Abstract
Southern rockhopper penguins (Eudyptes chrysocome chrysocome) have experienced severe population declines across their distribution area, potentially in response to bottom-up effects following elevated sea surface temperatures, changes in the food web and prey availability. We conducted stable isotope analysis to compare trophic levels and distribution patterns in the non-breeding period over three consecutive years, and between males and females, using egg membranes, blood cells and feathers of parent birds. Tissues representing the non-breeding season had lower δ13C values than prey sampled around the Falklands and red blood cells from breeding rockhopper penguins. In contrast, δ15N values were higher in red blood cells from the end of winter compared to those from the breeding season and compared to feathers. This indicated that rockhopper penguins left the Falkland Island area in the non-breeding season and foraged either around Burdwood Bank further south, or over the Patagonian Shelf. In winter, only males took more prey of higher trophic level than females. Inter-annual differences in isotopic values partly correlated with sea surface temperatures. However, as prey isotope samples were collected only in 1 year, inter-annual differences in penguin isotopic values may result from different foraging sites, different prey choice or different isotopic baseline values. Our study highlights the potential for stable isotope analyses to detect seasonal and gender-specific differences in foraging areas and trophic levels, while stressing the need for more sampling of isotopic baseline data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the main impacts of global climate change is the alteration of marine ecosystems by water temperature increase and ocean acidification (Behrenfeld et al. 2006; Beardall et al. 2009; Iglesias-Rodriguez et al. 2008). While ocean acidification primarily affects calcification processes and has only secondary effects on the phytoplankton composition and food (e.g. Riebesell et al. 2000), elevated sea surface temperatures influence the marine ecosystem directly. In warm surface waters, macronutrients are depleted and primary productivity decreases, leading to a lower carrying capacity of oceans with consequences for predators like seabirds (Schell 2000; Hirons et al. 2001; Weimerskirch et al. 2003; Jenouvrier et al. 2005; Jaeger and Cherel 2011). The southern ocean around the western Antarctic Peninsula undergoes one of the fastest changes worldwide (Clarke et al. 2007). Warming of the Antarctic circumpolar current may also affect marine ecosystems further north. The cold Falkland current originates from the Antarctic circumpolar current and transports nutrient-rich waters to the Patagonian Shelf and the Falkland Islands (Peterson and Whitworth 1989; see Fig. 1 for an overview of the area). Thus, changes in temperature or strength of this current may influence the food web in this productive marine area, as reflected in food availability to marine predators (Quillfeldt et al. 2007).
In this context, stable isotope analysis can be used as a non-invasive method to study food webs and the foraging behaviour of predators at the end of the food chain, e.g., in predatory seabirds (e.g. Quillfeldt et al. 2005, 2008b; Cherel et al. 2006; Gladbach et al. 2007). The use of stable isotope analysis in food web studies is based upon the assumption that the isotopic composition of prey items matches the consumer tissues with a predictable discrimination factor. Isotopic discrimination, i.e., the accumulation of heavy in relation to light isotopes from prey to predator during enzymatic metabolic processes, leads to an increase in nitrogen isotope ratios (δ15N) by 3–5‰ per trophic level (Minagawa and Wada 1984; Owens 1987). Thus, nitrogen isotopes can be used to assess the trophic level. In contrast, carbon isotope ratios (δ13C) are less affected by isotopic discrimination, but instead can be used to assess the origin of seabird prey because baseline δ13C varies from the benthic to the pelagic and also along latitudes (reviewed in Rubenstein and Hobson 2004).
Moreover, different tissues integrate over different time intervals. Feathers, as metabolically inert keratinous products, are similar in isotopic composition to the food that was taken up during moult. However, penguins fast during moult, and therefore, feathers reflect the diet within the last weeks prior to moult (Cherel et al. 2005a). Egg membranes also remain metabolically inert (Oppel et al. 2009) and therefore match in isotopic composition with the diet prior to or during egg formation (Quillfeldt et al. 2009). Additionally, compared to other egg components such as albumen, egg membranes can be obtained non-invasively (Quillfeldt et al. 2009). Red blood cells that are constantly renewed by the organism have an isotopic half-life of 12–20 days and therefore reflect the diet of the last weeks prior to sampling (Hobson and Clark 1992; Hobson 1999, 2007). As a consequence, stable isotope analysis can be used to determine the importance of certain prey types and the trophic level within the food web, but also provide an estimate of foraging areas at different times. However, as isotopic values of most tissues reflect the diet of several weeks, this value does not necessarily reflect an isotopic equilibrium, but could also integrate more than one foraging area when the animal moves through an isoscape (e.g. Hobson 1999, 2008).
The southern rockhopper penguin (Eudyptes chrysocome chrysocome) inhabits the southern Atlantic Ocean around the tip of South America including the Falkland Islands (Malvinas). In the last decades, the population has experienced a stepwise decline to less than 20% of its original size on the Falkland Islands, but also in other areas (Pütz et al. 2003a and literature therein). Elevated sea surface temperatures and their consequences are one of the factors that have been discussed as a reason for the decline (Birdlife International 2010). Hilton et al. (2006) found evidence for a decrease in primary productivity and a negative correlation between sea surface temperatures and trophic level, using stable isotope analysis of feather samples dating back to 1861. These data reflect a long time series. However, visible mortality in rockhopper penguins during the past decades occurred periodically during periods of unfavourable conditions (e.g. Lyster 1986; Cunningham and Moors 1994; Keymer et al. 2001;Huin 2003). Among the diving seabirds breeding in the Falkland Islands, rockhopper penguins occupy the lowest trophic level (Weiss et al. 2009, Masello et al. 2010), such that this species is likely to be directly and rapidly affected by changes at the base of the food webs.
Southern rockhopper penguins have a strongly synchronised breeding cycle and come ashore only to breed (September–February) and to moult (March–April) (Strange 1982; Williams 1995). Prey species and foraging areas during the breeding season are relatively well known (Boersma et al. 2002; Pütz et al. 2003b; Raya Rey and Schiavini 2005; Raya Rey et al. 2007b; Masello et al. 2010) but less data are available for the inter-breeding season between April and September (Pütz et al. 2002, 2006b; Raya Rey et al. 2007a). Seabirds at sea surveys from vessels revealed none or only few observations of rockhopper penguins around the Falkland Islands during the non-breeding season (White et al. 1999, 2001). Southern rockhopper penguins from the Falklands equipped with satellite transmitters dispersed mainly west and north along the Patagonian Shelf and less frequently towards Burdwood Bank south of the Falklands (Pütz et al. 2002). Inter-annual differences in these distribution patterns are thought to be related to sea surface temperatures and food availability, while there were also differences in the directions of satellite tracks of birds equipped in different colonies (Pütz et al. 2002). However, these satellite data only covered the first 3 months after moult (i.e. April–June), leaving a gap of about 3 months until the start of the next breeding season in October. A stable isotope study of adults arriving to breed in the Crozet Islands indicated that eastern rockhopper penguins (Eudyptes chrysocome filholi) spent the last weeks of winter in oceanic waters north of their breeding grounds (Cherel et al. 2007). Focussing on the Falkland Island population of southern rockhopper penguins, the present study aimed at collecting more information about foraging grounds and prey in the non-breeding period and the relationship with sea surface temperatures. In detail, we focussed on:
-
the end of summer, namely the weeks prior to moult (i.e. feathers of adults) and
-
the last months of winter, by using tissues that were built at the end of winter or from resources that were accumulated during that time (i.e. red blood cells at the beginning of the breeding period and egg membranes).
As rockhopper penguins exhibit some sexual size dimorphism (e.g. Williams 1995; Poisbleau et al. 2010) that might influence their foraging behaviour and prey choice (Forero et al. 2002; Kato et al. 2000), we also analysed sexual differences in stable isotope ratios.
Materials and methods
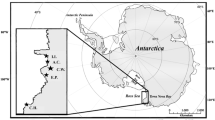
Fieldwork was conducted in the ‘Settlement Colony’ on New Island, Falkland Islands (51°43′S, 61°17′W) as part of an ongoing study on southern rockhopper penguins (e.g. Poisbleau et al. 2008, 2009). New Island, in the westernmost part of the Falkland Island archipelago, is situated close to the western branch of the Falkland current.
Sample collection
An overview of samples and sample sizes is given in Table 1. In the season 2005/06, we randomly collected eggshells in the colony after chicks had hatched. In 2006/07 and 2007/08, eggshells were collected from study nests once the chicks had hatched.
Red blood cells and feathers were taken from adults of study nests in the breeding seasons 2006/07 and 2007/08. Birds were captured by hand from their nests at the beginning of the incubation period. Blood samples were taken from the brachial vein within 3 min after capture (heparinised syringe, 23-gauge needle). Feathers were gently pulled out of the skin (2 black and 2 white feathers per individual). Blood samples were centrifuged, and plasma was removed for hormone analysis. Red blood cell samples were subsequently frozen (−20°C) and later dried in a drying furnace (60°C).
Additional prey samples
The Falkland Islands Fisheries Department kindly provided samples obtained during a survey cruise in February 2006 by bottom and plankton trawling close to the Falkland Islands (P. Brickle, personal communication). We grouped these samples in fish (muscle of southern blue whiting Micromesistius australis, hoki Macruronus magellanicus, pike icefish Champsocephalus esox, yellowfin rockcod Patagonotothen guntheri and Patagonian rockcod Patagonotothen ramsayi), squid (muscle samples of Argentine shortfin squid Illex argentinus, great hooked squid Moroteuthis ingens and Patagonian longfin squid Loligo gahi) and krill (Euphausia lucens and Thysanoessa macrura). All these species are abundant in Falkland waters and have been identified as major prey species for rockhopper penguins by conventional diet analysis (e.g. Croxall et al. 1985; Thompson 1989).
Sea surface temperatures
Sea surface temperature anomaly (SSTA) data in the Falkland current area (50–52°S, 61–63°W) were downloaded from http://ingrid.ldeo.columbia.edu/SOURCES/.NOAA/.NCEP/.EMC/.CMB/.GLOBAL/.Reyn_SmithOIv2/.weekly/.ssta/.
Stable isotope analysis
Stable isotope analysis of penguin tissues was carried out at the Leibniz Institute for Zoo and Wildlife Research, Berlin, Germany. We used stainless steel scissors and scalpels to cut small fragments of feathers and egg membranes. Both tissue types were free from any obvious contamination. As feather tips are synthesised already at sea and might differ in δ13C and δ15N compared to the rest of the feather (Cherel et al. 2005a), we only used the proximal parts of the barb for stable isotope analysis. Dried red blood cells were homogenised and ground to a fine powder. Stable carbon and nitrogen isotope analyses were carried out on 0.35 mg aliquots, weighed into tin cups. Stable carbon and nitrogen isotope ratios were measured simultaneously by continuous-flow isotope ratio mass spectrometry using a Flash Elemental Analyser (Thermo Finnigan, Bremen, Germany) linked to a Delta V Advantage Isotope Ratio Mass Spectrometer (Thermo Finnigan, Bremen, Germany). Two laboratory standards were analysed for every ten unknown samples, allowing any instrument drift over a typical 14-h run to be corrected. Stable isotope ratios were expressed in δ notation as parts per thousand (‰) deviation from the international standards V-Pee Dee Belemnite (carbon) and AIR (nitrogen), according to the following equation δX = [(R sample/R standard) − 1] × 1,000 where X is 15N or 13C and R is the corresponding ratio 15N/14N or 13C/12C. Based on internal standards (N = 165, tyrosin; Roth, Germany), the analytical precision (±1 SD) equalled ±0.16‰ and ±0.29‰ for δ15N and δ13C, respectively.
Feather stable isotope samples contained black and white parts in varying amounts, as we were not aware of possible differences in stable isotope values between black and white feathers caused most likely by melanin (Michalik et al. 2010). However, the described differences between black and white feathers of the same individual ranged only from 0.2 to 0.7 for δ13C and −0.3 to 0.3 for δ15N (Michalik et al. 2010). Applied to our data, this small range should not have a significant effect on the results, as the range is smaller than the standard deviation of the groups that were compared with each other.
All prey samples were fat extracted using a Soxhlet apparatus with a methanol-to-chloroform 2:1 solvent. Crustacean samples were additionally acid washed with HCl and analysed at the NERC Life Sciences Mass Spectrometry Facility, Glasgow, as described earlier (Weiss et al. 2009).
Statistics
Statistical testing was carried out using SPSS 16.0. Data were tested for normal distribution with Kolmogorov–Smirnoff tests and for homogeneity of variances with Levene’s tests. Adult blood data from the season 2006/07 did not show homogeneity of variances, and sample sizes were small; therefore, we tested these groups non-parametrically with Mann–Whitney U tests, but not with λ-Wilk tests.
To compare blood and feathers with each other and with prey types, we corrected our measured stable isotope values, using isotopic discrimination factors. Cherel et al. (2005b) presented discrimination factors for captive rockhopper penguins that were on a constant diet of herring and capelin and suggested to use these discrimination factors with care and rather to apply them to wild fish-eating birds. However, rockhopper penguins in the wild mainly feed on pelagic crustaceans, squid and small fish (e.g. Cooper et al. 1990; Thompson 1994; Raya Rey and Schiavini 2005). Moreover, due to changes in metabolism, body size and activity, discrimination factors obtained in captive individuals might differ from those in free-living animals (Carleton and de Rio 2005; Tierney et al. 2008 and literature therein). Therefore, we used mean isotopic discrimination rates of marine birds for blood (reviewed in Caut et al. 2009) that presumably had a mixed diet not only consisting of fish: Δ13Cdiet-blood = 0.6‰, Δ15Ndiet-blood = 2‰. For feathers, we applied the discrimination factors obtained by Bearhop et al. (2002) for skuas (Catharacta skua): Δ13Cdiet-feathers = 2‰, Δ15Ndiet-feathers = 4‰.
Results
End of summer—feathers
Stable isotope values from feather samples of adult birds differed significantly between the years (Fig. 2; Wilk’s λ = 0.625, P < 0.001). δ13C and δ15N were significantly lower in 2006/07 compared to 2007/08 only (t tests: δ13C: t = −3.5, df = 45, P = 0.001; δ15N: t = −4.9, df = 45, P < 0.001). Isotopic values in feathers did not differ between the sexes (t tests within seasons: all P > 0.3).
End of winter
Red blood cells
Red blood cell stable isotope values differed between years and sexes (Fig. 3).
For the year 2006/2007, δ15N values were higher in males compared to females, and the same trend was found in 2007/08, although not significant (Mann–Whitney U tests for 2006/07: U = 4.0, P = 0.014; 2007/08: U = 24.0, P = 0.052). In contrast, in both years, δ13C values did not differ between the sexes (Mann–Whitney U tests for 2006/07: U = 20.0, P = 0.945; 2007/08: U = 40.0, P = 0.481).
In 2007/08, adults of both sexes had lower δ13C values compared to the previous year (Fig. 3), while δ15N values did not differ significantly (Mann–Whitney U tests: δ13C for males U = 6.0, P = 0.007 and females U = 13.0, P = 0.033; δ15N for males U = 24.0, P = 0.562 and females U = 16.0, P = 0.070).
Egg membranes
We found differences in stable isotope values of egg membranes among the 3 years (Fig. 3; Wilk’s λ = 0.408, P < 0.001; ANOVA: δ13C: F 9, 2 = 26.5, P < 0.001; δ15N: F 18, 2 = 9.2, P < 0.001). Egg membranes in 2007/08 had lower δ13C values than in 2005/06 and 2006/07, but δ15N values differed only between 2006/07 and 2007/08 (Bonferroni post hoc tests) and not between 2005/06 and 2006/07 (Bonferroni post hoc tests).
Comparison of rockhopper tissues and their prey
Stable isotope values of blood cells and feathers from the non-breeding season that were corrected for trophic discrimination had lower δ13C values than prey species around the Falklands (Fig. 4). For comparative purposes, we added a red blood cell sample of adults collected during crèche (end of the breeding season; taken in January 2007; see Table 1). These samples had higher δ13C values compared to the non-breeding season samples and were in the range of isotope ratios recorded in prey species. Thus, rockhopper penguin tissues that were formed in late winter and late summer had lower δ13C values than tissues formed during the breeding season.
Corrected stable nitrogen and carbon isotope ratios (‰) of prey and rockhopper penguin red blood cells and feathers (means ± SD). We corrected for the discrimination of 13C and 15N in the tissue compared with prey values by subtracting the discrimination factors described for seabird tissues in the literature. The triangular-shaped lines connecting stable isotope ratios of potential prey give the isotopic range that would reflect foraging areas and prey around the Falklands. To better compare with the breeding season, we added a red blood cell sample from the breeding season 2006/07. RBC stands for red blood cells
Discussion
In the present study, the stable isotope ratios of rockhopper penguin tissues differed among the study years. Inter-annual changes were found for two time periods, the end of winter and end of summer. Moreover, we found sexual differences in nitrogen isotope ratios of red blood cells of birds returning to breed, indicating that, at the end of the winter 2006, males foraged on prey of higher trophic level than females. For the winter 2007, we found the same trend, but low sample sizes might have precluded finding significance. At the end of summer, feather samples revealed no differences in isotopic composition between foraging areas or trophic level of males and females. All rockhopper penguin tissues from the non-breeding season were less enriched in 13C than the prey species sampled around the Falklands and also than rockhopper penguin red blood cells that represented the breeding season. Our values for red blood cells from the end of winter were similar to isotopic values of rockhopper penguin muscle tissue collected in breeding colonies on the Patagonian Shelf (Ciancio et al. 2008; see Table 2). In contrast, rockhopper penguins from the Crozet Islands had similarly depleted δ13C values, but much lower δ15N values in blood throughout the year, while feathers had both lower δ13C and δ15N values compared to our data (Cherel et al. 2007; Jaeger and Cherel 2011; see Table 2). The difference in δ15N values between the Crozet Islands and the Falkland Islands/Patagonian Shelf might indicate that rockhoppers from the Crozet archipelago feed predominantly on crustaceans, i.e., on a lower trophic level. However, within the Falkland Island penguin assemblage, the rockhopper penguin clearly had the lowest trophic level during the breeding season, indicating that crustaceans had a higher proportion in the rockhopper penguin diet compared to Magellanic (Spheniscus magellanicus) and gentoo penguins (Pygoscelis papua) (Weiss et al. 2009; Masello et al. 2010). The diet of rockhopper penguins in the non-breeding season is unknown, and at this point, a higher dependence on fish such as sardines (see e.g. Raya Rey and Schiavini 2005 for diet in Patagonia) cannot be excluded. However, the strong differences in rockhopper penguin isotopic ratios between the Falkland Islands/Patagonian Shelf versus Crozet Islands could also be related to the oceanographic conditions: while the Crozet Islands are located in the middle of the Polar Frontal Zone, just north of the Polar Front, the Falkland Islands are located about 300 km north of the Polar Front (see Fig. 1). The southern ocean, south of the Antarctic Polar Front, is marked by depleted δ13C values (see Quillfeldt et al. 2010 for isoscape) as well as a crustacean-based food chain (Knox 2006).
Distribution in the non-breeding period
In comparison with prey and red blood cells from the breeding season 2006/07, lower δ13C values indicate that rockhopper penguins from New Island foraged during winter in different areas than during the breeding season. Quillfeldt et al. (2010) published an isoscape for the area around the Falkland Islands, based on isotopic values of phytoplankton. A comparison of our results with this map indicates two alternative foraging areas for rockhopper penguins from New Island during the non-breeding season: (1) the Patagonian Shelf in the west of the Falklands or (2) the Burdwood Bank, a shallow part of the Scotia Ridge, about 200 km south of the Falklands. Both areas would agree with the high δ15N values in rockhopper penguin blood and feathers that indicate productive neritic foraging areas.
While isotopic values from potential penguin prey species are not available for the Burdwood Bank, Ciancio et al. (2008) published values from breeding rockhopper penguins and prey species from the Patagonian Shelf (see Table 2). Potential rockhopper penguin prey on the Patagonian Shelf had comparatively lower δ13C values than prey around the Falkland Islands and could therefore explain the isotopic values in rockhopper penguins from the Falklands at the end of the winter.
Our results of neritic foraging areas, most likely on the Patagonian Shelf or around the Burdwood Bank, agree with the satellite tracking data of rockhopper penguins from the Falkland Islands, obtained by Pütz et al. (2002) for the beginning of the winter distribution. Rockhopper penguins from different breeding populations in the Falkland Islands foraged mainly on the Patagonian Shelf west and north of the Falkland Islands, although some individuals also went south towards the Burdwood Bank or to more easterly locations on the Falkland Shelf during their winter distribution (Pütz et al. 2002). Combining these results with our findings, rockhopper penguins from the Falkland Islands seem to use neritic waters as foraging ground throughout the winter. Moreover, it seems that the population from New Island in the westernmost part of the archipelago of the Falklands used more westerly or southerly foraging grounds either for the whole winter, or only for the end of winter, compared to conspecifics from other breeding colonies on the Falklands, that also went north along the Patagonian Shelf. Furthermore, our findings indicate that rockhopper penguins from New Island could use the same foraging areas in winter as during the breeding season (Boersma et al. 2002; Ludynia et al. unpublished data). These foraging areas overlap marginally with those of rockhopper penguins breeding on Staten Island, Argentina, during winter as well as during incubation trips (Pütz et al. 2006a, b; Raya Rey et al. 2007a).
After correction for trophic discrimination, feathers had δ15N values that were 2–3‰ lower than those of red blood cells, i.e., nearly a whole trophic level (e.g. Minagawa and Wada 1984; Ogden et al. 2004; Weiss et al. 2009). δ13C values of feathers were also lower than those of red blood cells, yet, only in the range of 1–1.5‰. Across species, feathers are enriched in 15N and 13C in relation to blood cells integrating the same time of assimilation (Quillfeldt et al. 2008a). In our data, however, the uncorrected values of red blood cells were already higher in δ15N compared with uncorrected values of feathers. Thus, even though the discrimination factors that we used were not previously applied to rockhopper penguins, our data still suggest a strong difference in the isotopic composition of prey at the end of winter compared to end of summer. The lower δ15N values in feathers indicate that rockhopper penguins prior to moult took prey of lower trophic levels (i.e. more crustaceans) than at the end of winter. The difference in δ13C values between feathers and red blood cells was not strong enough to reflect large-scale differences in foraging areas (compare with isoscape in Quillfeldt et al. 2010). However, the low δ13C values in feathers suggest that foraging areas were less productive at the end of summer compared to the end of winter, as δ13C tends to decrease when cell growth, e.g., of phytoplankton, takes place slowly (Hilton et al. 2006 and literature therein). This would also explain the lower trophic level of food, indicated by lower δ15N values prior to moult.
Sexual differences
We found gender-specific differences in the stable isotope ratios of red blood cells, but not in those of feathers. Incubating males had δ15N values, which were 0.5–1.0‰ higher than those of females, while δ13C was similar in both sexes. These results indicate that foraging grounds were similar for both sexes in all studied time periods, and males foraged on a higher trophic level than females only during winter, but not during the feed up to moult. Rockhopper penguin males are larger than females (Williams 1995; Poisbleau et al. 2010), and their longer and wider bill is likely to enable males to hold larger prey items. Even more important, however, in penguins as in other seabirds, body mass is correlated with diving capacity (Schreer and Kovacs 1997), and larger penguin species spend more time in deeper depths (Wilson et al. 1991). Thus, rockhopper penguin males can dive deeper and longer (e.g. see Pütz et al. 2006a and literature therein) and handle larger prey specimens than females. As larger fish are generally higher in trophic level (e.g. Fig. 6 in Jennings et al. 2001), this can explain the observed differences in trophic positions between male and female rockhopper penguins at the end of winter. Another possible explanation would be that male rockhopper penguins reach bottom prey that is known to have different isotopic values then prey of the pelagic food chain (Rubenstein and Hobson 2004). However, penguins tend to feed on schooling prey (Davis and Renner 2003; Wilson 1995), and water depths in foraging areas reach up to 200 m (see Fig. 1). As mean diving depths of male rockhopper penguins in the Falklands during the breeding season averaged 21 m (Pütz et al. 2006a), it is unlikely that male rockhopper penguins dive to the bottom to forage on benthic prey regularly.
Sexual differences in stable isotope values have also been described for Adélie (Pygoscelis adeliae), gentoo and Magellanic penguins (Forero et al. 2002; Bearhop et al. 2006; Beaulieu et al. 2010). In all three species, males had higher δ15N values than females, reflecting prey of higher trophic levels, and male Adélie penguins also foraged further offshore than females. In macaroni penguins (Eudyptes chrysolophus) from South Georgia, Bearhop et al. (2006) found two alternative foraging strategies in females, leading to a higher variation in δ13C values compared to males. Male isotopic values differed significantly from those of females that did short foraging trips (Bearhop et al. 2006). For the Crozet Islands, however, Cherel et al. (2007) did not find significant differences between male and female isotope signatures in macaroni penguins, nor for rockhopper penguins.
Yet, we did not find sexual differences in stable isotope samples of rockhopper penguins prior to moult, i.e., at the end of summer. Foraging areas of rockhopper penguins during this period seemed to be less productive, and penguins took food of lower trophic levels compared to the end of the winter. Under these changed and most likely less optimal foraging conditions, males and females obviously took similar prey, resulting in similar stable isotope values for both sexes.
Inter-annual differences
Our results for all analysed tissues revealed inter-annual differences in stable isotope values. For logistical reasons, however, our prey samples were collected during the breeding season around the Falkland Islands only in 1 year. Thus, inter-annual changes may have different possible explanations. Differences in rockhopper penguin tissue isotopes may result from changes in prey, changes in foraging areas or changes in prey isotope values caused by isotopic baseline changes with environmental conditions. We discuss these scenarios in the following paragraph.
End of summer
Feather samples from the season 2006/07 had lower δ15N and δ13C values than those from the following season. This result suggests that penguins used either slightly different foraging areas or that primary productivity was lower in the foraging grounds prior to moult in 2006. During this time, February to March 2006, sea surface temperatures were elevated (Fig. 5), which could have led to a decrease in primary productivity of ocean water. In contrast, the time prior to moult in 2007 was characterised by lower sea surface temperatures, which might have enhanced primary productivity, thus leading to higher δ13C values and the possibility of foraging in higher trophic levels. Therefore, our results are in line with the expectation that elevated sea surface temperatures lead to lower primary productivity and changes in the food composition for penguins. Yet, we do not know prey isotopic values from 2007/08 and thus cannot exclude the possibility that the geographic distribution of isotopic signatures differed with altered current conditions, and this was reflected at higher trophic levels.
Sea surface temperature anomaly data from January 2005 until December 2007 for the Falkland current area adjacent to the Falkland Islands (50–2°S, 61–63°W). Black-labelled periods correspond with the time prior to moult and light-grey-labelled periods mark the last months of winter, prior to the start of the breeding season
End of winter
Stable isotope signatures of egg membranes and red blood cells of adults differed among the seasons and both tissues showed the same general pattern: δ15N and δ13C were higher in the season 2006/07 than in the following year. Egg membrane isotopic signatures did not differ between the seasons 2005/06 and 2006/07.
Sea surface temperatures (Fig. 5) in the austral winter prior to the breeding season 2005/06 and 2006/07 were warmer compared with 2007/08. This can explain first of all, why egg membrane isotopic signatures were similar in 2005/06 and 2006/07. The season 2007/08 was characterised by a cold influx of more southerly waters, caused by a strong Falkland current. The low δ13C values from tissues grown in the austral winter 2007 are consistent with a shift of southern water masses further to the north.
Future studies should, therefore, include prey isotope samples of different areas and time periods. A combination with geolocation data recording positions of penguins during the winter would also be able to greatly enhance our knowledge on winter distribution and foraging ecology, especially in combination with stable isotope analyses (e.g. Bost et al. 2009).
Conclusion
Our data suggest that rockhopper penguins from New Island spend the inter-breeding season in neritic waters, west or south of the Falklands. We found a difference in isotopic values between feathers (end of summer) and red blood cells (end of winter), indicating that foraging grounds at the end of summer were less productive. In comparison with previously described winter distributions of the species, our results reinforce previous findings that rockhopper penguin populations from the Falklands utilise the Patagonian Shelf or the Burdwood Bank to forage.
We found sexual differences in stable isotope ratios from the end of the winter, indicating that males take more prey of higher trophic level in winter than females. The interpretation of inter-annual differences was partly impaired by the lack of baseline prey isotopic values of the different years. Thus, our study highlights the potential of stable isotope analyses to detect differences in foraging areas and trophic levels, while stressing the need for more detailed sampling of isotopic baseline data.
References
Beardall J, Stojkovic S, Larsen S (2009) Living in a high CO2 world: impacts of global climate change on marine phytoplankton. Plant Ecol Divers 2:191–205
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164
Beaulieu M, Spee M, Lazin D, Ropert-Coudert Y, Le Maho Y, Ancel A, Raclot T (2010) Ecophysiological response of Adélie penguins facing an experimental increase in breeding constraints. J Exp Biol 213:33–39
Behrenfeld MJ, O’Malley RT, Siegel DA, McClain CR, Sarmiento JL, Feldman GC, Milligan AJ, Falkowski PG, Letelier RM, Boss ES (2006) Climate-driven trends in contemporary ocean productivity. Nature 444:752–755
Birdlife International (2010) Rockhopper penguins. A plan for research and conservation to investigate and address population changes. In: Proceedings of an international workshop, Edinburgh, 3–5 June 2008
Boersma PD, Stokes DL, Strange IJ (2002) Applying ecology to conservation: tracking breeding penguins at New Island South Reserve, Falkland Islands. Aquatic Conserv Mar Freshw Ecosyst 12:63–74
Bost CA, Thiebot JB, Pinaud D, Cherel Y, Trathan PN (2009) Where do penguins go during the inter-breeding period? Using geolocation to track the winter dispersion of the macaroni penguin. Biol Lett 5:473–476
Carleton S, de Rio C (2005) The effect of cold-induced increased metabolic rate on the rate of 13C and 15 N incorporation in house sparrows (Passer domesticus). Oecologia 144:226–232
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Delta N-15 and Delta C-13): the effect of diet isotopic values and applications for diet reconstruction. J Applied Ecol 46:443–453
Cherel Y, Hobson KA, Bailleul FR, Groscolas R (2005a) Nutrition, physiology, and stable isotopes: new information from fasting and molting penguins. Ecology 86:2881–2888
Cherel Y, Hobson KA, Hassani S (2005b) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115
Cherel Y, Phillips RA, Hobson KA, McGill R (2006) Stable isotope evidence of diverse species-specific and individual wintering strategies in seabirds. Biol Lett 2:301–303
Cherel Y, Hobson KA, Guinet C, Vanpe C (2007) Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. J Anim Ecol 76:826–836
Ciancio JE, Pascual MA, Botto F, Frere E, Iribarne O (2008) Trophic relationships of exotic anadromous salmonids in the southern Patagonian Shelf as inferred from stable isotopes. Limn Oceanogr 53:788–798
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DKA, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Phil Trans R Soc B 362:149–166
Cooper J, Brown CR, Gales RP, Hindell MA, Klages NTW, Moors PJ, Pemberton D, Ridoux KR, Thompson KR, Van Heezik YM (1990) Diets and dietary segreation of crested penguins (Eudyptes). In: Davies LS, Darby JT (eds) Penguin Biology. Academic Press, San Diego, pp 131–156
Croxall JP, Prince PA, Baird A, Ward P (1985) The diet of the southern rockhopper penguin Eudyptes chrysocome chrysocome at Beauchene Island, Falkland-Islands. J Zool 206:485–496
Cunningham DM, Moors PJ (1994) The decline of Rockhopper penguins Eudyptes chrysocome at Campbell Island, Southern Ocean and the influence of rising sea temperatures. Emu 94:27–36
Davis LS, Renner M (2003) Penguins. Yale University Press, New Haven and London
Forero MG, Hobson KA, Bortolotti GR, Donazar JA, Bertellotti M, Blanco G (2002) Food resource utilisation by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299
Gladbach A, McGill RAR, Quillfeldt P (2007) Foraging areas of Wilson’s storm-petrel Oceanites oceanicus in the breeding and inter-breeding period determined by stable isotope analysis. Polar Biol 30:1005–1012
Hilton GM, Thompson DR, Sagar PM, Cuthbert RJ, Cherel Y, Bury SJ (2006) A stable isotopic investigation into the causes of decline in a sub-Antarctic predator, the rockhopper penguin Eudyptes chrysocome. Glob Change Biol 12:611–625
Hirons AC, Schell DM, Finney BP (2001) Temporal records of delta C-13 and delta N-15 in North Pacific pinnipeds: inferences regarding environmental change and diet. Oecologia 129:591–601
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Hobson KA (2007) An isotopoic exploration of the potential of avian tissues to track changes in terrestrial and marine ecosystems. In: Dawson TE, Siegwolf TW (eds) Stable isotopes as indicators of ecological change, 1st edn. Elsevier Academic Press, London, pp 129–144
Hobson KA (2008) Isotopic tracking of migrant wildlife. In: Michener RH, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Publishing Ltd., Oxford, pp 155–175
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I. Turnover of 13C in tissues. Condor 94:181–188
Huin N (2003) Falkland Island Seabird Monitoring Programme Annual Report 2002/03. Falklands Conservation
Iglesias-Rodriguez MD, Halloran PR, Rickaby REM, Hall IR, Colmenero-Hidalgo E, Gittins JR, Green DRH, Tyrrell T, Gibbs SJ, von Dassow P, Rehm E, Armbrust EV, Boessenkool KP (2008) Phytoplankton calcification in a high-CO2 world. Sci 320:336–340
Jaeger A, Cherel Y (2011) Isotopic investigation of contemporary and historic changes in penguin trophic niches and carrying capacity of the Southern Indian Ocean. PLoS ONE 6:e16484
Jennings S, Pinnegar JK, Polunin NVC, Boon TW (2001) Weak cross-species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J Anim Ecol 70:934–944
Jenouvrier S, Barbraud C, Weimerskirch H (2005) Long-term contrasted responses to climate of two Antarctic seabird species. Ecology 86:2889–2903
Kato A, Watanuki Y, Nishiumi I, Kuroki M, Shaughnessy P, Naito Y (2000) Variation in foraging and parental behavior of king cormorants. Auk 117:718–730
Keymer IF, Malcolm HM, Hunt A, Horsley DT (2001) Health evaluation of penguins (Sphenisciformes) following mortality in the Falklands (South Atlantic). Dis Aquat Org 45:159–169
Knox GA (2006) Biology of the Southern Ocean. CRC Press Taylor & Francis Group, Boca Raton
Lyster S (1986) Penguin mortality in the Falklands. Oryx 20:206–207
Masello JF, Mundry R, Poisbleau M, Demongin L, Voigt CC, Wikelski M, Quillfeldt P (2010) Diving seabirds share foraging space within and among species. Ecosphere 1:19
Michalik A, McGill RAR, Furness RW, Eggers T, van Noordwijk HJ, Quillfeldt P (2010) Black and white - does melanin change the bulk carbon and nitrogen isotope values of feathers? Rapid Commun Mass Spectrom 24:851–1112
Minagawa M, Wada E (1984) Stepwise enrichment of 15 N along food chains: further evidence and the relation between d 15 N and animal age. Geochim Cosmochim Acta 48:1135–1140
Ogden LJE, Hobson KA, Lank DB (2004) Blood isotopic (delta C-13 and delta N-15) turnover and diet- tissue fractionation factors in captive Dunlin (Calidris alpina pacifica). Auk 121:170–177
Oppel S, Powell AN, O’Brien DM (2009) Using eggshell membranes as a non-invasive tool to investigate the source of nutrients in avian eggs. J Ornithol 150:109–115
Owens N (1987) Natural variations in 15 N in the marine environment. Adv Mar Biol 24:389–451
Peterson RG, Whitworth T (1989) The sub-antarctic and polar fronts in relation to deep-water masses through the southwestern Atlantic. J Geophys Res Ocean 94:817–838
Poisbleau M, Demongin L, Strange IJ, Otley H, Quillfeldt P (2008) Aspects of the breeding biology of the southern rockhopper penguin Eudyptes c. chrysocome and new consideration on the intrinsic capacity of the A-egg. Polar Biol 31:925–932
Poisbleau M, Demongin L, Angelier F, Dano S, Lacroix A, Quillfeldt P (2009) What ecological factors can affect albumen corticosterone levels in the clutches of seabirds? Timing of breeding, disturbance and laying order in rockhopper penguins (Eudyptes chrysocome chrysocome). Gen Comp Endocrinol 162:139–145
Poisbleau M, Demongin L, van Noordwijk HJ, Strange IJ, Quillfeldt P (2010) Sexual dimorphism and use of morphological measurements to sex adults, immatures and chicks of rockhopper penguins. Ardea 98:217–227
Pütz K, Ingham RJ, Smith JG, Lüthi BH (2002) Winter dispersal of rockhopper penguins Eudyptes chrysocome from the Falkland Islands and its implications for conservation. Mar Ecol Prog Ser 240:273–284
Pütz K, Clausen AP, Huin N, Croxall JP (2003a) Re-evaluation of historical rockhopper penguin population data in the Falkland Islands. Waterbirds 26:169–175
Pütz K, Smith JG, Ingham RJ, Lüthi BH (2003b) Satellite tracking of male rockhopper penguins Eudyptes chrysocome during the incubation period at the Falkland Islands. J Avian Biol 34:139–144
Pütz K, Rey AR, Huin N, Schiavini A, Pütz A, Luthi BH (2006a) Diving characteristics of southern rockhopper penguins (Eudyptes c. chrysocome) in the Southwest Atlantic. Mar Biol 149:125–137
Pütz K, Rey AR, Schiavini A, Clausen AP, Lüthi BH (2006b) Winter migration of rockhopper penguins (Eudyptes c. chrysocome) breeding in the Southwest Atlantic: is utilisation of different foraging areas reflected in opposing population trends? Polar Biol 29:735–744
Quillfeldt P, McGill RAR, Furness RW (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304
Quillfeldt P, Strange IJ, Masello JF (2007) Sea surface temperatures and behavioural buffering capacity in thin-billed prions Pachyptila belcheri: breeding success, provisioning and chick begging. J Avian Biol 38:298–308
Quillfeldt P, Bugoni L, McGill RAR, Masello JF, Furness RW (2008a) Differences in stable isotopes in blood and feathers of seabirds are consistent across species, age and latitude: implications for food web studies. Mar Biol 155:593–598
Quillfeldt P, McGill RAR, Masello JF, Weiss F, Strange IJ, Brickle P, Furness RW (2008b) Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Mar Ecol Prog Ser 373:137–148
Quillfeldt P, McGill RAR, Masello JF, Poisbleau M, van Noordwijk H, Demongin L, Furness RW (2009) Differences in the stable isotope signatures of seabird egg membrane and albumen—implications for non-invasive studies. Rapid Commun Mass Spectrom 23:3632–3636
Quillfeldt P, Masello JF, McGill RAR, Adams M, Furness RW (2010) Moving polewards in winter: a recent change in the migratory strategy of a pelagic seabird? Front Zool 7:15
Raya Rey A, Schiavini A (2005) Inter-annual variation in the diet of female southern rockhopper penguin (Eudyptes chrysocome chrysocome) at Tierra del Fuego. Polar Biol 28:132–141
Raya Rey A, Trathan P, Pütz K, Schiavini A (2007a) Effect of oceanographic conditions on the winter movements of rockhopper penguins Eudyptes chrysocome chrysocome from Staten Island, Argentina. Mar Ecol Prog Ser 330:285–295
Raya Rey A, Trathan P, Schiavini A (2007b) Inter-annual variation in provisioning behaviour of southern rockhopper penguins Eudyptes chrysocome chrysocome at Staten Island, Argentina. Ibis 149:826–835
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407:364–367
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. Trends in Ecol Evol 19:256–263
Schell DM (2000) Declining carrying capacity in the Bering Sea: isotopic evidence from whale baleen. Limnol Oceanogr 45:459–462
Schreer JF, Kovacs KM (1997) Allometry of diving capacity in air-breathing vertebrates. Can J Zool 75:339–358
Strange IJ (1982) Breeding ecology of the Rockhopper penguin (Eudyptes crestatus) in the Falkland Islands. Gerfaut 72:137–188
Thompson KR (1989) An assessment of the potential for competition between seabirds and fisheries in the Falkland Islands. Falkland Islands Foundation Project Reports
Thompson KR (1994) Predation on Gonatus antarcticus by Falkland Islands seabirds. Antarct Sci 6:269–274
Tierney M, Southwell C, Emmerson LM, Hindell MA (2008) Evaluating and using stable-isotope analysis to infer diet composition and foraging ecology of Adélie penguins Pygoscelis adeliae. Mar Ecol Prog Ser 355:297–307
Weimerskirch H, Inchausti P, Guinet C, Barbraud C (2003) Trends in bird and seal populations as indicators of a system shift in the Southern Ocean. Antarct Sci 15:249–256
Weiss F, Furness RW, McGill RAR, Strange IJ, Masello JF, Quillfeldt P (2009) Trophic segregation of Falkland Islands seabirds: insights from stable isotope analysis. Polar Biol 32:1753–1763
White RW, Reid JB, Black AD, Gillon KW (1999) Seabird and marine mammal dispersion in the waters around the Falkland Islands 1998–1999. Joint Nature Conservation Committee, Peterborough, UK
White RW, Gillon KW, Black AD, Reid JB (2001) Vulnerable concentrations of seabirds in Falkland Islands waters. Joint Nature Conservation Committee, Peterborough, UK
Williams TD (1995) The penguins. Oxford University Press, Oxford
Wilson RP (1995) Foraging ecology. In: Williams TD (ed) The penguins. Oxford University Press, Oxford, pp 81–106
Wilson RP, Culik B, Spairani HJ, Coria NR, Adelung D (1991) Depth utilization by penguins and gentoo penguin dive patterns. J Ornithol 132:47–60
Acknowledgments
We are grateful to the New Island Conservation Trust for permission to work on the island. We thank Ian, Maria and Georgina Strange, and Dan Birch for their support during the field season. Thanks also to Helen Otley, Falkland Islands Government and British Antarctic Survey for their logistic help. Prey samples were provided by Dr. Paul Brickle, Falkland Islands Government Fisheries Department, fat extracted by Felix Weiss and analysed at SURC, UK, by Dr. Rona McGill, as part of a previous project. We thank Karin Sörgel and Anja Luckner from the IZW for isotope analysis of penguin samples. Dr. Katrin Ludynia helped to create a map of the study area. We would like to thank three anonymous reviewers for helpful comments on this manuscript. Our study was funded by a grant provided by the Deutsche Forschungsgemeinschaft DFG (Qu 148/1-ff) and an Environmental Studies Budget grant from the Falkland Islands Government. All work was approved by the Falkland Islands Government (Environmental Planning Office).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehnhard, N., Voigt, C.C., Poisbleau, M. et al. Stable isotopes in southern rockhopper penguins: foraging areas and sexual differences in the non-breeding period. Polar Biol 34, 1763–1773 (2011). https://doi.org/10.1007/s00300-011-1026-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1026-x