds: insights from stable isotope analysis. Mar Biol 158:2199–2208
Phillips RA, Lewis S, González-Solís J, Daunt F (2017) Causes and consequences of individual variability and specialization in foraging and migration strategies of seabirds. Mar Ecol Prog Ser 578:117–150
Pinet P, Jaquemet S, Phillips RA, Le Corre M (2012) Sex-specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim Behav 83:979–989
Podlesak DW, McWilliams SR (2006) Metabolic routing of dietary nutrients in birds: effect of diet quality and macronutrient composition revealed using stable isotopes. Physiol Biochem Zool 79:534–549
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Priddel D, Carlile N, Portelli D, Kim Y, O’Neill L, Bretagnolle V, Ballance LT, Phillips RA, Rayner MJ (2014) Pelagic distribution of Gould’s Petrel (Pterodroma leucoptera): linking sight records of seabirds with remote tracking data. Emu 114:360–370
Quillfeldt P (2002) Seasonal and annual variation in the diet of breeding and non-breeding Wilson’s storm-petrels on King George Island, South Shetland Islands. Polar Biol 25:216–221
Quillfeldt P, McGill RAR, Furness RW (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304
Quillfeldt P, Bugoni L, McGill RAR, Masello JF, Furness RW (2008a) Differences in stable isotopes in blood and feathers of seabirds are consistent across species, age and latitude: implications for food web studies. Mar Biol 155:593–598
Quillfeldt P, McGill RAR, Masello JF, Weiss F, Strange IJ, Brickle P, Furness RW (2008b) Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Mar Ecol Prog Ser 373:137–148
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ramos R, Ramírez F, Sanpera C, Jover L, Ruiz X (2009) Feeding ecology of yellow-legged gulls Larus michahellis in the western Mediterranean: a comparative assessment using conventional and isotopic methods. Mar Ecol Prog Ser 377:289–297
Ramos JA, Fagundes AI, Xavier JC, Fidalgo V, Ceia FR, Medeiros R, Paiva VH (2015) A switch in the Atlantic Oscillation correlates with inter-annual changes in foraging location and food habits of Macaronesian shearwaters (Puffinus baroli) nesting on two islands of the sub-tropical Atlantic Ocean. Deep Sea Res Part I 104:60–71
Ramos R, Carlile N, Madeiros J, Ramírez I, Paiva VH, Dinis H, Zino F, Biscoito M, Leal GR, Bugoni L, Jodice PGR, Ryan PG, González-Solís J (2017) It is the time for oceanic seabirds: tracking year-round distribution of gadfly petrels across the Atlantic Ocean. Divers Distrib 23:794–805
Ramos JA, Rodrigues I, Melo T, Geraldes P, Paiva VH (2018) Variation in ocean conditions affects chick growth, trophic ecology, and foraging range in Cape Verde Shearwater. Condor 120:283–290
Rayner MJ, Taylor GA, Gummer HD, Phillips RA, Sagar PM, Shaffer SA, Thompson DR (2012) The breeding cycle, year-round distribution and activity patterns of the endangered Chatham petrel (Pterodroma axillaris). Emu 112:107–116
Richoux NB, Jaquemet S, Bonnevie BT, Cherel Y, McQuaid CD (2010) Trophic ecology of grey-headed albatrosses from Marion Island, Southern Ocean: insights from stomach contents and diet tracers. Mar Biol 157:1755–1766
Ropert-Coudert Y, Wilson RP, Daunt F, Kato A (2004) Patterns of energy acquisition by a central place forager: benefits of alternating short and long foraging trips. Behav Ecol 15:824–830
Roscales JL, Gómez-Diaz E, Neves V, González-Solíz J (2011) Trophic versus geographic structure in stable signatures of pelagic seabirds breeding in the northeast Atlantic. Mar Ecol Prog Ser 434:1–13
Ruiz-Cooley RI, Garcia KY, Hetherington ED (2011) Effects of lipid removal and preservatives on carbon and nitrogen stable isotope ratios of squid tissues: implications for ecological studies. J Exp Mar Biol Ecol 407:101–107
Russell JC, Welch JR, Dromzée S, Bourgeois K, Thoresen J, Earl R, Greene B, Westbrooke I, McNutt K (2017) Developing a national framework for monitoring the grey-faced petrel (Pterodroma gouldi) as an indicator species. DOC research and development series 350. Department of Conservation, Wellington
Schoener TW (1971) Theory of feeding strategies. Annu Rev Ecol Syst 2:369–404
Sears J, Hatch SA, O’brien DM, (2009) Disentangling effects of growth and nutritional status on seabird stable isotope ratios. Oecologia 159:41–48
Shaffer SA, Costa DP, Weimerskirch H (2003) Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct Ecol 17:66–74
Shoji A, Aris-Brosou S, Fayet A, Padget O, Perrins C, Guilford T (2015) Dual foraging and pair coordination during chick provisioning by Manx shearwaters: empirical evidence supported by a simple model. J Exp Biol 218:2116–2123
Sibly RM, Witt CC, Wright NA, Venditti C, Jetz W, Brown JH (2012) Energetics, lifestyle, and reproduction in birds. Proc Natl Acad Sci USA 109:10937–10941
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Steen H, Vogedes D, Broms F, Falk-Petersen S, Berge J (2007) Dovekies (Alle alle) breeding in a High Arctic fjord system: bimodal foraging strategies as a response to poor food quality? Polar Res 26:118–125
Thabet I, Bourgeois K, Le Loc’h F, Abdennadher A, Munaron J-M, Gharsalli M, Romdhane MS, Ben Rais Lasram F (2019) Trophic ecology of Scopoli’s shearwaters during breeding in the Zembra Archipelago (northern Tunisia). Mar Biol 166:61
Thompson DR, Phillips RA, Stewart FM, Waldron S (2000) Low δ13C in pelagic seabirds: lipid ingestion as a potential source of 13C-depleted carbon in Procellariiformes. Mar Ecol Prog Ser 208:265–271
Tremblay Y, Cherel Y (2003) Geographic variation in the foraging behaviour, diet and chick growth of rockhopper penguins. Mar Ecol Prog Ser 251:279–297
Warham J (1977) The incidence, functions and ecological significance of petrel stomach oils. Proc N Z Ecol Soc 24:84–93
Warham J (1990) The petrels: their ecology and breeding systems. Academic Press, London
Warham J (1996) The behaviour, population, biology and physiology of the petrels. Academic Press, London
Weimerskirch H, Chastel O, Ackermann L, Chaurand T, Cuenotchaillet F, Hindermeyer X, Judas J (1994) Alternate long and short foraging trips in pelagic seabird parents. Anim Behav 47:472–476
Whittow GC (2001) Seabird reproductive physiology and energetics. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, Boca Raton, pp 409–437
Williams CT, Buck CL, Sears J, Kitaysky AS (2007) Effects of nutritional restriction on nitrogen and carbon stable isotopes in growing seabirds. Oecologia 153:11–18
Wood JR, Lawrence HA, Scofield RP, Taylor GA, Lyver POB, Gleeson DM (2017) Morphological, behavioural, and genetic evidence supports reinstatement of full species status for the Grey-faced Petrel, Pterodroma macroptera gouldi (Procellariiformes: Procellariidae). Zool J Linn Soc 179:201–216
Yamamoto T, Hoshina K, Nishizawa B, Meathrel CE, Phillips RA, Watanuki Y (2015) Annual and seasonal movements of migrating short-tailed shearwaters reflect environmental variation in sub-Arctic and Arctic waters. Mar Biol 162:413–424
Zango L, Navarro-Herrero L, García-Vendrell M, Safi K, González-Solís J (2020) Niche partitioning and individual specialization among age, breeding status and sex classes in a long-lived seabird. Anim Behav 170:1–14
Zimmer I, Ropert-Coudert Y, Poulin N, Kato A, Chiaradia A (2011) Evaluating the relative importance of intrinsic and extrinsic factors on the foraging activity of top predators: a case study on female little penguins. Mar Biol 158:715–722
Acknowledgements
We are very grateful to the University of Auckland, the Royal Forest and Bird Protection Society of New Zealand and the European Union for funding this research. We are also very thankful to the Department of Conservation and island owners for granting permission and providing support to conduct this research. We thank all those who helped us during fieldwork, especially Marek Lang, Nalini Singh and Hendrik Schultz. We are grateful to the reviewers for their constructive comments on and improvements to the manuscript.
Funding
Funds and support were provided by the School of Biological Sciences, University of Auckland (SBS Internal Research Fund) and the Royal Forest and Bird Protection Society of New Zealand (JS Watson Trust). KB was supported by a Marie Curie Individual Fellowship (PIOF-GA-2012-328309).
Author information
Authors and Affiliations
Contributions
KB and JCR conceived and designed the research. KB, JRW, SD and GAT carried out the fieldwork. KB and JRW analysed regurgitates. KB and JCR performed statistical analyses. KB wrote the manuscript and made the figures, with inputs from JCR and GAT. All authors contributed to and commented on manuscript drafts, and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Ethics approval
This work was conducted with ethics approval from the University of Auckland Animal Ethics Committee (AEC R898), and banding permit (#2010/021) and research permission (34780-RES and 38573-FAU) from the New Zealand Department of Conservation. Thus, all applicable international, national, and institutional guidelines for the sampling and the care of animals alive have been followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bourgeois, K., Welch, J.R., Dromzée, S. et al. Flexible foraging strategies in a highly pelagic seabird revealed by seasonal isotopic niche variation. Mar Biol 169, 28 (2022). https://doi.org/10.1007/s00227-021-04011-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-04011-w