Abstract
The ram’s horn squid, Spirula spirula (Spirulida, Coleoidea), inhabits subsurface waters of the tropical and subtropical oceans. Because of the presence of an internal chambered shell in this species, it has frequently been used as a model species to investigate the paleobiology of fossil coleoids. However, the feeding and dietary habits of S. spirula in the nature are poorly known. In this study, we applied a new method (amino acid nitrogen isotopic analysis) to estimate the trophic position of S. spirula specimens captured off Suriname, as well as three cuttlefish Sepia species (Sepia officinalis, S. latimanus, and S. esculenta), with a calcified chambered shell from the shallower water. The trophic position of S. spirula was estimated to be 2.5–2.8, which was significantly lower than that for the three Sepia species (3.4–3.6). The results and available data on the gastric contents of S. spirula suggest that it feeds mainly on detritus and zooplankton, including crustaceans, from the overlying water column. The method used in this study can potentially be applied to the estimation of the trophic position of the fossil cephalopods having calcified chambered shells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spirula spirula (Linnaeus 1758; ram’s horn squid) has an internal loosely coiled chambered shell and is one of the most enigmatic cephalopods. Although empty shells are commonly found on beaches of the Atlantic, Indian, and west Pacific oceans (Bruun 1943; Clarke 1966), living specimens of S. spirula have rarely been observed, and consequently, the ecology of this species in the wild remains unclear. It has not been observed or filmed in its habitat, but is presumed to inhabit mesopelagic (550–1,000 m deep) waters of tropical and subtropical Atlantic and Indo-West Pacific regions (Norman 2,000). The species is thought to live in groups and exhibits diel vertical migration, based on evidence using sampling with a closing net (Clarke 1969). During the day, it stays at 550–1,000 m depth and then rises to feed at 100–300 m depth during the night. Spawning has been suggested to occur in deep water close to the seafloor (Bruun 1943; Nesis 1987), a hypothesis supported by the observation of juveniles (0.5 cm in length) at depths of 1,000–1,750 m (Clarke 1970). The internal, loosely coiled chambered shell of S. spirula starting from a spherical initial chamber resembles the external chambered shells of Ammonoidea, the extinct cephalopod subclass that flourished for more than 340 million years from the Middle Devonian to the end of the Cretaceous (Bandel and von Bolezky 1979; Neige and Warnke 2010).
Spirula spirula has in recent years been a key species used in the reconstruction of the biological properties of extinct Coleoidea (e.g., Belemnitida) and Ammonoidea. For example, Tanabe (1989) postulated that the initial chamber of ammonoids (ammonitella) was briefly enveloped by the outer mantle during the late embryonic development, based on the fact that the tuberculate microornamentation seen on the surface of embryonic shells of ammonoids is also present during the early shell development in S. spirula. Based on morphological and molecular data, Warnke and Keupp (2005) concluded that S. spirula could serve as a model organism for understanding the embryonic development of Ammonoidea. With respect to the feeding and dietary habits of this species, Kerr (1931) and Young (1977) reported small crustaceans, including copepods and ostracods in the muscular gizzard of live-caught specimens, but not in the esophagus. A toothed tongue (radula) is absent (Nesis 1987) or vestigial in S. spirula (Nixon and Young 2003), which differs from other cephalopods. S. spirula has a relatively large jaw apparatus relative to its body size (~7 cm in total length), suggesting a microphagous feeding mode, but the details of the feeding habits of this species in nature remain to be fully elucidated.
Among modern coleoid cephalopods, only cuttlefish (Sepia spp.) and ram’s horn squid S. spirula have calcified chambered shells. As the latter inhabits the deep sea, its diet and feeding ecology are poorly understood. In contrast, available records of fossil cephalopods indicate that most had a calcified external or internal chambered shell (e.g., belemnoids and aulacocelids for fossil Coleoidea). Therefore, investigation of modern descendants should provide insights into the marine ecology of the geological past.
Knowledge on the feeding habits of modern cephalopods relies mainly on the analysis of esophagus and gut contents, and observations of feeding behavior in the wild and aquaria (Nixon 1987; Rodhouse and Nigmatullin 1996). However, these methods potentially involve complications. For example, identification of stomach contents is often difficult because cephalopods use their chitinous jaws to tear prey into small pieces. Furthermore, stomach contents do not necessarily reflect average dietary intake, but may reflect prey that is more resistant to digestion (Jackson et al. 2007). Direct observation of feeding behavior in natural habitats is difficult for cephalopods that live in deep pelagic environments.
In this context, assessing the nitrogen isotopic composition of soft tissues is a potentially useful approach to investigating the diet of S. spirula (DeNiro and Epstein 1981; Minagawa and Wada 1984; Fry 2006 and references therein; Cherel et al. 2009). However, obtaining precise estimates of trophic position using this tool is difficult because of problems in characterization of the δ15N values of primary producers. Even analytical results from phytoplankton samples usually represent a snapshot of the varying oceanographic environment (e.g., O’Reilly et al. 2002).
To address this problem, a method for trophic level assessment was recently developed using analysis of the nitrogen isotope composition of amino acids (Chikaraishi et al. 2009). This method is based on differences in the δ15N values of glutamic acid and phenylalanine and has an advantage over the commonly used bulk isotope method in that it does not require the characterization of δ15N values of primary producers, as described below. This method has been proven useful for estimating the food sources of aquatic organisms and has been applied in many ecosystems (McClelland and Montoya 2002; Popp et al. 2007; Chikaraishi et al. 2009, 2010; Hannides et al. 2009; Styring et al. 2010).
In this study, we report the first use of this amino acid method to reconstruct the trophic position of S. spirula, based on the analysis of the soft tissues of live-caught specimens and three cuttlefish species (S. officinalis, S. latimanus, and S. esculenta), the feeding ecologies of which are known from previous studies (Guerra 1985; Castro and Guerra 1990; Jereb et al. 2005). We also evaluated whether analysis of the calcified skeleton (cuttlebone) of Sepia species is useful for the reconstruction of trophic levels, through comparison of the nitrogen isotopic composition of amino acids from soft tissue and the cuttlebone of live-caught specimens.
Materials and methods
Samples
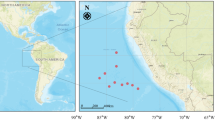
We collected soft tissues and shell from five specimens of the following four coleoid species: (1) Spirula spirula (Linnaeus, 1758) (Spirulidae, Spirulida): two specimens were caught live from 810 m depth off Suriname on April 21, 1980; (2) Sepia officinalis Linnaeus, 1758 (Sepiidae, Sepiida): the specimen was purchased at a market in Lisbon, Portugal, on December 28, 1989; (3) Sepia latimanus Quoy and Gaimard, 1832 (Sepiidae, Sepiida): the specimen was caught live from shallow waters of Ulugan Bay (Palawan, the Philippines) on June 29, 1988; (4) Sepia esculenta Hoyle, 1885 (Sepiidae, Sepiida): the specimen was purchased at a fish market at Tsukiji (Tokyo, Japan) in April 2009. The geographic distributions of the three Sepia species are more restricted than that of S. spirula, and the specimens of the above three species came from their main habitats (the northwest Pacific Ocean for S. esculenta, the Mediterranean Sea and northeast Atlantic Ocean for S. officinalis, and the southwest Pacific and Indian oceans for S. latimanus). All specimens were fixed with 10 % neutralized formalin solution soon after capture or purchase and then preserved in 99 % ethanol. It has been shown that preservation of samples in formic acid solution has little effect on the δ15N values of amino acids (Hannides et al. 2009; Ogawa et al. 2012). Furthermore, preservation in ethanol should not affect them, because ethanol neither contains nitrogen nor dissolves protein.
Nitrogen isotopic analysis of individual amino acids
Great care was taken to minimize contamination during sampling and processing. After ethanol-stored samples were washed with distilled water, the amino acids were extracted and separated using methods described by Chikaraishi et al. (2009); Takano et al. (2010). Briefly, for each specimen, a sample of mantle tissue was ground using a mortar and pestle, and approximately 10 mg was hydrolyzed with 12 M HCl at 100 °C. The hydrolyzate was washed with n-hexane/dichloromethane (6:5, v/v) to remove any hydrophobic constituents. For cuttlebone samples, following derivatization firstly with thionyl chloride/2-propanol (1:4, v/v) and then with pivaloyl chloride/dichloromethane (1:4, v/v), the pivaloyl/iso-propyl derivatives of the amino acids were extracted with n-hexane/dichloromethane (6:5, v/v).
The nitrogen isotopic composition of individual amino acids was determined by gas chromatography/combustion/isotope ratio mass spectrometry (GC/C/IRMS; Hayes et al. 1990), using a ThermoFinnigan Delta plus XP isotope ratio mass spectrometer coupled to an Agilent Technologies 6890N gas chromatography via combustion interface at the Japan Agency for Marine-Earth Science and Technology. Standard mixtures of eight δ15N-known amino acids (alanine, glycine, valine, leucine, aspartic acid, serine, glutamic acid, and phenylalanine) were analyzed every 4–5 GC/C/IRMS runs to confirm the reproducibility of the isotope measurements. The analytical errors (1σ) for the standards were always better than 0.5 ‰ when a minimum sample of 30 mg N was used.
In the case of calcareous cuttlebone samples, including those of the Sepia specimens, we conducted an additional procedure to remove interfering materials (Takano et al. 2010). A piece of cuttlebone (~1.5 g) sampled from the last-formed septum was powdered and soaked in a 30 % solution of hydrogen peroxide at 80 °C for 1 h with stirring. Thereafter, the solution was removed by the procedure of centrifuging and washing with distilled water. After washing three times in distilled water, each sample was hydrolyzed with 12 M HCl to remove calcium carbonate and then hydrolyzed with 6 M HCl at 110 °C for 12 h. The resulting hydrolyzate was filtered by a plastic Eppendorf tube to remove precipitates, subjected to liquid/liquid extraction with n-hexane/CH2Cl2 to remove lipophilic compounds, and then dried under a N2 stream prior to purification of the amino acids. The pH of the sample was adjusted to 1 using 0.1 M HCl, and a cation-exchange resin column (AG50 W-X8; 20–400 mesh) was used for desalting and the isolation of amino acids.
Estimation of trophic position
Based on detailed comparison of the δ15N values of individual amino acids between phytoplankton and their consumer zooplankton, McClelland and Montoya (2002) reported differences in the degree of 15 N-enrichment in each amino acid. They suggested that 15 N-enrichment in some amino acids (e.g., glutamic acid) showing a large variation in nitrogen isotopic compositions provides a greater scope for defining trophic level, while other amino acids (e.g., phenylalanine) showing little variation in nitrogen isotopic composition provides information on nitrogen sources at the base of the food web. Chikaraishi et al. (2007) discussed factors controlling the isotopic signature of each amino acid and concluded that deamination during metabolism as well as transamination could produce the variations in nitrogen isotopic ratios observed among individual amino acids. Moreover, they suggested the possibility of reconstructing the trophic levels of organisms using nitrogen isotopic differences between two amino acids. Chikaraishi et al. (2009) subsequently proposed that the following equation could be used for estimating the trophic levels of organisms.
In this equation, TP denotes the trophic position, where TP 1 = autotrophs, TP 2 = herbivore, and TP 3 = primary carnivore. δ15NGlu and δ15NPhe are the nitrogen isotopic compositions of glutamic acid (Glu) and phenylalanine (Phe), respectively. During metabolism, glutamic acid is systematically enriched in 15N as a result of C–N bond cleavage. In contrast, little 15N-enrichment of phenylalanine occurs during metabolism because, rather than C–N bond cleavage, a hydroxyl group is added to form tyrosine (Chikaraishi et al. 2007).
An advantage of the amino acid analysis method is that it does not require characterization of the δ15N values of primary producers or inorganic substrates (e.g., nitrate) when estimating the trophic position. Hence, this method is applicable to organisms in the absence of knowledge of the ecosystem from which they originated. Only nanomolar amounts of nitrogen are required for precise determination of the isotopic composition of individual amino acid using GC/C/IRMS; in this study, we used 0.7–1.0 mg of sample. The method is applicable to museum collections where specimens have been preserved in formalin for one year or less (Ogawa et al. 2012).
Results and discussion
Trophic positions of cephalopods
The δ15N values of 10 amino acids from mantle and cuttlebone of the S. officinalis, S. esculenta, and S. latimanus specimens ranged from 2 to 30 ‰ (Table 1; Fig. 1). In contrast, the amino acids from two soft tissues, mantle and head (the portion that bears eyes and mouthpart), from two specimens of S. spirula were somewhat depleted in 15N (range 2–23 ‰). However, the amino acid nitrogen isotopic patterns in the Sepia species and S. spirula were similar, with alanine, valine, leucine, isoleucine, proline, and glutamic acid being enriched in 15N relative to glycine, serine, methionine, and phenylalanine (Fig. 1). A similar pattern has been reported among various aquatic organisms in previous studies (e.g., McClelland and Montoya 2002; Popp et al. 2007; Chikaraishi et al. 2009).
Although we analyzed only two samples per Sepia specimen (i.e., mantle and cuttlebone), the estimated trophic positions of S. officinalis, S. latimanus, and S. esculenta were similar (range 3.4–3.6; Table 1). The potential uncertainty in the trophic position, calculated by taking into account the propagation of analytical errors, is 0.12 (Ogawa et al. 2012; for more details of the calculation protocol, see discussion in Chikaraishi et al. 2011). Therefore, although the specimens were collected from different regions, the trophic positions for the three Sepia species were statistically almost identical and indicated that they were carnivores. This interpretation is supported by previous studies of their gastric contents, which included various crustaceans, fishes, and mollusks (gastropods, bivalves, and other cephalopods) (Guerra 1985; Castro and Guerra 1990; Jereb et al. 2005). In contrast, the estimated trophic position of live-caught S. spirula ranges from 2.5 to 2.8 (Table 1); the substantially lower values strongly suggest that the diet of S. spirula is different from that of the Sepia species. The estimated trophic position of S. spirula is also lower than that of the modern nautilid. The trophic position of mature stage of Nautilus pompilius was estimated to be 3.7 using the same method (Kashiyama et al. 2010).
It should be noted that the trophic position does not indicate definite prey organisms, but represents a mean value for the various food sources, and thus simply reflects the average feeding ecology. Figure 2 illustrates the trophic positions of the four coleoid species investigated in this study, together with estimates of the trophic position of other marine organisms determined using the same method as that used in this study. The trophic positions of three copepod species (Euchaeta rimana, Pleuromamma xiphias, and Neocalanus robstior) from the tropical and subtropical Pacific Ocean were reported to be in the range 2.2–2.8 (recalculated from the data reported by Hannides et al. 2009). Various pelagic crustacean species collected from the northwest Pacific Ocean have trophic positions of 2.3–2.8 (Kitamura et al. submitted). Therefore, the estimated trophic position of crustaceans is similar to that of S. spirula. If the diet of S. spirula consists primarily of crustaceans, as proposed by Kerr (1931); Young (1977), its expected trophic position should be ~3.5, one level higher than that estimated in this study.
If our results are correct, they strongly suggest that S. spirula feeds on substantial amounts of autotrophic organisms, including algae and cyanobacteria. As S. spirula has rarely been observed at depths shallower than 100–300 m, direct feeding on living primary producers is unlikely. Rather, it may be that S. spirula feeds on detritus composed largely of phytoplankton fragments (marine snow) sinking from the overlying water column (McCarthy et al. 2007). Although the amino acid nitrogen isotopic composition of sinking particles in the oceanic water column has never been determined, its mean “trophic position” could be somewhat higher than 1.0 because of the presence of grazer fragments (McCarthy et al. 2007; Maki 2010).
Our results also suggest that the feeding ecology of S. spirula may be significantly different from that of other cephalopods. Most extant cephalopods are carnivores that generally capture relatively large prey. The microphagous feeding mode is rare, but has been reported to occur among some cephalopepod genera living in deeper waters (~900 m), including Vampyroteuthis, Spirula, Histioteuthis, and Japetella (Young 1977; Cherel et al. 2009). However, this feeding habit of the cephalopepod genera remains to be proven; at present, it remains speculation only, based on gut contents or specialized morphological features of the feeding apparatus (Young 1977; Scott 1910; Robson 1930).
Potential applications to fossil specimens
We compared the nitrogen isotopic composition of amino acids extracted from calcareous shell material (the cuttlebone) with those from soft tissue (mantle) for the three Sepia species, to evaluate the possibility of using hard body parts as a record of the biological properties of fossilized organisms. Although we evaluated only three specimens, the results from the cuttlebone of each species for glutamic acid, phenylalanine, alanine, proline, glycine, and valine were quite consistent with those from the mantle of the same specimen (Table 1; Fig. 3). This evidence suggests that the amino acids in the calcareous shell provide equivalent information on the dietary habit of these cephalopods as the amino acids from the mantle. Because empty shells are commonly found on shorelines of the world’s oceans, compound-specific nitrogen isotopic analysis of the calcareous septum from such material may contribute to understanding of the feeding ecology of shell-bearing coleoids, including S. spirula.
The calcified skeletal material of organism generally includes proteinaceous material that is synthesized at the time of skeleton formation. Thus, organic material incorporated into the calcified crystals of various types of organisms may preserve their feeding history, and this may also be the case for fossilized specimens. Kashiyama et al. (2010) recently reconstructed the trophic life history of modern Nautilus, based on the analysis of the nitrogen isotopic composition of amino acids obtained from its calcareous materials. They observed variation in the trophic position of the ventral shell wall in live-caught Nautilus specimens with growth and concluded that it resulted from the transitions in nutrition sources.
It has been reported that the calcified chambered shells of modern cephalopods, especially those of Sepia spp. and Spirula spirula, contain abundant organics present as organic sheets (Bandel and von Bolezky 1979). Therefore, the diagenetic loss of organic material from these specimens is likely to have less impact relative to other calcified materials. The rate of diagenetic loss of amino acids in the calcified shell should strongly depend on preservation environment, and it still remains to be carefully evaluated. In the extraction of amino acids from fossilized hard parts of organisms, special care has to be taken to completely remove contaminants (e.g., microbial fragments) that are potentially contained even in the crystalline structure during diagenesis. This is not easy, even in the case of cephalopods (Florkin et al. 1961; Dauphin and Denis 1999), and a reliable method still remains to be developed.
References
Bandel K, von Bolezky S (1979) A comparative study of the structure, development and morphological relationships of chambered cephalopod shells. Veliger 21:313–354
Bruun AF (1943) The biology of Spirula spirula (L.). Dana Rep 24:1–49
Castro BG, Guerra A (1990) The diet of Sepia officinalis (Linnaeus, 1758) and Sepia elegans (D’ Orbigny, 1853) (Cephalopoda, Sepioidea) from the Ria de Vigo (NW Spain). Scientia Marina 54:375–388
Cherel Y, Ridoux V, Spitz J, Richard P (2009) Stable isotopes document the trophic structure of a deep-sea cephalopod assemblage including giant octopod and giant squid. Biol Lett 5:364–367
Chikaraishi Y, Kashiyama Y, Ogawa NO, Kitazato H, Ohkouchi N (2007) Metabolic control of nitrogen isotope composition of amino acids in macroalgae and gastropods: implications for aquatic food web studies. Mar Ecol Prog Ser 342:85–90
Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Method 7:740–750
Chikaraishi Y, Ogawa NO, Ohkouchi N (2010) Further evaluation of the trophic level estimation based on nitrogen isotopic composition of amino acids. In: Ohkouchi N, Tayasu I, Koba K (eds) Earth, Life, and Isotopes. Kyoto University Press, Kyoto, pp 37–51
Chikaraishi Y, Ogawa NO, Doi H, Ohkouchi N (2011) 15N/14N ratios of amino acids as a tool for studying terrestrial food webs: a case study of terrestrial insects (bees, wasps, and hornets). Ecol Res 26:835–844
Clarke MR (1966) A review of the systematics and ecology of oceanic squids. Adv Mar Biol 4:91–300
Clarke MR (1969) Cephalopoda collected on the Sond cruise. J Mar Biol Assoc UK 49:961–976
Clarke MR (1970) Growth and development of Spirula spirula. J Mar Biol Assoc UK 50:53–64
Dauphin Y, Denis A (1999) Comparison of the diagenesis of the mineral part and of the soluble organic matrices in aragonitic tests of nautiloids and ammonites. Bull de la Soc Geol de France 170:355–365
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Florkin M, Grégoire C, Bricteux-Grégoire S, Schoffeniels E (1961) Conchiolines de nacres fossils. Compt Rend 252:440–442
Fry B (2006) Stable Isotope Ecology. Springer, Berlin-Heidelberg
Guerra A (1985) Food of the cuttlefish Sepia officinalis and S. elegans in the Ria de Vigo (N.W. Spain) (Mollusca: Cephalopoda). J Zool Lond (A) 207:511–519
Hannides CCS, Popp BN, Landry MR, Graham BS (2009) Quantification of zooplankton trophic position in the North Pacific Subtropical Gyre using stable nitrogen isotopes. Limnol Oceanogr 54:50–61
Hayes JM, Freeman KH, Popp BN, Hoham CH (1990) Compound-specific isotopic analyses: a novel tool for reconstruction of ancient biochemical processes. Org Geochem 16:1115–1128
Jackson GD, Bustamante P, Cherel Y, Fulton EA, Grist EPM, Jackson CM, Nichols PD, Pethybridge H, Phillips K, Ward RD, Xavier JC (2007) Applying new tools to cephalopod trophic dynamics and ecology: perspectives from the Southern Ocean Cephalopods. Rev Fish Biol Fish 17:79–99
Jereb P, Roper CFE, (eds) (2005) Cephalopods of the World, vol 1. Chambered Nautiluses and Sepioids, Food and Agriculture Organization of the United Nations, Rome
Kashiyama Y, Ogawa NO, Chikaraishi Y, Kashiyama N, Sakai S, Tanabe K, Ohkouchi N (2010) Reconstructing the life history of modern and fossil nautiloids based on the nitrogen isotopic composition of shell organic matter and amino acids. In: Tanabe K, Shigeta Y, Sasaki T, Hirano H (eds) Cephalopods: Present and Past. Tokai University Press, Tokyo, pp 67–75
Kerr JG (1931) Notes upon the Dana specimens of Spirula and upon certain problems of cephalopod morphology. Dana Rep 8:1–34
Maki K (2010) Stable isotopic analyses of organic matter dynamics in aquatic ecosystems. PhD Thesis, University of Tokyo
McCarthy MD, Benner R, Lee C, Fogel ML (2007) Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochim Cosmochim Acta 71:4727–4744
McClelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidences and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Neige P, Warnke K (2010) Just how many species of Spirula are there? A morphometric approach. In: Tanabe K, Shigeta Y, Sasaki T, Hirano H (eds) Cephalopods: Present and Past. Tokai University Press, Tokyo, pp 77–84
Nesis KN (1987) Cephalopods of the world. Squid, cuttlefishes, octopuses and allies. TFH Publ, Neptune City, 351 pp
Nixon M (1987) Cephalopod diets. In: Boyle PR (ed) Cephalopod Life Cycles, Vol.II. Academic Press, London, pp 201–219
Nixon M, Young JZ (2003) The brains and lives of cephalopods. Oxford University Press, New York, 392 pp
O’Reilly CM, Hecky RE, Cohen AS, Plisnier PD (2002) Interpreting stable isotopes in food webs: recognizing the role of time averaging at different trophic levels. Limnol Oceanogr 47:306–309
Ogawa NO, Chikaraishi Y, Ohkouchi N (2012) Trophic position estimates of formalin-fixed samples with nitrogen isotopic compositions of amino acids: an application to gobiid fish (Isaza) in Lake Biwa. Japan. Ecol Res. doi:10.1007/s11284-012-0967-z
Popp BN, Graham BS, Olson RJ, Hannides CCS, Lott MJ, López-Ibarra GA, Galván-Magaña F, Fry B (2007) Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson T and Siegwolf R (eds) Stable isotopes as indicators of ecological change, Elsevier Academic Press, Terrestrial Ecology Series, pp 173–190
Robson GC (1930) Cephalopoda, I. Octopoda. Discov Rep 2:373–401
Rodhouse PG, Nigmatullin CM (1996) Role as consumers. Phil Trans Royal Soc Lond B 351:1003–1022
Scott T (1910) Notes on Crustacea found in the gizzard of a deep-sea cephalopod. Ann Mag Nat Hist 5:51–54
Styring AK, Sealy JC, Evershed RP (2010) Resolving the bulk δ15N values of ancient human and animal bone collagen via compound-specific nitrogen isotope analysis of constituent amino acids. Geochim Cosmochim Acta 74:241–251
Takano Y, Kashiyama Y, Ogawa NO, Chikaraishi Y, Ohkouchi N (2010) Isolation and desalting with cation-exchange chromatography for compound-specific nitrogen isotope analysis of amino acids: application to biogeochemical samples. Rapid Comm Mass Spectrom 24:2317–2323
Tanabe K (1989) Endocochliate embryo model in the Mesozoic Ammonitida. Hist Biol 2:183–196
Warnke K, Keupp H (2005) Spirula—a window to the embryonic development of ammonoids? Morphological and molecular indications for a palaeontological hypothesis. Facies 51:60–65
Young JZ (1977) Brain, behavior and evolution of cephalopods. Symp Zool Soc Lond 38:377–434
Acknowledgments
We thank Dr. T. Kubodera of the National Museum of Nature and Science, Tokyo and Dr. T. Okutani at JAMSTEC for kindly providing specimens used in this study. We also thank two anonymous reviewers for comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by C. Harrod.
Rights and permissions
About this article
Cite this article
Ohkouchi, N., Tsuda, R., Chikaraishi, Y. et al. A preliminary estimate of the trophic position of the deep-water ram’s horn squid Spirula spirula based on the nitrogen isotopic composition of amino acids. Mar Biol 160, 773–779 (2013). https://doi.org/10.1007/s00227-012-2132-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2132-1