Abstract
Extractives of tree barks have long been considered as a rich source of novel bioactive secondary metabolites, which are by far not well-explored. In the current work, bark extracts of Catalpa bungei were investigated for the first time and five extractives were isolated and purified, including a new triterpene saponin derivative, namely 3-O-β-D-glucuronopyranosyl-6′-methyl-21-O-cis-caffeoyl machaerinic acid (4), two known flavonoids [(+)-gallocatechin (1) and isoquercitrin-6″-gallate (2)], a known oleanane-type triterpene [machaerinic acid (3)] and a known phytosterol [stigmasterol (5)]. Chemical structural elucidation of extractives 1–5 was carried out mainly on the basis of their physicochemical and spectroscopic (IR, NMR, MS) evidences and analysis, as well as by detailed comparison of the analytical evidence with those in the literature. To the best of the authors' knowledge, this is the first time to find the occurrence of extractives 1–5 in the tree of C. bungei. It is noteworthy that the five constituents have never previously been reported in any species of Catalpa genus. Compound 4, a previously undescribed triterpene saponin derivative, was isolated and elucidated in this work for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant extractives still present unrevealed novelties, which are relevant in terms of biosynthesis routes (Hu et al. 2017; Mangindaan et al. 2017; Si et al. 2001), markers for chemotaxonomical differentiation of wood species (Wang et al. 2016; Si et al. 2011), and various biological activities (Hu et al. 2016). Tree barks, long treated as wastes or residues in pulping and forestry industries, contain valuable bioactive substances, which could be commercialized as high value-added products, for example, in medicines and cosmetics.
Catalpa bungei C.A. Mey, a deciduous woody plant in Bignoniaceae family, is native to China. As an important ornamental arbor species, C. bungei is extensively used in urban forests in northern and central cities of China due to its straight stems, beautiful flowers and moderate efficiency in particulate matter removal (Xu et al. 2014; Lu et al. 2019). In addition to its value in landscaping, C. bungei wood exhibits superior mechanical properties and high durability that can resist the corrosion caused by insects and microorganisms (Lu et al. 2019). Plant materials of the tree have also been used in folk medicines to treat, alleviate or prevent various diseases, including nephritis, edema, cystitis, leprosy, eczema and gastric cancer. Previous biological and pharmaceutical studies showed that C. bungei extracts possess significant antioxidant and anti-cervical cancer effects (Xu et al. 2014, 2018), as well as inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities (Tang et al. 2016).
Earlier phytochemical investigations of C. bungei leaves and seeds resulted in the isolation of several types of constituents such as lignans, triterpenoids, flavonoids, iridoids and phenylethanoid glycosides (Machida et al. 2004; Kanai et al. 1996; Xu et al. 2014, 2018; Tang et al. 2016). However, to the best of the authors` knowledge, no study has ever been performed to screen the extractives of C. bungei barks. In the present systematic search for chemical extractives, which may be responsible for the biological and pharmacological activities of this tree, the isolation, separation and structural characterization of one new and four known natural extractives from the stem barks of C. bungei were described in the current work.
Materials and methods
General experimental procedure
1D and 2D nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance DPX 400 instrument (Rheinstetten, Germany) using deuterated solvent MeOH-d4 with tetramethylsilane as an internal standard. Positive fast atom bombardment mass (FAB MS) experiments were done on a Micromass Autospec M363 spectrometer (Manchester, UK). IR spectra were acquired by KBr disk method on a FTIR-8400S spectrometer (Shimadzu, Kyoto, Japan). A SGW-2 automatic polarimeter (Shanghai INESA Physico Optical Instrument Co., Ltd., Shanghai, China) was employed to determine the optical rotations. Melting points (M.P., uncorrected) were measured with the Electro Thermal 9100 apparatus (Electrothermal Engineering Ltd., Essex, UK).
Silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical plant, Qingdao, China) and Sephadex LH-20 (Sigma) were used as packing materials for open column chromatography (OCC). Vacuum liquid chromatography (VLC) was performed using ODS (50 µm, YMC) and D-101. SBS-160 fraction collectors (Shanghai Huxi Analysis Instrument Factory Co., Ltd., Shanghai, P.R. China) were employed to collect the eluents. Thin layer chromatography (TLC) experiments were conducted with DC-Plastikfolien Cellulose F (Merck, Darmstadt, Germany) plates, with H2O-HOAc (47:3, v/v, solvent A) and t-BuOH-H2O-HOAc (3:1:1, v/v/v, solvent B) used as developing solvents. TLC spots visualizations were carried out by UV light exposure (365 and 254 nm) and by spraying with 1% FeCl3 (in EtOH) solution, followed by heating. Analytical grade solvents were used for isolation and separation procedures.
Plant material
In the current work, tree stems of C. bungei (9 years old) were obtained in January of 2018 from a forest in Luoyang of Henan Province, P.R. China, which was constructed by Research Institute of Forestry, Chinese Academy of Forestry. Fresh barks were debarked from C. bungei stems and then shade-dried at room temperature. A voucher herbarium specimen with ID number Cb-20180305 was deposited in the herbarium of Tianjin Key Laboratory of Pulp and Paper, Tianjin University of Science and Technology.
Extraction, fractionation and purification
Stem barks of C. bungei (labeled as CbB, 4935 g) were dried in the shade at room temperature, and then finely ground with a Wiley mill (40-mesh sieve), followed by extraction at room temperature for more than 72 h with H2O-EtOH (5:95, v/v, each 15 L) for four times. The extracts were combined together, filtered and concentrated to give a crude residue (496.46 g, yield 9.97%) and then suspended in distilled water. Petroleum ether (54.20 g, yield 1.10%), CHCl3 (52.32 g, yield 1.06%) and EtOAc (70.38 g, yield 1.43%) soluble fractions, as well as EtOAc insoluble fraction (319.56 g, yield 6.48%) were obtained after successive solvent partitions, concentration and lyophilization.
As shown in Fig. 1, a portion of the above obtained CHCl3 soluble fraction of extract from C. bungei barks (CbBC, 49.10 g) was chromatographed on a column packed with silica gel (200 ~ 300 mesh), eluted with a solvent gradient system of EtOAc–MeOH-CHCl3 (1:4:95 → 2:19:79 → 2:49:49 → 5:90:5) to yield seven main fractions (CbBC1–CbBC7), which were monitored and grouped by TLC detection. The third main fraction (CbBC3, 12.32 g) and the sixth one (CbBC6, 18.05 g) were further subjected to silica gel (100–200 mesh) OCC elution with MeOH-CHCl3-H2O (1:7:2 → 3:6:1, v/v) to produce five (CbBC31–CbBC35) and three subfractions (CbBC61–CbBC63), respectively. Subfraction CbBC32 (8.06 g) was then applied to VLC on ODS, eluted successively with 5%, 15%, 25% and 35% EtOH in H2O, to yield five fractions CbBC321–CbBC325, respectively. Fraction CbBC321 (952.24 mg) was further purified by Sephadex LH-20 OCC and eluted with MeOH-H2O (4:1, v/v) to give compound 1 (123.15 mg) as white amorphous powder. Subfraction CbBC324 (3.42 g) was further loaded over Sephadex LH-20 OCC eluted with EtOH-n-hexane (3:1 and 1:2, v/v) to yield four fractions CbBC3241–CbBC3244. In the same way, subfraction CbBC3242 (1.26 g) was purified through Sephadex LH-20 OCC, with MeOH-H2O (2:1, 1:2, 1:5, v/v) used as washing solvent system to get 88.38 mg of white amorphous compound 2. Subfraction CbBC62 (12.17 g) was also subjected to Sephadex LH-20 OCC eluted with EtOH-n-hexane (2:1, v/v) to give five fractions CbBC621–CbBC625. Compound 3 (46.73 mg) was obtained from CbBC622 as an amorphous powder by recrystallization. CbBC624 (5.73 g) was further separated by D101 VLC with EtOH-H2O (1:4 → 4:1) used as eluting solvents to give 41.62 mg of amorphous compound 4, together with fractions CbBC6241 and CbBC6243. Compound 5 (72.27 mg) was purified from fraction CbBC6243 (2.18 g) through OCC as Sephadex LH-20 used as packing material, while MeOH-H2O (2:1 and 1:3, v/v) and EtOH-n-hexane (1:3, v/v) used as flowing phase successively.
Compound 4
Obtained as a whitish amorphous powder in this work with M.P. 289–291 °C and \(\left[ \alpha \right]_{{\text{D}}}^{20}\)−15.6° (MeOH, c 0.5), compound 4 presented its IR (KBr) νmax at 1510, 1605, 1700 and 3415 cm−1, respectively. While its Rf values appeared at 0.19 and 0.80 in solvents A and B, respectively. In positive FAB MS spectrum, compound 4 gave [M + K]+ at m/z 863, [M + Na]+ at m/z 847, and [M + H]+ at m/z 825, corresponding to molecular weight 824 and calculated for C46H64O13. The 1H (400 MHz, δ, MeOH-d4), 13C (100 MHz, δ, MeOH-d4) and partial 2D NMR information are presented in Table 1.
Results and discussion
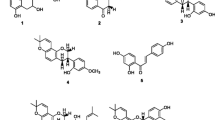
In the current study, the 95% ethanolic extract of C. bungei stem barks was fractionated with a serious of polar solvents to get several soluble parts. By means of a sequence of chromatographic techniques, purification and separation of the obtained chloroform soluble fraction led to the isolation of a new oleanane-type triterpene glycoside called 3-O-β-D-glucuronopyranosyl-6′-methyl-21-O-cis-caffeoyl machaerinic acid (4), together with four known extractives: a flavan-3-ol [(+)-gallocatechin, 1] (Agrawal 1989), a flavonol glycoside (isoquercitrin-6″-gallate, 2) (Collins et al. 1975), an oleanane-type triterpene (machaerinic acid, 3) (Delgado et al. 1984) and a phytosterol compound (stigmasterol, 5) (Forgo and Kövér 2004). The chemical structures of the five extractives are shown in Fig. 2. The known compounds (1–3, 5) were identified by comparison of their spectroscopic evidences and physiochemical data with those in the literature.
Compound 4 was isolated as a whitish amorphous powder showing \(\left[ \alpha \right]_{{\text{D}}}^{20}\)−15.6° in MeOH at c 0.5 and M.P. 289–291 °C. Its molecular formula was calculated to be C46H64O13 by Positive FAB MS spectrum, for its [M + K]+, [M + Na]+ and [M + H]+ ion peaks at m/z 863, 847, and 825, respectively, corresponding to its molecular weight 824. In compound 4, the presence of phenolic hydroxyl groups was confirmed from gray-green color through TLC experiment when spraying 1% ethanolic FeCl3 (Rf values 0.19 and 0.80 in developing solvents A and B, respectively) (Imakura et al. 1985; Si et al. 2011, 2018). For IR (KBr) spectrum, compound 4 exhibited absorptions for aromatic ring at 1510 and 1605 cm−1 (Si et al. 2008). While the bands of α,β-unsaturated carbonyl and hydroxyl groups were observed at 1700 and 3415 cm−1, respectively (Peng et al. 2019).
For the aglycone part of compound 4, its 1H NMR spectrum revealed signals for seven methyl groups including five singlets [δH 1.06, 0.86, 0.97, 0.83 and 1.20] ascribable to the methyl protons H-23, H-24, H-25, H-26 and H-27 (each 3H), respectively, as well as two doublets with coupling constant of J = 6.1 Hz due to the two methyl protons at δH 0.96 (3H, H-29) and 1.10 (3H, H-30) (Wang et al. 2018). The aglycone also presented a double doublet centering at δH 2.98 (1H, dd, J = 4.0 and 11.5 Hz, H-18) assignable to a methine proton, together with olefinic proton signal resonating at δH 5.32 (1H, t-like, J = 3.7, H-12), which typically indicated that the aglycone moiety is a 3β,21β-dihydroxy oleanolic-type triterpene (Lehbili et al. 2018). The 13C NMR spectrum of compound 4 additionally supported the above conclusion, for its carbons of C-12, 13, 18, 19, 20 and 21 on the aglycone unit gave characteristic peaks at δC 123.08, 143.81, 41.59, 47.56, 35.64 and 76.16, respectively (Bitchi et al. 2019). By a more detailed inspection, the 1D and 2D NMR spectrum data of the aglycone part of triterpene skeleton in compound 4 (in Table 1) were very similar to those in compound 3, an oleanolic acid named machaerinic acid (Mair et al. 2018), which are also summarized in Table 1. Thus, the identification led to the confirmation of the aglycone part of compound 4 to be machaerinic acid.
Besides the aglycone part signals, in 1H NMR spectrum of compound 4, a pair of ABX style proton signals [δH 7.35 (1H, d, J = 2.1 Hz, H-2″), δH 6.66 (1H, d, J = 7.9 Hz, H-5″) and δH 7.04 (1H, d, J = 2.1 & 7.9 Hz, H-6″)] corresponding to a catechol ring (Hu et al. 2017), and a set of AB type signals for cis olefinic protons exhibiting distinctive doublets at δH 6.73 (1H, H-α″) and δH 5.74 (1H, H-β″) with coupling constant of J = 12.2 Hz were ascribable to a cis-caffeoyl group (Si et al. 2016; Yahagi et al. 2012).
Furthermore, in 1H NMR spectrum of compound 4, the proton signals including an anomeric proton irritating at δH 4.65 (1H, d, J = 7.1 Hz, H-1′) and others ranging from δH 3.70 to δH 4.09 (4H, m, H-2′, 3′, 4′, 5′), were assignable to a β-D-glucuronic acid residue (Si et al. 2009). In 13C NMR spectrum, the carboxyl carbon C-6′ of the β-D-glucuronic acid moiety characteristically resonated at δC 170.52 (Fig. 3). The proton signals at δH 3.70 (3H) as a singlet in 1H NMR and a carbon signal at δC 51.91 in 13C NMR spectra indicated the existence of a methoxy in compound 4 (Li et al. 2020).
As for the HMBC spectrum of compound 4, long-range correlations were observed between the anomeric proton at δH 4.65 (1H, d, J = 7.1 Hz, H-1′) and the carbon at δC 89.72 (C-3), between proton peak at δH 4.94 (1H, dd, J = 5.0 and 11.6 Hz, H-21) and carbon signal at δC 167.96 (C-γ''), between proton signals at δH 3.70 (3H, s, H-6′-Me) and carbon at δC 170.52 (C-6′), which confirmed that the glucuronic acid and cis-caffeoyl residues were combined to sites C-3 and C-21 of the triterpene aglycone, respectively (Yoshikawa et al. 2001; Bitchi et al. 2019; Si et al. 2017), while the methoxy group attached to position C-6′ of glucuronic acid moiety (Li et al. 2020).
In compound 4, the 13C NMR spectrum gave resonances for 46 carbons, which were assigned to the presence of 8 methyl, 9 methene, 16 methine and 13 tertiary carbons, as shown in Table 1, by analysis of DEPT spectrum. More detailed analysis of the 1D and 2D spectroscopic data combined with careful comparison to other known literature data, led to the structural elucidation of extractive 4 as 3-O-β-D-glucuronopyranosyl-6′-methyl-21-O-cis-caffeoyl machaerinic acid, which is a new triterpene saponin derivative and has not previously been isolated from any other plant species.
Conclusion
To date, this is the first chemical investigation of extractives in the stem barks C. bungei. Successive silica gel OCC, VLC and Sephadex LH-20 separation and purification of CHCl3 fraction of H2O-EtOH (5:95, v/v) extracts from C. bungei stem barks resulted in the isolation of five low-molecular-weight extractives. Structures of the isolated extractives were identified and elucidated extensively on the basis of their spectroscopic evidences, chemical data and a careful comparison with those in the literature. Among them, 3-O-β-D-glucuronopyranosyl-6′-methyl-21-O-cis-caffeoyl machaerinic acid (4) is a new triterpene saponin derivative isolated and established for the first time here. The two flavonoids, (+)-gallocatechin (1) and isoquercitrin-6″-gallate (2), together with machaerinic acid (3), an oleanane-type triterpene, as well as stigmasterol (5), a phytosterol, have never been reported from Catalpa genus previously.
References
Agrawal PK (1989) Carbon-13 NMR of flavonoids. Elsevier, New York
Bitchi MB, Magid AA, Yao-Kouassi PA, Kabran FA, Harakat D, Martinez A, Morjani H, Tonzibo FZ, Voutquenne-Nazabadioko L (2019) Triterpene saponins from the roots of Parkia bicolor A. Chev Fitoter 137:104264. https://doi.org/10.1016/j.fitote.2019.104264
Collins FW, Bruceand AB, Cornelius KW (1975) Flavonol glycoside gallates from Tellima grandiflora. Phytochemistry 14(4):1099–1102. https://doi.org/10.1016/0031-9422(75)85195-8
Delgado MCC, Silva MSD, Fo RB (1984) 3β-hydroxy-21β-e-cinnamoyloxyolean-12-en-28-oic acid, a triterpenoid from enterolobium contorstisiliquum. Phytochemistry 23:2289–2292. https://doi.org/10.1016/S0031-9422(00)80537-3
Forgo P, Kövér KE (2004) Gradient enhanced selective experiments in the 1H NMR chemical shift assignment of the skeleton and side-chain resonances of stigmasterol, a phytosterol derivative. Steroids 69:43–50. https://doi.org/10.1016/j.steroids.2003.09.012
Hu LQ, Wang K, Li GB, Zhang RY, Luo YY, Si CL, Wang JH (2017) Isolation and structural elucidation of heartwood extractives of Juglans sigillata. Holzforschung 71:785–792. https://doi.org/10.1515/hf-2017-0036
Hu WC, Wang XF, Wu L, Shen T, Ji LL, Zhao XH, Si CL, Jiang YY, Wang GC (2016) Apigenin-7-O-β-D-glucuronide inhibits LPS-induced inflammation through the inactivation of AP-1 and MAPK signalling pathways in RAW 264.7 macrophages and protects mice against endotoxin shock. Food Funct 7:1002–1013. https://doi.org/10.1039/c5fo01212k
Imakura Y, Kobayashi S, Mima A (1985) Bitter phenyl propanoid glycosides from Campsis chinensis. Phytochemistry 24:139–146. https://doi.org/10.1016/S0031-9422(00)80823-7
Kanai E, Machida K, Kikuchi M (1996) Studies on the constituents of Catalpa species. I. Iridoids from Catalpae fructus, Chem Pharm Bull 44:1607–1609. https://doi.org/10.1248/cpb.44.1607
Lehbili M, Magid AA, Kabouche A, Voutquenne-Nazabadioko L, Morjani H, Harakat D, Kabouche Z (2018) Triterpenoid saponins from Scabiosa stellata collected in North-eastern Algeria. Phytochemistry 150:40–49. https://doi.org/10.1016/j.phytochem.2018.03.005
Li DQ, Liu D, Lv MC, Gao PY, Liu XG (2020) Isolation of triterpenoid saponins from Medicago sativa L. with neuroprotective activities. Bioorg Med Chem Lett 30:126956. https://doi.org/10.1016/j.bmcl.2020.126956
Lu N, Zhang MM, Xiao Y, Han DH, Liu Y, Zhang Y, Yi F, Zhu TQ, Ma WJ, Fan EQ, Qu GZ, Wang JH (2019) Construction of a high-density genetic map and QTL mapping of leaf traits and plant growth in an interspecific F1 population of Catalpa bungei × Catalpa duclouxii Dode. BMC Plant Biol 19:596. https://doi.org/10.1186/s12870-019-2207-y
Machida K, Hishinuma E, Kikuchi M (2004) Studies on the constituents of Catalpa species. IX. Iridoids from the fallen leaves of Catalpa ovata G DON. Chem Pharm Bull 52:618–621. https://doi.org/10.1248/cpb.52.618
Mair CE, Grienke U, Wilhelm A, Urban E, Zehl M, Schmidtke M, Rollinger JM (2018) Anti-influenza triterpene saponins from the bark of Burkea africana. J Nat Prod 81:515–523. https://doi.org/10.1021/acs.jnatprod.7b00774
Mangindaan B, Matsushita Y, Aoki D, Yagami S, Kawamura F, Fukushima K (2017) Analysis of distribution of wood extractives in Gmelina arborea by gas chromatography and time of-flight secondary ion mass spectrometry. Holzforschung 71:299–305. https://doi.org/10.1515/hf-2016-0129
Peng WW, Huo GH, Zheng LX, Xiong ZH, Shi XJ, Peng DY (2019) Two new oleanane derivatives from the fruits of Leonurus japonicus and their cytotoxic activities. J Nat Med 73:252–256. https://doi.org/10.1007/s11418-018-1244-4
Si CL, An LL, Xie DN, Liu CY, Chen XQ, Wang GH, Huo D, Yang QL, Hong YM (2016) New acylated flavonol glycosides with antibacterial activity from root barks of Sophora Japonica. Wood Sci Technol 50(3):645–659. https://doi.org/10.1007/s00226-016-0809-1
Si CL, Gao Y, Wu L, Liu R, Wang GH, Dai L, Li XH, Hong YM (2017) Isolation and characterization of triterpenoids from the stem barks of Pinus massoniana. Holzforschung 71:697–703. https://doi.org/10.1515/hf-2016-0228
Si CL, Liu Z, Su YF, Kim JK, Bae YS (2008) Coumarins and secoiridoid glucosides from bark of Fraxinus rhynchophylla Hance. Holzforschung 62:553–555. https://doi.org/10.1515/HF.2008.086
Si CL, Wu L, Zhu ZY, Kim JK, Kwon DJ, Bae YS (2009) Apigenin derivatives from Paulownia tomentosa Steud. var. tomentosa stem barks. Holzforschung 63:440–442. https://doi.org/10.1515/HF.2009.063
Si CL, Xu J, Kim JK, Bae YS, Liu PT, Liu Z (2001) Antioxidant properties and structural analysis of phenolic glucosides from bark of Populus ussuriensis Kom. Wood Sci Technol 45(1):5–13. https://doi.org/10.1007/s00226-009-0286-x
Si CL, Xu J, Lu YY, Su Z, Su YF, Bae YS (2011) Hydrolysable tannins from Juglans sigillata stem barks. Biochem Syst Ecol 39:225–227. https://doi.org/10.1016/j.bse.2011.02.010
Si CL, Yang XH, Li ZJ, Lu JS, Tao X, Zhang JY, Liu W, Bae YS (2018) Extractives of Cercidiphyllum japonicum twigs: isolation and structural elucidation of a new galloylflavonol glycoside, anomeric tannins and flavonoids. Holzforschung 72:719–725. https://doi.org/10.1515/hf-2018-0029
Tang HY, Bai MM, Tian JM, Pescitelli G, Lvšić T, Huang XH, Lee H, Son YN, Kim JH, Kim YH, Gao JM (2016) Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv 6:40706–40716. https://doi.org/10.1039/c6ra04207d
Wang R, Wang K, Si CL, Luo YY, Liu W, Zhang XX, Tian YY, Wang JH (2018) Triterpene saponins from branches of Pinus massoniana. Chem Nat Compd 54:717–720. https://doi.org/10.1007/s10600-018-2453-5
Wang SN, Zhang FD, Huang AM, Zhou Q (2016) Distinction of four Dalbergia species by FTIR, 2nd derivative IR, and 2D-IR spectroscopy of their ethanol-benzene extractives. Holzforschung 70:503–510. https://doi.org/10.1515/hf-2015-0125
Xu HY, Hu GG, Dong J, Wei Q, Shao HB, Lei M (2014) Antioxidative activities and active compounds of extracts from Catalpa plant leaves. Sci World J 2014:857982. https://doi.org/10.1155/2014/857982
Xu H, Zhou Z, Dong J, Lei M (2018) Suppression of cervical cancer cell survival by ursolic acid extracted from Catalpa bungei leaves. Pharmacog Mag 14:425–431. https://doi.org/10.4103/pm.pm_408_17
Yahagi T, Daikonya A, Kitanaka S (2012) Flavonol acylglycosides from flower of Albizia julibrissin and their inhibitory effects on lipid accumulation in 3T3-L1 cells. Chem Pharm Bull 60:129–136. https://doi.org/10.1248/cpb.60.129
Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H (2001) Medicinal flowers. III. Marigold. (1): hypoglycemic, gastric emptying inhibitory, and gastroprotective principles and new oleanane-type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula officinalis. Chem Pharm Bull 49:863–870. https://doi.org/10.1248/cpb.49.863
Acknowledgements
This work was kindly supported by National Key Research and Development Program of China (2017YFD0600604), Key Technology Research and Development Program of Tianjin (19YFZCSN00950), and Tianjin Enterprise Technology Commissioner Project (19JCTPJC52800). The plant samples were authenticated by Professor J.H. Wang from Research Institute of Forestry, Chinese Academy of Forestry, Beijing, P.R. China.
Funding
The funder had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, J., Lu, N., Liu, K. et al. Bark extractives of Catalpa bungei: isolation, purification and structural elucidation of triterpene, phytosterol and flavonoid derivatives. Wood Sci Technol 55, 231–241 (2021). https://doi.org/10.1007/s00226-020-01239-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-020-01239-5