Abstract
In pelagic seabirds, who often explore distant food resources, information is usually scarce on the level of trophic segregation between parents and their offspring. To investigate this issue, we used GPS tracking, stable isotopes and dietary information of Cory’s shearwaters Calonectris diomedea breeding in contrasting environments. Foraging trips at Selvagem Grande (an oceanic island) mainly targeted the distant African coast, while at Berlenga island (located on the continental shelf), shearwaters foraged mainly over nearby shelf waters. The degree of isotopic segregation between adults and chicks, based on δ13C, differed markedly between the two sites, indicating that adult birds at Selvagem fed their chicks with a mixture of shelf and offshore pelagic prey but assimilated more prey captured on coastal shelf waters. Isotopic differences between age classes at Berlenga were much smaller and may have resulted from limited dietary segregation or from age-related metabolic differences. The diet of shearwaters was also very different between the two colonies, with offshore pelagic prey only being detected at Selvagem Grande. Our findings suggest that spatial foraging constraints influence resource partitioning between pelagic seabirds and their offspring and can lead to a parent–offspring dietary segregation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To optimize reproductive effort, central-place foragers, including colonial seabirds, are predicted to be selective in relation to prey delivered to their offspring (Orians and Pearson 1979; Ydenberg et al. 1994), and this may lead to dietary differences between adults and nestlings (e.g., Davoren and Burger 1999; Wilson et al. 2004; Dänhardt et al. 2011). Inshore seabirds often deliver larger amounts of or higher-quality prey items to their chicks than those they consume themselves, thereby reducing the flight and transport costs associated with provisioning and at the same time fulfilling the chick nutritional and energetic needs (Baird 1991; Davoren and Burger 1999; Wilson et al. 2004; Dänhardt et al. 2011). Additionally, as the ingestion capacity (related to gape-size) and the energetic requirements of chicks vary during growth, parents are known to provide different prey types and sizes along the nestling period (Pedrocchi et al. 1996; Ramos et al. 1998). However, adults should adjust their foraging behavior according to not only the dietary requirements of chicks, but also their own energetic requirements and the distance to the foraging grounds (Orians and Pearson 1979; Weimerskirch 1998; Catard et al. 2000). Comparatively to inshore seabirds, little is known on the existence of dietary segregation in most oceanic seabirds. Several studies, based on conventional methods, have suggested the existence of an age-related dietary segregation in species that adopt a dual-foraging strategy, reflecting a spatially related trophic segregation between adults and chicks (Chaurand and Weimerskirch 1994; Weimerskirch and Cherel 1998; Catard et al. 2000). The composition of regurgitates of chicks or of provisioning adults, however, only provides snapshot information of recently ingested food (Barrett et al. 2007) and may not be representative of the adult diet when foraging at distant places.
Stable isotopes analysis (SIA) has proved to be a powerful tool to study the diet of seabirds, as it can give insight into the origin and type of prey consumed over a broader timescale, depending on the analyzed tissue (Kelly 2000; Inger and Bearhop 2008). Carbon isotopes show evidence of an increasing enrichment in inshore as compared to offshore food chains (Cherel and Hobson 2007) and also present a surface to benthic gradient (Hobson et al. 1994). Nitrogen stable isotopes increase in a predictable manner between trophic levels and thus indicate the trophic position of consumers (Kelly 2000; Bearhop et al. 2004). Recently, SIA has provided evidence for a dietary segregation between adults and chicks in a wide range of pelagic seabirds (Hodum and Hobson 2000; Forero et al. 2005; Cherel et al. 2005a, 2008; Navarro et al. 2009; Bond et al. 2010; Richoux et al. 2010). However, most of the mentioned studies could not assess the biological significance of the isotopic differences between adults and growing chicks (but see Cherel et al. 2005a; Forero et al. 2005) as isotopic fractionation differs between them, as a result of growth or nutritional stress effects (Williams et al. 2007; Sears et al. 2009). Additionally, the ingestion of a lipid-rich diet, as stomach oil, can deplete carbon isotopic signatures of chicks, and this has been suggested as an alternative explanation for age differences in carbon signatures (Thompson et al. 2000).
The Cory’s shearwater Calonectris diomedea borealis is a pelagic seabird that nests on several islands of the Eastern North Atlantic (Cramp and Simmons 1977). This long-lived shearwater (Order Procellariiformes) rears only one chick during a long chick-rearing period of about 3 months (Cramp and Simmons 1977) and can forage over vast oceanic areas, searching mostly for epipelagic fishes and cephalopods (Granadeiro et al. 1998a; Paiva et al. 2010a). We investigated the feeding ecology of Cory’s shearwaters in two distinct colonies, Selvagem Grande (SG) and Berlenga (BE), using stable isotope analysis, conventional diet methods and tracking data obtained with GPS-loggers. Selvagem Grande is surrounded by deep oceanic waters, and the Cory’s shearwaters are known to use, to some extent, the distant (~350 km) African Coast when provisioning chicks (Paiva et al. 2010b, c). As for Berlenga, the breeding colony is located in the Portuguese continental shelf, an upwelling zone, and the Cory’s shearwaters depend almost exclusively on that area to forage during the chick-rearing period (Paiva et al. 2010b, c). Our objective was to determine whether adult Cory’s shearwaters were using distant foraging areas for self-feeding or to provision their chicks. In that respect, we wanted to address the following questions: (1) Do adult shearwaters from Selvagem Grande and Berlenga use different foraging strategies and are those differences reflected in chick body condition? Oceanographic features in the vicinity of both islands are extremely different, and therefore, we expect shearwaters from Selvagem Grande to make regular trips to productive areas at the African coast, while at Berlenga, birds should mainly forage in the productive continental shelf. The reliance on distant food resources has the potential to induce a lower body condition in the chicks of Selvagem Grande. (2) Do adult shearwaters use different areas for self-feeding and for provisioning, when depending on long-distance resources? We expect adult Cory’s shearwaters from Selvagem Grande to use different foraging areas for self-feeding and provisioning, when exploiting distant foraging grounds, while at Berlenga, birds should make use of the same foraging area to self-feeding and chick provisioning. (3) Does the diet of chicks differ between the two colonies, due to differences in their main foraging areas? We expect the diet of chicks from Selvagem Grande to be more based on oceanic prey, while at Berlenga, chicks should mainly feed on coastal prey.
Materials and methods
Study sites
Our study was carried out at Selvagem Grande (30°09′N, 15°52′W) and at Berlenga (39°24′N, 9°30′W) islands (see Fig. 1), during the chick-rearing period of 2010. After a 55-day incubation period, Cory’s shearwater chicks hatch in the end of July, and the chick-rearing period extends until the end of October, when chicks fledge (Granadeiro 1991). Selvagem Grande supports a population estimated at ca. 30,000 breeding pairs (Granadeiro et al. 2006), and the Berlenga archipelago has a colony of ca. 850 breeding pairs (Lecoq et al. 2011).
Diet and GPS tracking data
Diet samples were obtained from breeding adults returning to the nest to feed their chick using the “water offloading” technique (Wilson 1984); 65 diet samples were collected from 17 to 31 August on Selvagem Grande, and 23 diet samples were collected on Berlenga, between 16 and 18 August. During a preliminary screening, items were quantified, fresh fishes were identified using specialized guides (Whitehead et al. 1986; Quéro et al. 2003), and samples were stored in ethanol (70%) until further analysis.
In the laboratory, digested fish were identified from their hard-part remains, mainly from vertebrae, using our own reference collection and published information (Granadeiro and Silva 2000). Cephalopods were quantified based on number of mantles, other fresh remains and fresh beaks. Eroded beaks were not quantified, since they can be retained in the stomach (Barrett et al. 2007), and birds with an empty stomach were not considered in the analysis (28% of all birds captured at Berlenga and 19% at Selvagem Grande). We calculated frequencies of occurrence as the number of samples with a given prey type, expressed as a percentage of the total number of samples, and numerical frequencies as the number of individuals of a given taxon as percentage of the total number of individuals.
From 11 to 28 August, we tracked 52 breeding Cory’s shearwaters, from different nests, with GPS-loggers, 23 on Selvagem Grande and 29 on Berlenga. We used two types of GPS-loggers, GPSlog (Earth & Ocean Technologies, Germany) and customized CatTrack Loggers (Perthold Engineering LLC, USA), that were programmed to collect one location every 10–15 min and weighed either 15 or 17 g (used at Selvagem Grande and at Berlenga, and representing 1.5–2.1% or 1.6–2.7% of the body mass, respectively). The GPS devices were attached to the bird’s back feathers or to the four central tail feathers using Tesa tape (Wilson et al. 1997). The procedure took less than 10 min, and birds were thereafter returned to their nest.
Stable isotopes analysis
Blood sampling was carried out on 27 August on Berlenga and between 2 and 4 September on Selvagem Grande. We collected whole blood samples from a total of 50 adults (not the same birds that carried GPS devices or from who were collected the diet samples) and 50 chicks (mostly ca 3–6 weeks old). On most cases, we took the blood samples from parent–offspring pairs (16 pairs on Berlenga and 28 pairs on Selvagem Grande). Whole blood isotopic signature is expected to mainly represent the diet during the month prior to the sampling (Bearhop et al. 2002), so our chick data should correspond to the chick diet in the period between the first and the sixth week of age. Birds were captured by hand, and ~0.1 ml of blood was collected from the foot vein and preserved in absolute ethanol, for subsequent isotopic analysis. By using the same brand and batch for all sampling groups, we expected to minimize ethanol storage effects on stable isotope signatures (Bugoni et al. 2008). In order to compare the condition of chicks between the colonies, biometric measurements were collected from all blood-sampled chicks by the same person. We measured chick tarsus (to the nearest 0.1 mm) with a caliper and wing length (to the nearest 1 mm) with a ruler. Chicks were weighed (to the nearest 5 g) in a bag using a spring balance.
Whole blood generally has low lipid content and does not require lipid extraction (Cherel et al. 2005b). Blood samples were dried in a hood at 50°C, during approximately 40 h, and then ground into powder. Sub-samples of 1.0 ± 0.1 mg of homogenous whole blood were weighed into 8 × 5 mm tin capsules and combusted at 1,000°C in a Euro EA Elemental Analyser. Resultant CO2 and N2 gases were analyzed using a continuous-flow isotope ratio mass spectrometer IsoPrime (MicroMass), with unknowns separated by laboratory standards. Stable isotope abundances were expressed in δ-notation as the deviation from standards in parts per thousand (‰) according to the following equation:
where X is 13C or 15N and R is the corresponding ratio of 13C/12C or 15N/14N. R standard values were based on PeeDee Belemnite for 13C, or atmospheric nitrogen (N2) for 15N. Replicate measurements of laboratory standards showed measurement errors of ±0.1‰ and ±0.2‰ for stable nitrogen and carbon isotope measurements, respectively. Quality control samples were run before and after each sequence.
Oceanographic features
To characterize the oceanographic conditions in the surroundings of the two colonies (see Fig. 2), we used the chlorophyll-a concentration (Chl-a, mg C m−3) in the seawater surface, the sea surface temperature (SST, °C) and the seafloor depth (depth, m). Both Chl-a and the SST are monthly composites for August 2010. Aqua MODIS products of Chl-a and SST were downloaded from OceanColor Web site (http://oceancolor.gsfc.nasa.gov/), as level 3 HDF products at a spatial resolution of 0.04° (approx. 4 × 4 km). Depth composite was downloaded from ETOPO Web site (http://www.ngdc.noaa.gov/mgg/global/global.html), as a binary product at a spatial resolution of 0.01° (approx. 1 km). The files were then converted to raster images using the Marine Geospatial Ecology Tools (GeoEco) for ArcGIS 9.2 (Roberts et al. 2010).
Statistical analysis
To assess the differences between colonies in foraging trip length and duration, we used Mann–Whitney tests, considering only one trip per bird (using the median value of all the foraging trips of each individual). Spearman’s rank correlations were used to examine the relationship between foraging trip duration and distance travelled during the trip, in both colonies. Mixed-effects ANOVAs were used to test for differences in δ13C and δ15N between adults and their offspring, between colonies and the interaction between both, with nest used as a random factor. Although the hatching dates of Selvagem Grande and Berlenga are generally similar (Catry et al. 2009), the age of our sampled chicks was unknown, and we considered that wing and tarsus length should give us a reasonable indication of chick age at both colonies (Granadeiro 1991). An analysis of covariance (ANCOVA) was used to test for differences in chick weight between the colonies, using wing length as a covariate.
Chi-square tests were performed to test differences in the occurrence and of numerical frequencies of the main preys in both islands. In order to meet basic statistical assumptions, we grouped prey in three major groups: oceanic, neritic and others. Oceanic prey included mainly open-ocean species: Pilot-fish Naucrates ductor, Flying-fishes (Exocoetidae) and Cephalopods (mostly Flying-squids; Ommastrephidae); neritic prey included species that are known to be much more abundant in coastal than in oceanic waters: Sardine Sardina pilchardus, Garfish Belone belone and Atlantic saury Scomberesox saurus; and others included Chub mackerel Scomber sp/colias (which is abundant both in oceanic and coastal waters) and all the other fishes found in the diet sampling.
All the analyses were performed using R software (R Development Core Team 2009), and GLMM was carried out with package nlme (Pinheiro et al. 2011). Results are expressed as means ± SD, unless otherwise stated.
Results
Foraging trips
We tracked 127 and 34 foraging trips from 29 and 23 individual Cory’s shearwaters in Berlenga (hereafter termed BE) and Selvagem Grande (hereafter termed SG), respectively. Four foraging trips were incomplete (due to the loss of battery in the returning way to the colony) and were only used to assess the final destination of the birds. In BE, foraging trips lasted 1.3 days (median; interquartile range =0.9–2.9 days, N = 29) (Fig. 3), and birds travelled a distance of 404 km (median; interquartile range = 218–670 km, N = 29), in each trip. At SG, the shearwaters engaged in foraging trips that lasted 2.5 days (median; interquartile range = 1.6–3.9 days, N = 23) (Fig. 3) and travelled a distance of 1,067 km (median; interquartile range 801–1,538 km, N = 23). Foraging trips differed between the two colonies, both in duration and in distance travelled (Mann–Whitney U = 4.85, P = 0.028, N = 52 and U = 13.18, P < 0.001, N = 52, respectively). The distance travelled per bird in each foraging trip was highly correlated with the duration of the same trip, either at BE (r s = 0.80, N = 127, P < 0.001) or at SG (r s = 0.92, N = 30, P < 0.001). Most (88%, N = 127) of the foraging trips from BE birds were performed along the Portuguese coastal shelf (Fig. 4), where the colony is located. At SG, 68% (N = 34) of the foraging trips reached the African shelf (Fig. 4), 11% were to seamounts located to the northeast of the Canary islands, and 21% of the trips were restricted to the oceanic waters around the island.
Stable isotopes
There were significant differences between colonies, age classes and their interaction in stable isotope ratios (Manova, λcolony = 0.09, F (2,85) = 455.4, P < 0.001; λage = 0.49, F (2,85) = 44.5, P < 0.001; λage*colony = 0.71, F (2, 85) = 17.7, P < 0.001), which means that nitrogen and carbon ratios should be analyzed separately. Significant differences were found between the whole blood isotopic signatures of adult Cory’s shearwaters and chicks (Fig. 5; Table 1), as well as a significant interaction between age and colony, in both δ13C (mixed-effect ANOVA, Age: F (1,94) = 97.5, P < 0.001; Colony: F (1,94) = 1.1, P = 0.3; Age × Colony: F (1,94) = 9.6, P = 0.003) and δ15N (Age: F (1,94) = 10.1, P = 0.002; Colony: F (1,94) = 242.2, P < 0.001; Age × Colony: F (1,94) = 39.4, P < 0.001). C/N mass ratios of chicks were similar between the two study colonies (see Table 1; ANOVA, F (1,47) = 0.4, P = 0.52). We did not find any correlation between wing length (a proxy for age) and isotopic signatures of chicks, either at SG (δ13C: r = −0.11, N = 30, P = 0.55; δ15N: r = 0.17, N = 30, P = 0.36) or at BE (δ13C: r = 0.24, N = 19, P = 0.32; δ15N: r = 0.28, N = 19, P = 0.24).
Diet
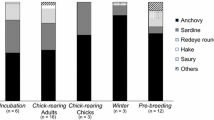
Fish was the major component of the Cory’s shearwater diet at both colonies, but cephalopods were also a frequent prey at SG (Table 2). Scomber colias and Sardina pilchardus were the most frequent fish prey at SG, but strictly oceanic prey, such as the Naucrates ductor and Exocoetidae, were also common. The main prey of Cory’s shearwaters at BE was Belone belone. Oceanic prey species occurred more frequently in the diet samples of SG than at BE (Fisher’s exact test, P < 0.001). The numerical frequency of oceanic, neritic and others prey also differed between colonies (chi-square test, χ² = 27.9, P < 0.001), with significantly more oceanic prey in SG.
Chick condition
At the time of measurements, mean chick wing length did not differ between colonies (SG: 109.5 ± 25.1 mm, N = 30; BE: 98.9 ± 18.5 mm, N = 19; ANOVA, F (1,47) = 2.52, P = 0.12). The same result was found for tarsus length: 53.2 ± 3.7 mm (N = 30) and 52.9 ± 3.6 mm (N = 19), in SG and BE, respectively (ANOVA, F (1,47) = 0.07, P = 0.79). Chick body mass from the two colonies differed significantly, after correcting for wing length (ANCOVA, Colony effect, t 46 = 6.32, P = 0.001; Wing × Colony interaction t 46 = 1.31, P = 0.20), with chicks being heavier at Berlenga.
Discussion
The present study documents the occurrence of a dietary segregation between adults and their offspring in the Cory’s shearwater, a pelagic seabird. This segregation is probably related to spatial foraging constraints and to the distance between nesting sites and productive feeding grounds.
Foraging strategy and chick condition
Cory’s shearwaters in SG engaged in longer foraging trips than birds of BE, both in terms of distance travelled and duration. Most foraging trips at BE were made along the Portuguese coast, where the colony is located. When compared to offshore waters, the Portuguese shelf is an area of enhanced marine productivity, due to a strong summer upwelling (Sousa et al. 2008), and BE shearwaters are known to explore this profitable area (Paiva et al. 2010b, c). At SG, shearwaters performed much longer trips, reaching in most cases the Western African shelf, also an important upwelling zone (Davenport et al. 2002). This last result indicates that the food availability around SG may be scarce, which agrees with previous studies that reported lower feeding frequencies (Granadeiro et al. 1998b) and longer foraging trips at this colony (Catry et al. 2009; Paiva et al. 2010b), when compared to BE. Indeed, the length of foraging trips is known to be largely influenced by the local food availability (Uttley et al. 1994) and by the distance to the most profitable areas (Paiva et al. 2010c). Moreover, as the number of breeding shearwaters at SG is much larger than at BE (Granadeiro et al. 2006; Lecoq et al. 2011), intraspecific competition could also lead to differences in the foraging strategies between colonies (Lewis et al. 2001; Grémillet et al. 2004). The exploration of distant profitable waters, during chick rearing, has also been described in other similar-sized pelagic seabirds, like sooty shearwaters Puffinus griseus (Weimerskirch 1998) and short-tailed shearwaters Puffinus tenuirostris (Weimerskirch and Cherel 1998; Einoder et al. 2011) and is usually linked to the use of a dual-foraging strategy (Weimerskirch 1998; Weimerskirch and Cherel 1998).
Previous studies at SG also showed that shearwaters use the African coast during chick rearing, employing a dual-foraging strategy (Granadeiro et al. 1998b; Paiva et al. 2010c). With this strategy, adults are predicted to use the long trips mainly to self-feeding and recover their body condition and the short trips to allocate food to their offspring (Chaurand and Weimerskirch 1994; Catard et al. 2000). Perhaps unexpectedly, we did not find a bimodal pattern in the frequency of foraging trips of different durations at this colony (see Fig. 3), and our results suggest that shearwaters of SG were not using a dual-foraging strategy. However, the dual-foraging strategy is not a species-specific trait but rather a facultative one and known to vary, both spatially and temporarily, possibly depending on food availability and distribution (Granadeiro et al. 1998b; Baduini and Hyrenbach 2003; Phillips et al. 2009).
To study movements at sea, we used GPS devices that weighed less than 3% of the Cory’s shearwaters body mass, which is the commonly accepted threshold for deleterious effects on the foraging performance of pelagic seabirds (Phillips et al. 2003). However, we cannot rule out the possibility that our devices could still somehow affect the study birds, for example, by prolonging the time at sea (see Passos et al. 2010), given the scarcity of short trips recorded in this study (see, for example, Granadeiro et al. 1998b). Any effect, however, should not greatly impact our results and conclusions. In fact, Cory’s shearwaters at BE carried the heaviest GPS and still made shorter foraging trips than SG individuals. If the extra-weight had an effect on the birds, then the difference between the two colonies should be even larger than reported here. Furthermore, it should be noted that birds sampled for stable isotopes were not the same that carried the devices.
When correcting for wing length, chicks at BE were heavier than at SG. Body mass corrected for wing length (the later considered to be well correlated with age) is expected to be a good measure of chick body condition (Benson et al. 2003), and it is well established that this parameter is mainly affected by feeding frequency and meal size, reflecting overall foraging behavior of adults (Pinaud et al. 2005; Magalhães et al. 2008). We suggest that differences in chick body condition mainly reflect the better feeding conditions around BE and the shorter distance to the most important feeding grounds, in comparison with SG.
Dietary segregation
We found significant differences in the δ13C signatures of chicks and adults, in both colonies, but the difference was much larger at SG than at BE, which strongly suggests a parent–offspring dietary segregation at SG. In fact, chicks of SG had lower values in δ13C than adults, which is in agreement with a more oceanic signature and suggests that they were assimilating more oceanic prey than their parents. Similar age differences in δ13C signatures were found in short-tailed shearwaters Puffinus tenuirostris that exploited an oceanic-neritic gradient (Cherel et al. 2005a). Moreover, δ13C signatures of SG adults were closer to the values found for adults from the Canary Islands, known to almost exclusively exploit the African coastal waters (Navarro et al. 2007; Navarro and González-Solís 2009). The foraging trips also showed an intensive use of an oceanic-neritic gradient by SG shearwaters, and the occurrence of oceanic items on the chick diet was confirmed by conventional dietary analysis. There were also significant (although much smaller) differences in the carbon signatures of chicks and adults of BE, but with the available information, it is unclear whether such differences reflect an age segregation in diet or just metabolic effects (see below).
The occurrence of oceanic species in the SG chick diet was similar to that recorded in previous years, when birds were performing a dual-foraging strategy (unpubl data; Paiva et al. 2010a, c). In contrast, the sardine, a mainly coastal prey, was scarce in other years but found frequently in 2010 diet samples. Sardine is one of the most abundant pelagic species off the NW African Coast, particularly on the Moroccan shelf (Machu et al. 2009), and is expected to be an important prey for adults when foraging along the coast (Paiva et al. 2010a). In contrast, in oceanic islands like the Azores, sardine is not consumed at all by Cory’s shearwaters (Granadeiro et al. 1998a; Paiva et al. 2010a; Xavier et al. 2011). Despite its high frequency in the chick diet at SG, sardine was persistently found in a highly digested state (pers obs). This suggests that its contribution to chick diet could have been overestimated by the diet sampling method (Duffy and Jackson 1986), because an important part of sardines was probably assimilated by adults prior to delivery. The small overlap of adult and chick carbon isotope signatures at SG also suggests that chicks could have been receiving only a small part of their food from coastal waters. Moreover, individual variation in foraging behavior could have also contributed to the isotopic overlap found, as SG adults showed a high variance in δ13C values (see Bearhop et al. 2006). This idea is supported by the isotopic signatures of Cory’s shearwaters from BE and Canaries, since adults from those colonies forage mainly along the coast and present much lower δ13C variances (Navarro and González-Solís 2009).
δ15N isotopes are strongly affected by diet and usually represent the trophic position of the consumer (Kelly 2000; Bearhop et al. 2004). We found significant differences in the δ15N signatures of Cory’s shearwaters between SG and BE and smaller age differences in both colonies. Paiva et al. (2010a) studied the foraging ecology of Cory’s shearwaters at several Atlantic islands, including BE and SG, and found that cephalopods had much lower δ15N values than the fish prey species (Paiva et al. 2010a). Indeed, in the present study, Cory’s shearwaters chicks from SG had much lower δ15N values than at BE, where chicks were not eating cephalopods. However, δ15N differences between birds from the two colonies may also reflect different baseline values at different latitudes and oceanic habitats.
Metabolic issues
In the last decade, SIA has been widely used to investigate resource partitioning in pelagic seabirds, namely between adults and their offspring. Chicks often exhibit higher δ15N signatures than adults, usually between 1.0 and 2.0 ‰, and these age differences are often quoted as an indication that adult fed their chicks with higher-trophic-level prey than they assimilated themselves (Hodum and Hobson 2000; Cherel et al. 2005a, 2008; Forero et al. 2005; Richoux et al. 2010). Age differences in δ13C signatures do not present any obvious trend and generally are of a smaller magnitude (less than 1 ‰) (Hodum and Hobson 2000; Forero et al. 2005; Cherel et al. 2008, Navarro et al. 2009; Bond et al. 2010; Richoux et al. 2010; but see Cherel et al. 2005a). The uncritical interpretation of differences in chick and adult isotope levels as evidence of dietary segregation may present some problems, as explained below.
Physiological and metabolic differences among adults and chicks influence isotopic fractionation (Williams et al. 2007; Harding et al. 2008; Sears et al. 2009) and can also contribute to parent–offspring differences in the isotopic signatures. δ15N values can gradually change during chick growth (Williams et al. 2007; Sears et al. 2009) presumably due to changes in tissue turnover rates or in changes on the use of the nitrogen stored during growth (Harding et al. 2008; Sears et al. 2009). There are also potential carryover effects from maternal nutrients in eggs on chick isotopic signatures, and little attention has been given to this issue (Bond and Jones 2009). In contrast, it is known that the amount of uric acid in blood, which is supposed to be higher during growth, can deplete the δ15N in that tissue, because nitrogenous wastes are enriched in 14N (Bearhop et al. 2000; but see Cherel et al. 2005c). By comparing similarly aged chicks of two colonies, we controlled for the effects of chick growth and of the aforementioned age-specific metabolism. We also avoided carryover maternal or egg effects by collecting blood samples relatively late in the chick-rearing period (~1 month of age). The fact that there was no evidence for severe nutritional stress at either of the study sites further reinforces the validity of our comparisons (Williams et al. 2007; Sears et al. 2009).
Beyond the diet itself, the ingestion of stomach oil can also have an effect on the δ13C signatures of chicks, potentially contributing to age-related differences. The incorporation of dietary lipids is supposed to decrease δ13C values, since lipids are depleted in 13C in relation to proteins or carbohydrates (Thompson et al. 2000). We did not quantify the amount of stomach oil in the diet of chicks, but the occurrence of stomach oil during the diet sampling was of small magnitude, either at SG or at BE. Moreover, C/N ratios of shearwaters chicks were low (less than 3.5) and similar between the two colonies, suggesting that chick blood had a low lipid content (Post et al. 2007) and consequently that stomach oil had no major effect on the δ13C signatures of chicks (Thompson et al. 2000). In a recent study, Richoux et al. (2010) compared the diet of gray-headed albatross Thalassarche chrysostoma adults and chicks, using stomach contents and stable isotope analysis, and found no age-related differences in the δ13C signatures of whole blood (or in the C/N ratio), despite the fact that they had collected stomach oil from a majority of the diet samples. This population forages mainly in Subantarctic and Antarctic oceanic waters and does not explore an oceanic-neritic gradient (Richoux et al. 2010). The effect of the ingestion of stomach oil on δ13C chick signatures should be higher when using plasma to analyze stable isotopes, since plasma is richer in lipids than in whole blood (Cherel et al. 2005a), and we argue that the use by foraging seabirds of a strong oceanic-neritic gradient is more determinant for age-related differences in δ13C signatures in whole blood than the ingestion of small quantities of stomach oil.
Final considerations
Our study provides evidence that resource partitioning among adults and their offspring in offshore seabirds is conditioned by spatial foraging constrains. At SG, where adult Cory’s shearwaters depended on distant food resources, a dietary segregation was evident. The integrated study of individual-foraging behavior, resource allocation and body condition seems now essential to an improved understanding of the foraging strategies of Cory’s shearwaters and, more generally, of pelagic seabirds. Stable isotopes proved to be a useful method to study resource allocation between parents and their offspring, but there is a clear need for more studies of age-metabolic effects on isotopic signatures, especially on the specific magnitude of those effects, for a better understanding of the overall picture.
References
Baduini CL, Hyrenbach KD (2003) Biogeography of procellariiform foraging strategies: does ocean productivity influence provisioning? Mar Ornithol 31:101–112
Baird PH (1991) Optimal foraging and intraspecific competition in the tufted puffin. Condor 93:503–515
Barrett RT, Camphuysen CJ, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Hüppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691
Bearhop S, Teece MA, Waldron S, Furness RW (2000) Influence of lipid and uric acid on δ13C and δ15N values of avian blood: implications for trophic studies. Auk 117:504–507
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164
Benson J, Suryan RM, Piatt JF (2003) Assessing chick growth from a single visit to a seabird colony. Mar Ornithol 31:181–184
Bond AL, Jones IL (2009) A practical introduction to stable-isotope analysis for seabird biologists: approaches, cautions and caveats. Mar Ornithol 37:183–188
Bond AL, McClelland GTW, Jones IL, Lavers JL, Kyser TK (2010) Stable isotopes confirm community patterns in foraging among Hawaiian Procellariiformes. Waterbirds 33:50–58
Bugoni L, McGill RAR, Furness RW (2008) Effects of preservation methods on stable isotope signatures in bird tissues. Rapid Commun Mass Spectrom 22:2457–2462
Catard A, Weimerskirch H, Cherel Y (2000) Exploitation of distant Antarctic waters and close shelf-break waters by white-chinned petrels rearing chicks. Mar Ecol Prog Ser 194:249–261
Catry P, Matias R, Vicente L, Granadeiro JP (2009) Brood-guarding behaviour in Cory’s shearwaters Calonectris diomedea. J Ornithol 150:103–108
Chaurand T, Weimerskirch H (1994) The regular alternation of short and long foraging trips in the Blue petrel Halobaena caerulea: a previously undescribed strategy of food provisioning in a pelagic seabird. J Anim Ecol 63:275–282
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287
Cherel Y, Hobson KA, Hassani S (2005a) Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiol Biochem Zool 78:106–115
Cherel Y, Hobson KA, Weimerskirch H (2005b) Using stable isotopes to study resource acquisition and allocation in procellariiform seabirds. Oecologia 145:533–540
Cherel Y, Hobson KA, Bailleul F, Groscolas R (2005c) Nutrition, physiology, and stable isotopes: new information from fasting and molting penguins. Ecology 86:2881–2888
Cherel Y, Le Corre M, Jaquemet S, Ménard F, Richard P, Weimerskirch H (2008) Resource partitioning within a tropical seabird community: new information from stable isotopes. Mar Ecol Prog Ser 366:281–291
Cramp S, Simmons KE (1977) Handbook of the birds of Europe, the Middle East and North Africa, vol 1. Oxford University Press, Oxford
Dänhardt A, Fresemann T, Becker PH (2011) To eat or to feed? Prey utilization of Common Terns Sterna hirundo in the Wadden Sea. J Ornithol 152:347–357
Davenport R, Neuer S, Helmke P, Perez-Marrero J, Llinas O (2002) Primary productivity in the northern Canary Islands region as inferred from SeaWiFS imagery. Deep Sea Res II 49:3481–3496
Davoren GK, Burger AE (1999) Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim Behav 58:853–863
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colon Waterbirds 9:1–17
Einoder LD, Page B, Goldsworthy SD, De Little SC, Bradshaw CJA (2011) Exploitation of distant Antarctic waters and close neritic waters by short-tailed shearwaters breeding in South Australia. Aust Ecol 36:461–475
Forero MG, Gonzalez-Solis J, Hobson KA, Doncazar JA, Bertellotti M, Blanco G, Bortolotti GR (2005) Stable isotopes reveal trophic segregation by sex and age in the southern giant petrel in two different food webs. Mar Ecol Prog Ser 296:107–113
Granadeiro JP (1991) The breeding biology of Cory’s shearwater Calonectris diomedea borealis on Berlenga Island, Portugal. Seabird 13:30–33
Granadeiro JP, Silva MA (2000) The use of otoliths and vertebrae in the identification and size-estimation of fish in predator-prey studies. Cybium 24:383–393
Granadeiro JP, Monteiro LR, Furness RW (1998a) Diet and feeding ecology of Cory’s shearwater Calonectris diomedea in the Azores, north-east Atlantic. Mar Ecol Prog Ser 166:267–276
Granadeiro JP, Nunes M, Silva MC, Furness RW (1998b) Flexible foraging strategy of Cory’s shearwater, Calonectris diomedea, during the chick-rearing period. Anim Behav 56:1169–1176
Granadeiro JP, Dias MP, Rebelo R, Santos CD, Catry P (2006) Numbers and population trends of Cory’s Shearwater Calonectris diomedea at Selvagem Grande, northeast Atlantic. Waterbirds 29:56–60
Grémillet D, Dell’Omo G, Ryan PG, Peters G, Ropert-Coudert Y, Weeks SJ (2004) Offshore diplomacy, or how seabirds mitigate intra-specific competition: a case study based on GPS tracking of cape gannets from neighbouring colonies. Mar Ecol Prog Ser 268:265–279
Harding AMA, Hobson KA, Walkusz W, Dmoch K, Karnovsky NJ, Van Pelt TI, Lifjeld JT (2008) Can stable isotope (δ13C and δ15N) measurements of little auk (Alle alle) adults and chicks be used to track changes in high-Arctic marine foodwebs? Polar Biol 31:725–733
Hobson KA, Piatt JF, Pitocchelli J (1994) Using stable isotopes to determine seabird trophic relationships. J Anim Ecol 63:786–798
Hodum PJ, Hobson KA (2000) Trophic relationships among Antarctic fulmarine petrels: insights into dietary overlap and chick provisioning strategies inferred from stable-isotope (δ15N and δ13C) analyses. Mar Ecol Prog Ser 198:273–281
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Lecoq M, Catry P, Granadeiro JP (2011) Population trends of Cory’s shearwaters Calonectris diomedea borealis breeding at Berlenga Islands, Portugal. Airo 20:36–41
Lewis S, Sherratt TN, Hamer KC, Wanless S (2001) Evidence of intra-specific competition for food in a pelagic seabird. Nature 412:816–819
Machu E, Ettahiri O, Kifani S, Benazzouz A, Makaoui A, Demarcq H (2009) Environmental control of the recruitment of sardines (Sardina pilchardus) over the western Saharan shelf between 1995 and 2002: a coupled physical/biogeochemical modelling experiment. Fish Oceanogr 18:287–300
Magalhães MC, Santos RS, Hamer KC (2008) Dual-foraging of Cory’s shearwaters in the Azores: feeding locations, behaviour at sea and implications for food provisioning of chicks. Mar Ecol Prog Ser 359:283–293
Navarro J, González-Solís J (2009) Environmental determinants of foraging strategies in Cory’s shearwaters Calonectris diomedea. Mar Ecol Prog Ser 378:259–267
Navarro J, González-Solís J, Viscor G (2007) Nutritional and feeding ecology in the Cory’s shearwater (Calonectris diomedea) during breeding. Mar Ecol Prog Ser 351:261–271
Navarro J, Louzao M, Igual JM, Oro D, Delgado A, Arcos JM, Genovart M, Hobson KA, Forero MG (2009) Seasonal changes in the diet of a critically endangered seabird and the importance of trawling discards. Mar Biol 156:2571–2578
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 154–177
Paiva VH, Geraldes P, Ramírez I, Meirinho A, Garthe S, Ramos JA (2010a) Foraging plasticity in a pelagic seabird species along a marine productivity gradient. Mar Ecol Prog Ser 398:259–274
Paiva VH, Geraldes P, Ramírez I, Meirinho A, Garthe S, Ramos JA (2010b) Oceanographic characteristics of areas used by Cory’s shearwaters during short and long foraging trips in the North Atlantic. Mar Biol 157:1385–1399
Paiva VH, Xavier J, Geraldes P, Ramírez I, Meirinho A, Garthe S, Ramos JA (2010c) Foraging ecology of Cory’s shearwaters in different ecological environments of the North Atlantic. Mar Ecol Prog Ser 410:257–268
Passos C, Navarro J, Giudici A, González-Solís J (2010) Effects of extra mass on the pelagic behavior of a seabird. Auk 127:100–107
Pedrocchi V, Oro D, González-Solís J (1996) Differences between diet of adult and chick Audouin’s gulls Larus audouinii at the Chafarinas Islands, SW Mediterranean. Ornis Fenn 73:124–130
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Phillips RA, Wakefield ED, Croxall JP, Fukuda A, Higuchi H (2009) Albatross foraging behaviour: no evidence for dual foraging, and limited support for anticipatory regulation of provisioning at South Georgia. Mar Ecol Prog Ser 391:279–292
Pinaud D, Cherel Y, Weimerskirch H (2005) Effect of environmental variability on habitat selection, diet, provisioning behaviour and chick growth in yellow-nosed albatrosses. Mar Ecol Prog Ser 298:295–304
Pinheiro JC, Bates DM, DebRoy S, Sarkar D, R Development Core Team (2011) nlme: linear and nonlinear mixed effects models. R package version 3.1-102
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Quéro J-C, Porché P, Vayne JJ (2003) Guide des Poissons de l’Atlantique Européen. Delachaux & Niestlé, Paris
Ramos JA, Sola E, Monteiro LR, Ratcliffe N (1998) Prey delivered to roseate tern chicks in the Azores. J Field Ornithol 69:419–429
Richoux NB, Jaquemet S, Bonnevie BT, Cherel Y, McQuaid CD (2010) Trophic ecology of grey-headed albatrosses from Marion Island, Southern Ocean: insights from stomach contents and diet tracers. Mar Biol 157:1755–1766
Roberts JJ, Best BD, Dunn DC, Treml EA, Halpin PN (2010) Marine geospatial ecology tools: an integrated framework for ecological geoprocessing with ArcGIS, Python, R, MATLAB, and C++. Environ Model Softw 25:1197–1207
Sears J, Hatch SA, O’Brien DM (2009) Disentangling effects of growth and nutritional status on seabird stable isotope ratios. Oecologia 159:41–48
Sousa FM, Nascimento S, Casimiro H, Boutov D (2008) Identification of upwelling areas on the sea surface temperature images using fuzzy clustering. Remote Sens Environ 112:2817–2823
R Development Core Team (2009) R: a language and environment for statistical computing (2.10.1). R Foundation for Statistical Computing, Vienna
Thompson DR, Phillips RA, Stewart FM, Waldron S (2000) Low δ13C signatures in pelagic seabirds: lipid ingestion as a potential source of 13C-depleted carbon in the Procellariiformes. Mar Ecol Prog Ser 208:265–271
Uttley JD, Walton P, Monaghan P, Austin G (1994) The effects of food abundance on breeding performance and adult time budgets of Guillemots Uria aalge. Ibis 136:205–213
Weimerskirch H (1998) How can a pelagic seabird provision its chick when relying on a distant food resource? Cyclic attendance at the colony, foraging decision and body condition in sooty shearwaters. J Anim Ecol 67:99–109
Weimerskirch H, Cherel Y (1998) Feeding ecology of short-tailed shearwaters: breeding in Tasmania and foraging in the Antarctic? Mar Ecol Prog Ser 167:261–274
Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E (1986) Fishes of the North-eastern Atlantic and the Mediterranean. UNESCO, Paris
Williams CT, Buck CL, Sears J, Kitaysky AS (2007) Effects of nutritional restriction on nitrogen and carbon stable isotopes in growing seabirds. Oecologia 153:11–18
Wilson RP (1984) An improved stomach pump for penguins and other seabirds. J Field Ornithol 55:109–112
Wilson RP, Pütz K, Peters G, Culik BM, Scolaro JA, Charrassin JB, Ropert-Coudert Y (1997) Long-term attachment of transmitting and recording devices to penguins and others seabirds. Wildl Soc Bull 25:101–106
Wilson LJ, Daunt F, Wanless S (2004) Self-feeding and chick provisioning diet differ in the common guillemot Uria aalge. Ardea 92:197–208
Xavier JC, Magalhães MC, Mendonça AS, Antunes M, Carvalho N, Machete M, Santos RS, Paiva V, Hamer KC (2011) Changes in diet of Cory’s Shearwaters Calonectris diomedea breeding in the Azores. Mar Ornithol 39:129–134
Ydenberg RC, Welham CVJ, Schmid-Hempel R, Schmid-Hempel P, Beauchamp G (1994) Time and energy constraints and the relationship between currencies in foraging theory. Behav Ecol 5:28–34
Acknowledgments
Parque Natural da Madeira and, particularly Paulo Oliveira, Dília Menezes and Carolina Santos, provided permission for us to work on Selvagem Grande, and the Instituto de Conservação da Natureza e Biodiversidade provided the permission to work on Berlenga. We are grateful to all who gave us a valuable help in field work, namely Ana Almeida, Maria Dias, Teresa Catry, Filipe Moniz, Rui Rebelo and Mariana Marques. We are also thankful to the logistical support provided by the wardens of both Nature Reserves during our stays: Paulo Crisóstomo, Jorge Mourato, Ricardo Cabral, Carlos Santos, Maurício Paixão and Jacques da Mata. This study was financed by Fundação para a Ciência e Tecnologia (FCT-Portugal) through Project PTDC/MAR/71927/2006, as part of the Programa Plurianual (UI&D 331/94), and through a doctoral fellowship to H. Alonso (BD/47055/2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Garthe.
Rights and permissions
About this article
Cite this article
Alonso, H., Granadeiro, J.P., Paiva, V.H. et al. Parent–offspring dietary segregation of Cory’s shearwaters breeding in contrasting environments. Mar Biol 159, 1197–1207 (2012). https://doi.org/10.1007/s00227-012-1900-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1900-2