Abstract
Long-term use of sevoflurane, an inhalation anesthetic, could negatively impact cognitive function. Current studies have suggested that cognitive impairment induced by sevoflurane may be associated with neuroinflammation. Sestrin2 (SESN2), which belongs to a family of stress-inducible genes, has been reported to exert neuroprotective effects against brain injury. However, its role and underlying mechanisms in sevoflurane-induced cognitive dysfunction in aged rats remain unknown. A sevoflurane-induced aging rat injury model with or without SESN2 overexpression was constructed. The learning and memory abilities of rats were evaluated by the MWM test. ELISA assay and qRT-PCR were conducted to analyze the level of pro-inflammatory factors in the hippocampus. Levels of oxidative stress markers were measured by DHE staining or kit methods. Neuronal apoptosis in the hippocampus was detected using TUNEL assay. Expression of proteins were analyzed by western blot. Sevoflurane exposure caused elevated protein level of SESN2 in hippocampus and cognitive impairment of aged rats. Importantly, overexpression of SESN2 alleviated sevoflurane-induced cognitive dysfunction and inhibited the production of pro-inflammatory factors, oxidative stress, and neuronal apoptosis in the hippocampus. Furthermore, SESN2 overexpression suppressed NLRP3 inflammasome activation induced by sevoflurane. These findings suggested that SESN2 could exert neuroprotective against sevoflurane-induced nerve injury of aged rats through anti-oxidant and anti-inflammatory effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postoperative cognitive dysfunction (POCD), a common nervous system complication after surgery, especially in the elderly, is characterized by lack of concentration, memory loss, slow thinking, etc. (Dong et al. 2018). POCD is not only linked to surgical trauma-induced oxidative stress and neuroinflammation, but anesthesia drugs such as sevoflurane utilized during surgery can also elicit cognitive impairment, reported by precious studies (Netto et al. 2018; Rump and Adamzik 2022; Skvarc et al. 2018).
Sestrin2 (SESN2) is a member of a stress-responsive gene family, and its encoded protein has been documented to exert a significant impact on encephalopathy (R. Li et al. 2019; X. Liu et al. 2021). For example, SESN2 mitigates sepsis-associated encephalopathy by promoting autophagy in the hippocampus (Luo et al. 2020); SESN2 protects nerve cells against cerebral ischemia–reperfusion injury by inhibiting inflammation, oxidative stress, and apoptosis (J. Liu et al. 2020). In addition, a previous study showed that SESN2 exhibited a suppressive effect against sevoflurane-induced neuroapoptosis in neuronal cells in vitro (Yi, 2015). However, the effects of SESN2 on cognitive dysfunction in animal models exposed to sevoflurane and its underlying mechanisms remain elusive.
Herein, a cognitive impairment model was established by exposing aged rats to sevoflurane. Next, the effects of SESN2 on cognitive function of rats and inflammation, oxidative stress, and neuronal apoptosis in hippocampus were investigated. A possible mechanism was also explored.
Materials and methods
Viral vectors construction
An adeno-associated viral vector (AAV) expressing SESN2 tagged with enhanced green fluorescent protein (EGFP) (AAV-EGFP-SESN2, denoted as SESN2) or AAV-GFP (Control, denoted as Vector) was constructed by Addgene (Watertoen, MA, USA). Subsequently, SESN2 or Vector was injected into the CA1 region of the rats hippocampus using stereotaxic surgery as described previously (Yu, 2017). Briefly, Rats were anesthetized with sodium pentobarbital (40 mg/kg body weight) and then secured in a stereotaxic apparatus. As per the predetermined coordinates (− 3.6 mm AP, ± 1.8 mm ML, and − 3.0 mm DV), bilateral holes were drilled in the skull. Next, SESN2 or Vector was injected bilaterally with 1.5 µL per side into the CA1 region of the hippocampus. The final titers of the viral particles were 1 × 1013/mL. After injection, the syringe needles were left inserted for another 10 min prior to being slowly removed. Afterwards, Rats were allowed to recover under a heated blanket.
Sevoflurane-induced cognitive dysfunction rats model creation
Twenty-four 24-month-old male Sprague–Dawley (SD) rats, that obtained from Vital River Laboratory Animal Technology Co., Ltd (Beijing, China), were adaptively raised for 1 week under identical conditions before the experiment. The animal experimental procedures were authorized by the Animal Care Committee of the Affiliated Huai'an No. 1 People’s Hospital of Nanjing Medical University.
The rats were randomly divided into 4 groups (6 animals each). For the Sev group, the rats were exposed to a 2.6% concentration of sevoflurane, administered through a humidified 30% O2 carrier gas, for a duration of 4 h at a flow rate of 2 L/min, utilizing an anesthetic apparatus equipped with a multi-gas monitor. The rats were exposed to the carrier gas without the inclusion of sevoflurane for an equivalent duration, as the Control group. For the Sev + Vector group, the rats were intracerebrally injected with Vector 10 days before being exposed to 2.6% sevoflurane. For the Sev + SESN2 group, the rats were intracerebrally injected with SESN2 10 days before being exposed to 2.6% sevoflurane.
Morris water maze (MWM) test
MWM test was conducted as previously described (Peng et al. 2020). Briefly, one day after sevoflurane exposure, the rats were trained to find a hidden platform underwater in the maze. Context test began on the third day after sevoflurane exposure, initially, the rats underwent a detection experiment lasting 120 s to identify the concealed platform, and the duration of their escape was documented. Subsequently, the platform was eliminated, and the frequency with which the rats identified the platform’s location within a specific timeframe of 60 s was tallied. Finally, the duration of time the rats expended searching for the platform in the target quadrant and the distance covered in the target quadrant were recorded.
Western blot
The hippocampal tissues samples were obtained after MWM from the rats. For western blot analysis, the hippocampal tissues were homogenized using RIPA buffer (Sigma-Aldrich, Milan, Italy). Protein extracts of equal amounts were subjected to 12% SDS-PAGE and subsequently transferred onto PVDF membranes. Primary antibodies were used as follows: anti-SESN2 (ab178518, 1:1000, Abcam), anti-Bax (ab32503, 1:2000, Abcam), anti-Bcl-2 (ab196495, 1:1000, Abcam), anti-NLRP3 (ab263899, 1:1000, Abcam), anti-ASC (ab309497, 1:1000, Abcam), anti-IL-18 (ab191860, 1:500, Abcam), anti-IL-1β (ab254360, 1:1000, Abcam) and anti-β-actin (ab8227, 1:1000, Abcam). Next, an HRP-conjugated secondary antibody was used. The protein band was developed using an ECL kit (PIERCE, Rockford, IL, USA) and then quantitated using Image J software.
Enzyme-linked immunosorbent assay (ELISA)
Levels of interleukin 6 (IL-6), tumor necrosis factor α (TNF-α) and IL-1β in supernatants of homogenized hippocampal tissues were evaluated through ELISA kits (Beyotime, Shanghai, China).
Quantitative real time PCR (qRT-PCR)
Total RNA from hippocampal tissues was extracted using TRIzol reagent (Takara, Beijing, China). cDNA was generated with a reverse transcription kit (Takara, Beijing, China). Next, using SYBR Green methods, qRT-PCR was performed on an ABI 7500FAST system (Applied Biosystems, CA, USA), and the primers were listed in Table 1.
Dihydroethidium (DHE) staining
ROS production in hippocampal tissues was assessed using DHE staining. 5 frozen fresh tissue sections were taken per rat in each group of 6 rats. Sections were treated with 10 µM DHE (Sigma-Aldrich, Milan, Italy) for 30 min at 37℃, and then washed with PBS. The sections were subjected to a slight drying process, followed by staining with DAPI solution at ambient temperature. After washing and drying, the sections were visualized under a fluorescence microscope (E400, Nikon, Tokyo, Japan).
Oxidative stress markers detection
The malondialdehyde (MDA) levels, superoxide dismutase (SOD) activity and catalase levels in supernatants of homogenized hippocampal tissues were detected using corresponding kits (Beyotime, Shanghai, China).
TUNEL assay
For TUNEL assay, 5 paraffin sections of the hippocampal tissues were taken per rat in each group of 6 rats. Sections were subjected to dewaxing with xylene, followed by hydration with gradient ethyl alcohol. Subsequently, the sections were cleaned with proteinase K. Post washing with PBS, the sections were incubated with TUNEL detection solution (Beyotime, Shanghai, China) at 37℃ for 1 h. Next, the sections were stained with DAPI to visualize the nucleus, and a fluorescence microscope (E400, Nikon, Tokyo, Japan) was employed to observe apoptosis.
Statistical analysis
Experiments were repeated thrice, each time in triplicate. Statistical analysis was conducted utilizing GraphPad Prism 8.0 software, and the data were presented as means ± SD. The comparison of multiple groups was based on a one-way ANOVA test with a Tekey’s multiple-comparisons test. A P < 0.05 was considered significant.
Results
SESN2 attenuates sevoflurane-induced cognitive impairment of aged rats
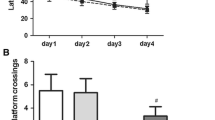
To determine the effect of SESN2 on sevoflurane-induced cognitive impairment, rats received bilateral injections of AAV-SESN2 or Vector into the CA1 region of the hippocampus of rats before sevoflurane treatment. Figure 1A showed the schematic diagram of the bilateral virus injections. The AAV-SESN2 successfully transduced the CA1 region as shown by the EGFP fluorescence (Fig. 1B). The results of western blot showed that SESN2 protein level in the rat hippocampus was increased in the Sev group compared with the Control group (0.130 ± 0.016 vs 0.03 ± 0.009, p < 0.001). Moreover, the Sev + SESN2 group had a higher protein level of SESN2 compared to the Sev + Vector group (0.341 ± 0.042 vs 0.111 ± 0.015, p < 0.001) (Fig. 1C). Subsequently, MWM test was performed to assess the spatial learning and memory abilities of rats. The results showed that rats in the Sev group exhibited a longer time for searching route compared with those in the Control group, whereas SESN2 overexpression greatly reversed this effect. Consistently, SESN2 overexpression abridged the escape latency time, increased the number of platform crossings (3.167 ± 0.983 vs 1.500 ± 0.548, p < 0.05), extended the time in target quadrant (36.730 ± 6.242 vs 20.000 ± 3.688, p < 0.001), and increased the percentage of the distance in target quadrant in aged rats induced by sevoflurane (43.600 ± 4.820 vs 25.160 ± 5.059 p < 0.001) (Fig. 1D). These findings indicated that SESN2 improved cognitive functions after sevoflurane exposure in aged rats.
SESN2 attenuated sevoflurane-induced cognitive impairment of aged rats. A Schematic diagram showing bilateral virus injection. B EGFP Fluorescence of EGFP in CA1 region showing the expression of AAV. C Western blot analysis determined SESN2 protein level in hippocampal tissues of rats. D Learning and memory abilities of rats detected by MWM test. ***p < 0.001, compared with the Control group; #p < 0.05, ###p < 0.001, compared with the Sev + Vector group
SESN2 suppresses sevoflurane-induced neuroinflammation of aged rats
ELISA and qRT-PCR were conducted to analyze the levels of IL-6, TNF-α and IL-1β in the hippocampus of rats in Control, Sev, Sev + Vector, and Sev + SESN2 groups. The results (Fig. 2A, B) showed an elevated levels of IL-6, TNF-α and IL-1β in the Sev group compared with those in the Control group (106.000 ± 17.630 vs 26.220 ± 3.826, p < 0.001; 125.100 ± 11.660 vs 51.670 ± 8.551, p < 0.001; 162.500 ± 22.500 vs 29.880 ± 5.666, p < 0.001; 4.113 ± 0.544 vs 1.056 ± 0.392, p < 0.001, 3.506 ± 0.421 vs 1.016 ± 0.201, p < 0.001; 5.384 ± 1.074 vs 1.030 ± 0.281, p < 0.001). However, those indexes were reduced in the Sev + SESN2 group compared to the Sev + Vector group (65.090 ± 8.791 vs 102.500 ± 7.167, p < 0.001; 80.150 ± 4.997 vs 118.400, p < 0.001; 96.940 ± 18.780 vs 160.800 ± 22.990, p < 0.001; 2.512 ± 0.606 vs 3.887 ± 0.471, p < 0.001; 2.043 ± 0.205 vs 3.166 ± 0.588, p < 0.001; 3.353 ± 0.684 vs 4.816 ± 0.629, p < 0.05). These data suggested that SESN2 mitigated the neuroinflammation of aged rats induced by sevoflurane.
SESN2 reduced sevoflurane-induced pro-inflammatory factors production. A ELISA was used to examine IL-6, TNF-α and IL-1β levels in hippocampal tissues of rats. B IL-6, TNF-α and IL-1β mRNA levels were determined by qRT-PCR. ***p < 0.001, compared with the Control group; #p < 0.05, ###p < 0.001, compared with the Sev + Vector group
SESN2 diminishes oxidative stress in the hippocampus of aged rats caused by sevoflurane
Next, the effects of SESN2 on sevoflurane-induced oxidative stress were evaluated. The results of DHE staining showed that ROS production was increased in the hippocampus in the Sev group compared with the Control group, while SESN2 overexpression reversed this effect (Fig. 3A). In addition, notably elevated MDA levels and reduced SOD and CAT levels were observed in the Sev group (19.780 ± 2.458 vs 10.780 ± 0.844, p < 0.001; 3.612 ± 0.722 vs 13.220 ± 2.687, p < 0.001; 6.222 ± 1.762 vs 16.550 ± 2.297, p < 0.001). Similarly, overexpression of SESN2 greatly reversed the changes of those enzymes caused by sevoflurane in the hippocampus (16.220 ± 2.588 vs 21.190 ± 2.762, p < 0.01; 8.190 ± 1.469 vs 3.302 ± 0.614, p < 0.001; 10.050 ± 1.755 vs 5.545 ± 1.446, p < 0.01) (Fig. 3B). These results indicated that SESN2 mitigated the sevoflurane-induced oxidative stress in the hippocampus of aged rats.
SESN2 alleviated oxidative stress in the hippocampus of aged rats induced by sevoflurane. A ROS production in hippocampus was assayed by DHE staining. B Levels of MDA, SOD and CAT in hippocampus were measured using kits. ***p < 0.001, compared with the Control group; ##p < 0.01, ###p < 0.001, compared with the Sev + Vector group
SESN2 reduces neuronal apoptosis induced by sevoflurane in aged rats
Further, to investigate the effect of SESN2 on hippocampal cell apoptosis after sevoflurane exposure, TUNEL staining and Bax/Bcl-2 immunoblotting were conducted. As shown in Fig. 4A, Sev group exhibited increased TUNEL+ staining cells compared with the Control group. However, Sev + SESN2 group presented a reduced TUNEL+ staining cells compared with the Sev + Vector group. Furthermore, rats exposed to sevoflurane showed a higher level of Bax protein and a lower level of Bcl-2 protein in the hippocampus in comparison with the Control group (0.555 ± 0.088 vs 0.120 ± 0.018, p < 0.001; 0.566 ± 0.111 vs 1.062 ± 0.061, p < 0.001), and rats overexpressing SESN2 showed a decreased Bax protein and an increased Bcl-2 protein in comparison with the Sev + Vector group (0.213 ± 0.055 vs 0.504 ± 0.060, p < 0.001; 0.812 ± 0.073 vs 0.498 ± 0.094, p < 0.001) (Fig. 4B). These data indicated that SESN2 inhibited sevoflurane-induced neuronal apoptosis in the hippocampus of aged rats.
SESN2 reduced neuronal apoptosis induced by sevoflurane in aged rats. A TUNEL staining was utilized to examine neuronal apoptosis in hippocampus. B Bax and Bcl-2 protein expression in hippocampus detected by western blot. ***p < 0.001, compared with the Control group; ###p < 0.001, compared with the Sev + Vector group
SESN2 inhibits the NLRP3 inflammasome activation
Prior studies showed that an excess of ROS can activate the NLRP3 inflammasome, resulting in heightened production of inflammatory cytokines and cell apoptosis. In this study, western blotting was performed to analyze the expression of NLRP3 inflammasome markers in the hippocampus of aged rats. The results showed a significant increase in the protein levels of key NLRP3 inflammasome components (NLRP3, ASC, IL-18, and IL-1β) in the Sev group compared to the Control group (0.741 ± 0.053 vs 0.050 ± 0.015, p < 0.001; 1.172 ± 0.127 vs 0.404 ± 0.085, p < 0.001; 0.963 ± 0.075 vs 0.461 ± 0.033, p < 0.001; 1.121 ± 0.168 vs 0.475 ± 0.111, p < 0.001). However, SESN2 overexpression led to a marked decrease in proteins of the NLRP3 components (0.111 ± 0.021 vs 0.684 ± 0.087, p < 0.001; 0.528 ± 0.091 vs 1.115 ± 0.119, p < 0.001; 0.631 ± 0.126 vs 0.954 ± 0.109, p < 0.001; 0.760 ± 0.080 vs 1.056 ± 0.169, p < 0.01) (Fig. 5A, B). These data suggested that SESN2 suppressed sevoflurane-induced activation of the NLRP3 inflammasome in the hippocampus of aged rats.
SESN2 inhibited the NLRP3 inflammasome activation. A Western blot detected the expression of NLRP3 protein in hippocampal tissues. B Western blot detected the expression of ASC, IL-18 and IL-1β proteins in hippocampal tissues. ***p < 0.001, compared with the Control group; ##p < 0.05, ###p < 0.001, compared with the Sev + Vector group
Discussion
In this study, the results indicated that exposure to sevoflurane significantly impaired the learning and memory abilities of aged rats. Interestingly, SESN2 has the potential to impede the sevoflurane-induced hippocampal inflammation, oxidative stress, and neuronal apoptosis in aged rats. Furthermore, SESN2 suppressed the NLRP3 inflammasome activation in the hippocampus after sevoflurane exposure.
Sevoflurane, an inhalation anesthetic, is widely used in surgery (Qiu et al. 2021). However, studies have demonstrated that the long-term use of sevoflurane could negatively impact the cognitive function of patients, especially aging adults (Kantonen et al. 2023; Kuzminskaite et al. 2023). Tang et al. reported that the neonatal mice exposed to 3% sevoflurane at 2 h/day for 3 days exhibited significant neurotoxicity (X. L. Tang et al. 2021). Zhao et al. found that the cell viability of the neurons in aging mice was reduced and cell apoptosis was increased after exposure to 2.5% or 5% sevoflurane for 4 h (Zhao et al. 2021). In the present study, the learning and memory were notably impaired in aged rats exposed to 2.6% sevoflurane for 4 h, which was consistent with the studies of Peng et al. (Peng et al. 2020).
SESN2 modulates diverse biological processes, ranging from cell metabolism, oxidative stress, inflammation, and cell apoptosis (Xu et al. 2023). The regulation role of SESN2 in brain damage has been illuminated by multiple studies (L. Li et al. 2016; X. Liu et al. 2021; Luo et al. 2020; L. L. Zhang and Zhang 2018). Herein, we firstly demonstrated that SESN2 was upregulated in hippocampus of aged rats exposed to sevoflurane. Importantly, overexpression of SESN2 significantly attenuated sevoflurane-induced cognitive impairment. Neuroinflammation has been suggested as a significant mechanism underlying sevoflurane-induced neurotoxicity (Y. Zhang et al. 2022). Moreover, oxidative stress and apoptosis are other mechanisms contributing to sevoflurane-induced neurotoxicity (Tian et al. 2018; Wang et al. 2021). In this study, our results further showed that the increased release of proinflammatory factors and the enhanced oxidative stress in hippocampus caused by sevoflurane were reversed by SESN2 overexpression, which also reduced neuronal apoptosis. These findings suggest that the protective effects of SESN2 against sevoflurane-induced neurotoxicity in aged rats may be related to anti-inflammation, anti-oxidative stress and anti-apoptosis.
Inflammatory cytokines can activate oxidative stress, and inflammation and oxidative stress showed robust synergism in promoting apoptosis (Y. Tang et al. 2022; Teleanu et al. 2022). Therefore, blocking the sevoflurane-induced inflammation reaction may be beneficial to nerve injury. The NLRP3 inflammasome is composed of NLRP3, ASC and caspase-1, and its activation has the effect of initiating and amplifying proinflammatory responses, rendering it a promising therapeutic target for inflammatory diseases (Coll et al. 2022; Kelley et al. 2019; Wu et al. 2020). Previous studies showed that SESN2 could suppress NLRP inflammasome activation, for example, SESN2 mitigated sepsis by inhibiting NLRP3 inflamasome activation (Kim et al. 2016); SESN2 conferred protection against cholestatic liver injury through the prevention of NLRP3 inflammasome-mediated pyroptosis (Han et al. 2022). The results in this study additionally validated the ability of SESN2 to effectively counteract the activation of NLRP3 inflammasome in the hippocampus of rats induced by sevoflurane. This may be one possible mechanism by which SESN2 exerts its neuroprotective role in the sevoflurane exposure model in rats.
In conclusion, our findings demonstrated that SESN2 alleviated the sevoflurane-induced cognitive impairment in aged rats. This effect may be achieved through the inactivation of the NLRP3 inflammasome, further reducing inflammation, oxidative stress, and neuronal apoptosis in the hippocampus.
Data availability
All data generated or analyzed during this study are included in this published article. The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
Coll RC, Schroder K, Pelegrín P (2022) NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci 43:653–668. https://doi.org/10.1016/j.tips.2022.04.003
Dong P, Zhao J, Li N, Lu L, Li L, Zhang X, Li D (2018) Sevoflurane exaggerates cognitive decline in a rat model of chronic intermittent hypoxia by aggravating microglia-mediated neuroinflammation via downregulation of PPAR-γ in the hippocampus. Behav Brain Res 347:325–331. https://doi.org/10.1016/j.bbr.2018.03.031
Han D, Kim H, Kim S, Le QA, Han SY, Bae J, Park HW (2022) Sestrin2 protects against cholestatic liver injury by inhibiting endoplasmic reticulum stress and NLRP3 inflammasome-mediated pyroptosis. Exp Mol Med 54:239–251. https://doi.org/10.1038/s12276-022-00737-9
Kantonen O, Laaksonen L, Alkire M, Scheinin A, Långsjö J, Kallionpää R, Scheinin H (2023) Decreased thalamic activity is a correlate for disconnectedness during anesthesia with propofol, dexmedetomidine and sevoflurane but not S-Ketamine. J Neurosci. https://doi.org/10.1523/jneurosci.2339-22.2023
Kelley N, Jeltema D, Duan Y, He Y (2019) The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. https://doi.org/10.3390/ijms20133328
Kim MJ, Bae SH, Ryu JC, Kwon Y, Oh JH, Kwon J, Yoon JH (2016) SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 12:1272–1291. https://doi.org/10.1080/15548627.2016.1183081
Kuzminskaite V, Kontrimaviciute E, Kauzonas E, Slauzgalvyte I, Bukelyte G, Bruzyte-Narkiene G, Jatuzis D (2023) Sevoflurane and desflurane effects on early cognitive function after low-risk surgery: a randomized clinical trial. Brain and Behavior 13:e3017. https://doi.org/10.1002/brb3.3017
Li L, Xiao L, Hou Y, He Q, Zhu J, Li Y, Zhao Y (2016) Sestrin2 silencing exacerbates cerebral ischemia/reperfusion injury by decreasing mitochondrial biogenesis through the AMPK/PGC-1α pathway in rats. Sci Rep 6:30272. https://doi.org/10.1038/srep30272
Li R, Huang Y, Semple I, Kim M, Zhang Z, Lee JH (2019) Cardioprotective roles of sestrin 1 and sestrin 2 against doxorubicin cardiotoxicity. Am J Physiol Heart Circ Physiol 317:H39-h48. https://doi.org/10.1152/ajpheart.00008.2019
Liu J, Li Y, Mei C, Ning X, Pang J, Gu L, Wu L (2020) Phytic acid exerts protective effects in cerebral ischemia-reperfusion injury by activating the anti-oxidative protein sestrin2. Biosci Biotechnol Biochem 84:1401–1408. https://doi.org/10.1080/09168451.2020.1754158
Liu X, Li M, Zhu J, Huang W, Song J (2021) Sestrin2 protects against traumatic brain injury by reinforcing the activation of Nrf2 signaling. Hum Exp Toxicol 40:1095–1111. https://doi.org/10.1177/0960327120984224
Luo L, Wu J, Qiao L, Lu G, Li J, Li D (2020) Sestrin 2 attenuates sepsis-associated encephalopathy through the promotion of autophagy in hippocampal neurons. J Cell Mol Med 24:6634–6643. https://doi.org/10.1111/jcmm.15313
Netto MB, de Oliveira JAN, Goldim M, Mathias K, Fileti ME, da Rosa N, Petronilho F (2018) Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun 73:661–669. https://doi.org/10.1016/j.bbi.2018.07.016
Peng S, Li P, Liu P, Yan H, Wang J, Lu W, Zhou Y (2020) Cistanches alleviates sevoflurane-induced cognitive dysfunction by regulating PPAR-γ-dependent antioxidant and anti-inflammatory in rats. J Cell Mol Med 24:1345–1359. https://doi.org/10.1111/jcmm.14807
Qiu J, Zhang Y, Xie M (2021) Chrysotoxine attenuates sevoflurane-induced neurotoxicity in vitro via regulating PI3K/AKT/GSK pathway. Signa Vitae 17:185–191. https://doi.org/10.22514/sv.2021.107
Rump K, Adamzik M (2022) Epigenetic mechanisms of postoperative cognitive impairment induced by anesthesia and neuroinflammation. Cells. https://doi.org/10.3390/cells11192954
Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, Gray L (2018) Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev 84:116–133. https://doi.org/10.1016/j.neubiorev.2017.11.011
Tang XL, Wang X, Fang G, Zhao YL, Yan J, Zhou Z, Li SY (2021) Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-κB pathway in neonatal mice. J Nutr Biochem 90:108579. https://doi.org/10.1016/j.jnutbio.2020.108579
Tang Y, Zhou X, Cao T, Chen E, Li Y, Lei W, Liu S (2022) Endoplasmic reticulum stress and oxidative stress in inflammatory diseases. DNA Cell Biol 41:924–934. https://doi.org/10.1089/dna.2022.0353
Teleanu DM, Niculescu AG, Lungu II, Radu CI, Vladâcenco O, Roza E, Teleanu RI (2022) An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. https://doi.org/10.3390/ijms23115938
Tian Y, Chen KY, Liu LD, Dong YX, Zhao P, Guo SB (2018) Sevoflurane exacerbates cognitive impairment induced by Aβ (1–40) in rats through initiating neurotoxicity, neuroinflammation, and neuronal apoptosis in rat hippocampus. Mediators Inflamm 2018:3802324. https://doi.org/10.1155/2018/3802324
Wang CM, Chen WC, Zhang Y, Lin S, He HF (2021) Update on the mechanism and treatment of sevoflurane-induced postoperative cognitive dysfunction. Front Aging Neurosci 13:702231. https://doi.org/10.3389/fnagi.2021.702231
Wu D, Chen Y, Sun Y, Gao Q, Li H, Yang Z, Yu B (2020) Target of MCC950 in inhibition of NLRP3 inflammasome activation: a literature review. Inflammation 43:17–23. https://doi.org/10.1007/s10753-019-01098-8
Xu L, Liu Z, Wang H, Lu J, Xu J, Meng Y, Liu B (2023) SESN2 could be a potential marker for diagnosis and prognosis in glioma. Genes (basel). https://doi.org/10.3390/genes14030701
Yi W, Zhang Y, Guo Y, Li D, Li X (2015) Elevation of Sestrin-2 expression attenuates Sevoflurane induced neurotoxicity. Metab Brain Dis 30:1161–1166. https://doi.org/10.1007/s11011-015-9673-1
Yu XW, Curlik DM, Oh MM, Yin JC, Disterhoft JF (2017) CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. Elife. https://doi.org/10.7554/eLife.19358
Zhang LL, Zhang ZJ (2018) Sestrin2 aggravates oxidative stress of neurons by decreasing the expression of Nrf2. Eur Rev Med Pharmacol Sci 22:3493–3501. https://doi.org/10.26355/eurrev_201806_15176
Zhang Y, Gao Y, Yang F, Wu X, Tang Z, Liu H (2022) Neuroglobin alleviates the neurotoxicity of sevoflurane to fetal rats by inhibiting neuroinflammation and affecting microglial polarization. Brain Res Bull 183:142–152. https://doi.org/10.1016/j.brainresbull.2022.03.006
Zhao L, Gong H, Huang H, Tuerhong G, Xia H (2021) Participation of mind bomb-2 in Sevoflurane anesthesia induces cognitive impairment in aged mice via modulating ferroptosis. ACS Chem Neurosci 12:2399–2408. https://doi.org/10.1021/acschemneuro.1c00131
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LS designed the study, completed the experiment and supervised the data collection, YL analyzed the data, interpreted the data, DW and XH prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
Ethics approval
Ethical approval was obtained from the Ethics Committee of the Affiliated Huai'an No. 1 People's Hospital of Nanjing Medical University.
Additional information
Communicated by Sreedharan Sajikumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, L., Li, Y., Wang, D. et al. SESN2 attenuates sevoflurane-induced cognitive impairment and neuroinflammation in rats. Exp Brain Res 242, 375–384 (2024). https://doi.org/10.1007/s00221-023-06757-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06757-9