Abstract
Human locomotion may result from monotonic shifts in the referent position, R, of the body in the environment. R is also the spatial threshold at which muscles can be quiescent but are activated depending on the deflection of the current body configuration Q from R. Shifts in R are presumably accomplished with the participation of proprioceptive and visual feedback and responsible for transferring stable body balance (equilibrium) from one place in the environment to another, resulting in rhythmic activity of multiple muscles by a central pattern generator (CPG). We tested predictions of this two-level control scheme. In particular, in response to a transient block of vision during locomotion, the system can temporarily slow shifts in R. As a result, the phase of rhythmical movements of all four limbs will be changed for some time, even though the rhythm and other characteristics of locomotion will be fully restored after perturbation, a phenomenon called long-lasting phase resetting. Another prediction of the control scheme is that the activity of multiple muscles of each leg can be minimized reciprocally at specific phases of the gait cycle both in the presence and absence of vision. Speed of locomotion is related to the rate of shifts in the referent body position in the environment. Results confirmed that human locomotion is likely guided by feedforward shifts in the referent body location, with subsequent changes in the activity of multiple muscles by the CPG. Neural structures responsible for shifts in the referent body configuration causing locomotion are suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From posture to a single step and to continuous locomotion
In a classic study, Zatsiorsky and Duarte (2000) found that during quiet standing, the nervous system actively shifts body balance [i.e., the equilibrium point (EP) of the body] from one place to another relative to the ground. Although the time spent by the body at each EP is relatively small (“transient EPs”), it is sufficient to prevent falling. This behavior is further ensured by posture-stabilizing position- and velocity-dependent reactions to deviations from each current EP due to intrinsic muscle properties called pre-flexes (Loeb 1995) and proprioceptive, visual, and vestibular reflexes. Reflex influences on motoneurons (MNs) are transmitted with delays. However, due to their contractile mechanisms, the intrinsic muscle properties directly influence muscle forces practically without delays, which is sufficient to overcome the destabilizing effects of reflex delays (Pilon and Feldman 2006). Thus, although transient, the EPs during quiet standing are stable. In terms of the dynamic systems theory (e.g., Andronov and Khajkin 1949), stable EPs are attractors, implying that in response to a shift in a stable EP, the system automatically generates muscle forces and torques tending to bring the body to the shifted EP. This principle underlies the EP hypothesis (Feldman 1986), now advanced to the theory of referent control of motor actions (Feldman 2015). In this theory, all possible body postures, Q, are considered as comprising a spatial frame of reference (FR) or system of coordinates. The origin or referent point of this FR is a specific body posture called the referent body configuration, R. It is also the threshold body posture at which muscles of the body can be quiescent but are activated depending on the deflection of the current body configuration Q from R. Thus, motor actions result from shifts in R, causing agonist muscle activation and a decrease in the activity of antagonist muscles (reciprocal pattern). In the referent control framework, the R position is surrounded by a range of actual body configurations, Q, called the C zone in which agonist–antagonist muscles can be coactive. The C zone is shifted together with the R position. In this way, muscle coactivation contributes to acceleration of the body to the targeted position instead of resisting motion from the initial position (see Zhang et al. 2022). These notions are helpful in the explanation of not only how body postures are maintained during standing (Zatsiorsky and Duarte 2000), but also how single steps or continuous locomotion are produced. Specifically, during standing, starting from any intermediate referent position, Ri, the nervous system can shift the body EP by changing R, such that the vertical projection of the center of the body mass (COM) will appear outside the initial base of support (BOS), an area between the feet on the ground. According to the conventional view, when the COM is outside the BOS, the body begins to fall and then somehow catches itself (MacKinnon and Winter 1993; Winter 2009; Day and Bancroft 2018). However, this view has been challenged (Feldman et al. 2011, 2021): the body will be attracted to a new stable EP located outside the BOS, and a step will be produced bringing the body to a new stable posture in the environment. By prolonging shifts in R, the system can produce continuous walking.
Two-level structure and indirect control of locomotion
Traditionally, rhythmic muscle activation is usually considered as being produced directly by central pattern generators (CPGs) of motor actions. In contrast, in the referent control framework, rhythmic muscle activation during locomotion is a secondary, emergent effect of a monotonic shift in stable body balance in the environment (Feldman et al. 2011, 2021). There is an important physical principle of how equilibrium states can be set and changed in dynamical systems. A clarifying example: in isotonic conditions, muscle and external forces are balanced at each elbow angle, but the choice of a specific joint angle at which forces are balanced is defined not by muscle forces or other kinematic and kinetic variables and EMG patterns describing the motor outcome, but independently of them, by parameters of physical and physiological laws.
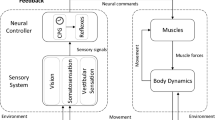
This principle can also be applied to locomotion. It was identified in thermodynamics of dynamical systems (Glansdorff and Prigogine 1971). The applicability of this principle to human motor control was demonstrated empirically, more than half a century ago, when parameter λ, the spatial threshold at which proprioceptive reflexes become functional in activating muscles, was identified (Asatryan and Feldman 1965). Thus voluntary actions result from changes in λ independently of variables describing the motor outcome, whereas in involuntary motor actions such as the unloading reflex, λ remains invariant (Ilmane et al. 2013). The notion of parametric, threshold control was generalized to multi-muscle systems by suggesting that, rather than defined directly by CPGs, multiple muscle activation is produced indirectly, due to the deflection of the emergent, actual body configuration from the threshold, referent body configuration. If necessary, the latter is modified until the emergent action meets the task demands. Aside from rare exceptions (e.g., Günther and Ruder 2003), this principle has been poorly understood and has not been considered in most studies of human or animal locomotion. Based on the notion of parametric, referent control, it has been suggested that a two-level neural structure underlies unperturbed human locomotion. At the first level, central and peripheral neural processes produce feedforward shifts in the referent body configuration, thus transferring stable body equilibrium from one place in the environment to another at a specified rate, r (Fig. 1). At the second, subordinated level, the CPG elicits rhythmical activity of multiple muscles, forcing the body to follow the shifts in equilibrium. There are several important consequences of this control scheme.

Reproduced with permission from Feldman et al. (2021)
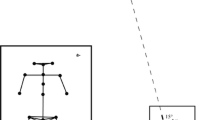
The production of a single step, continuous walking, and minimization of EMG activity of multiple muscles of the legs at specific phases of the walking cycle, in the context of referent control of motor actions. At body position Qi, during quiet standing, the ankles are in the neutral position (90°) with the feet flat on the ground. In contrast, in the centrally specified referent configuration Ri, the ankles are in virtual plantarflexion, such that the feet virtually penetrate the ground (red outline). The solid ground prevents the feet from reaching their referent, threshold positions at which they can be deactivated. Due to the difference between Qi and Ri, antigravity muscles (gastrocnemius, soleus, and peroneus longus) are activated and produce forces that compensate for the ground reaction forces. Before the onset of swing of the right leg, the system changes the referent body configuration to transfer body weight to the left leg. The swing phase of the right leg starts from changes in the referent ankle position from plantarflexion to dorsiflexion with subsequent ankle and hip flexion. Referent and actual toe trajectories are qualitatively shown with dashed blue and solid black thick curves, respectively. Green dots show the step phases at which referent and actual body configurations transiently match each other, resulting in the minimization of EMG activity of multiple leg muscles. This occurs during the transition from the stance to the swing phase, as symbolically shown by the left green dot. The right green dot shows that secondary EMG minimization can also occur during swing. The referent leg configuration not only changes in time but is also shifted spatially in the direction of locomotion (“place resetting”). Gait speed depends on parameter r, the rate of the translation of the referent body configuration in the environment, thus shifting body balance and stability. Thus, in this scheme, the primary cause of locomotion is a non-rhythmic but monotonic shift of body equilibrium in the environment. Rhythmic activity of multiple body muscles by the central pattern generator is the secondary, emerging effect of the shifts in body balance.
There are several important consequences of this control scheme. First, the referent control strategy does not require an internal representation and computation of COM motion based on hypothetical internal models. Moreover, it has been shown that computational control schemes of direct preprograming of motor actions are incompatible with the well-established nonlinear properties of MNs. In particular, motoneuronal input/output functions are irreversible (Feldman and Zhang 2020) and, based on computations, the CNS cannot invert these functions to deliver input signals to MNs and elicit the required output in terms of EMG patterns or muscle forces, even under isometric conditions: internal inverse models do not exist in such conditions (Feldman et al. 2021; Zhang et al. 2022). Second, while shifting body balance, the system, respectively, relocates the BOS (“place resetting”). As a result, the COM leaves the initial BOS and the body is propelled toward the new BOS at a certain speed. Normally, this speed is sufficient for the body to move from the initial BOS and reach the new area of body balance at the shifted BOS without falling. In the area outside of the BOS, called the dynamic stability area, falling is prevented, since the COM steadily moves toward the new BOS that plays the role of an attractor to which the body is forced to move (Feldman et al. 2011). Third, depending on the motor task, the R concept can be applied to multiple muscles of the entire body or its parts (e.g., arms or legs), with respective changes in the term—the referent arm or leg configuration. For example, in Fig. 1, we consider the referent leg-foot configuration to explain how ground reaction forces and single steps are produced. Fourth, humans spontaneously switch from walking to running as locomotor speed increases (Kubo et al. 2004), a phenomenon called phase transition. In the referent control framework, the speed of locomotion is controlled indirectly by influencing parameter r, the rate of shift in R. Therefore, in the referent control framework, the transition from walking to running and to jumping forward occurs by increasing the rate r, of the shift in body equilibrium.
Vision in the context of referent control of locomotion
In this study, we focused on the role of vision in the control of walking. Indeed, the role of vision in human locomotion has been analyzed in many studies, in particular by Patla (2011) who investigated stepping over different obstacles during walking. A substantial role of vision was also emphasized by Gibson (1966) in his ecological approach to locomotion (see also Turvey 2019; Shoja et al. 2020). The role of vision in locomotion has not been studied in the context of referent control of motor actions and, in the present study, we address this issue by formulating and testing some consequences (predictions) of the referent control framework for walking in stationary or perturbed visual conditions. The role of vision is substantially different during walking on a treadmill or overground (Feldman et al. 2021). Consider walking on a treadmill without a railing (as was the case in our study; Fig. 2). In this case, subjects should perceive that the environment or the room in which the treadmill is located remains motionless during walking, thus providing visual constancy and minimizing changes in the body position on the treadmill to prevent sliding off it, which usually occurs as soon as vision is blocked. In contrast, during overground walking, subjects need to perceive their own motion in the changing environment (Gibson 1966). In both cases, visual perception should be modulated depending on the walking speed. In the context of parametric, referent control, speed of walking can only be controlled indirectly (see above), by changing parameter r that defines the rate (speed) of shift in the R in the environment (Fig. 1). Thus, in both cases, the r should be continuously adjusted to ensure the required visual perception. In other words, vision can be involved in the control of locomotion at the primary level of the two-level structure of the referent control of locomotion. Visual perturbation can affect the rate, r, of shift in R and body balance, resulting in a phase resetting. In this study, the phase φ of a rhythmic process with cycle duration T is defined, in radians, as φ = ωt + φ0 where ω = 2π/T, is natural or circular frequency, t, time, and φ0 is the initial phase. After visual perturbation, the phase can be restored to its pre-perturbation value (transient phase resetting) or it will persist as long-lasting phase resetting, even though the rhythm and other characteristics of locomotion will be fully restored after perturbation. We tested the hypothesis (Hypothesis 1) that long-lasting phase resetting involving all four limbs can occur in response to visual perturbation. The phenomena of long-lasting phase resetting has been previously observed in response to a mechanical perturbation of gait (Feldman et al. 2011). In the present study, we tested whether a similar, although quantitatively different effect can be elicited by changes in parameter r in response to visual perturbations of locomotion.
Experimental setup. Motion of the body on a treadmill was recorded using an optometric system (Optotrak) with active markers (LEDs). EMG activity of 16 muscles was recorded using a telemetric system (Noraxon) with active electrodes. Vision was blocked by an electric pulse making the liquid crystal glasses worn by subject opaque
We formulated other hypotheses according to additional aspects of referent control of standing and walking. We addressed the question, not sufficiently explained in biomechanical textbooks (e.g., Winter 2009) of how ground reaction forces are produced and compensated by antigravitational muscles during standing (Fig. 1) and we predict that there will be a minimization of multi-muscle EMG activity at specific phases of the gait cycle, reciprocally for muscles of each leg (Hypothesis 2).
To clarify how ground reaction forces are produced, consider quiet standing when the body weight is transferred to the right leg and the right ankle is in the neutral position (90°), with the feet flat on the ground—the initial actual leg configuration, Q. At the same time, in the referent configuration R, the ankles are in virtual plantar flexion, such that the feet virtually penetrate the ground (Fig. 1, red outline). The solid ground prevents the feet from reaching the virtual position, and due to the difference between Q and R, antigravity muscles (gastrocnemius, soleus, and peroneus longus) are activated and produce forces that compensate for the ground reaction forces. To initiate a step with the right leg, the referent body configuration changes to transfer the body weight to the left leg and moves the referent posture of the right leg into the swing phase. This transition from stance to swing starts from changes in the referent ankle position from plantarflexion to dorsiflexion with subsequent ankle and hip flexion. In Fig. 1, referent and actual toe trajectories are qualitatively shown with dashed blue and solid black thick curves, respectively. The referent trajectory is formed in advance of the actual position during the swing phase. During the transition from stance to swing, the actual and referent positions may transiently match each other (Q≈R) as symbolically shown by the left green dot in Fig. 1, bringing EMG activity of numerous muscles of the right leg to a minimum. The depth of minimization can be limited by a coactivation (C) command responsible for coactivation of agonist and antagonist muscles (Feldman and Zhang 2020). After the first transient EMG minimization (Fig. 1), the referent and actual toe trajectories diverge, such that the referent toe trajectory overtakes the actual toe trajectory. At about mid-swing, the referent toe trajectory is redirected toward the ground to cause a transition of the right leg to the next stance phase, thus completing the gait cycle. Following the redirection of the referent trajectory, a second minimum may occur when the referent and actual trajectories intersect once more (Fig. 1, right green dot). This qualitative analysis shows that EMG minima in the activity of leg muscles can occur twice in each gait cycle for each leg. Also note that according to this scheme, with each gait cycle, the referent body configuration associated with EMG minimization is spatially translated (ΔR) in the direction of walking (Fig. 1), implying a shift in balance and stability in the environment. A reciprocal inhibitory interaction between MNs of left and right legs is likely responsible for the rhythmical transfer of body weight from one leg to another. Minimization of multi-muscle EMG activity of each leg has been observed in previous studies (St-Onge and Feldman 2004; Ivanenko et al. 2006; Feldman et al. 2011), for both forward and backward overground walking (Feldman et al. 2011). One can assume that a similar EMG minimization can also be observed during gait when vision is available or blocked to prevent falling (Hypothesis 2).
Finally, according to Fig. 1, the rate, r, of gait speed depends on the rate of shifts in the referent body configuration in the environment starting from any intermediate referent configuration (Ri) during standing:
Thus, we tested Hypothesis 3 that the rate (speed) of shifts in the referent body configuration and body balance in the environment would resemble the speed of walking, both in the presence of vision or when vision is occluded.
Materials and methods
Subjects
Ten healthy adults (6 females, 4 males, aged 31.5 ± 3.8 years), participated in the study. Each subject signed a consent form approved by the ethics committee of the Centre for Interdisciplinary Research in Rehabilitation (CRIR). All subjects had normal or corrected to normal vision (20/40 or better) and had no cardiovascular, respiratory, neurological, or musculoskeletal problems assessed by a general medical history questionnaire.
Experimental procedure
Subjects wore a safety harness and walked on a motorized treadmill without handrails (Fig. 2, Bertec Corp., USA) at a comfortable speed (1.09 ± 0.18 m/s). In preliminary experiments, it was found that subjects could deviate to the edges of the treadmill. Therefore, all subjects were instructed to walk in the sagittal direction coinciding with the direction of the treadmill, such that deviations to its edges during experimental sessions were excluded in all subjects. During walking, subjects wore liquid crystal glasses (Translucent Co, Toronto) that became opaque or fully transparent at the onset of the toe-off phase of the right leg. After at least ten cycles of walking with vision (pre-perturbation), vision was blocked as subjects continued walking (during-perturbation). Then, vision was restored as the subjects continued walking (post-perturbation). Eight gait cycles in each visual condition (pre-, during-, and post-visual perturbation) were analyzed for each subject.
Data recording
Infrared light-emitting diodes (LEDs) were placed on the arms, legs, trunk, and head (Fig. 2) to record kinematic data using a three-dimensional motion analysis system (2 camera-bar Optotrak Certus, Northern Digital Inc, Canada) at a sampling rate of 60 Hz. Three rigid bodies were placed on each arm and leg segment and joint centers were probed with a digitizing probe to determine the respective joint centers. These data were put in a 3D chain model together with individual anthropometric data (body weight, height, lengths, and circumference of body segments). The segmental and global positions of the COM were computed, as described by Winter (2009) and Dubreucq et al. (2017). Rigid bodies were also placed on the lateral midpoint of the foot as well as individual markers on the heel and lateral 5th metatarsal bone.
EMG activity was recorded bilaterally from 8 muscles of each leg (16 muscles in total): tibialis anterior (TA), medial gastrocnemius (MG), vastus medialis (VM), rectus femoris (RF), biceps femoris (BF), tensor fascia latae (TFL), gluteus maximus (GM), and erector spinae (ES) (Fig. 2). Prior to electrode placement, the skin was shaved and rubbed with alcohol at the electrode sites. Bipolar surface electrodes (Ag/AgCl¸ BlueSensor M) were placed 2 cm apart on the muscle bellies. EMG signals were sampled at 1200 Hz, pre-amplified (500x) using a 16-channel surface EMG Wireless System (Noraxon, USA). Two force plates under the treadmill belts were used to record vertical ground reaction forces (GRFs; sampling rate 600 Hz down-sampled to 60 Hz to align with kinematic data) and to determine the times of heel contact (HC) and toe-off (TO).
Data analysis
Force and kinematic data were filtered (4th-order Butterworth zero-lag at cut-off frequency of 10 and 6 Hz, respectively). Gait cycles were determined as the intervals when vertical ground reaction forces (GRFs) crossed a 10 N threshold.
Testing hypothesis 1
Markers on the heel and distal part of second metacarpal bone were used to determine sagittal displacements of legs and arms before, during and after perturbation (Feldman et al. 2011; Krasovsky et al. 2012, 2013, 2014).
Since the frequencies of arm and leg swinging could be different, Hilbert transformations were used to determine the phases of arm and leg oscillations (Costa et al. 2006; Schelter et al. 2006; Baghdadi and Nasrabadi 2012). First, we determined the average phase shifts for all the main frequency components of arm and leg motion. Then, the eight pre-perturbation cycles were shifted (“projected”) forward in time, so that they were aligned with the post-perturbation cycles. Phase shifts, \(\Delta \varphi ,\) were then computed by determining the time difference between the peaks of the background and the projected oscillations. This was done for projected and during-perturbation cycles as well as projected and post-perturbation cycles, as follows:
where \({\varphi }^{\left(i\right)}(t)\) is the phase of time series i and mod2 \(\pi\) means comparison of signals over period 2 \(\pi .\)
A negative phase shift meant a phase advance: a positive shift meant a phase delay. We compared the mean phase resetting between the first 4 and second 4 post-perturbation cycles to identify long-lasting phase resetting, for all four limbs (Feldman et al. 2011). In addition, to compare the phase shifts of all 4 limbs, we normalized the values of the phase shifts for legs and arms by considering their minimal and maximal sagittal displacements (\({x}_{i})\): \(\frac{{x}_{i}-\mathrm{min}(x)}{\mathrm{max}(x)-\mathrm{min}(x)}\).
Subjects were instructed to walk about 10–11 cycles and an equal number of 8 walking cycles were chosen to analyze transient responses in pre-, during-, and post-perturbation cycles in each subject. Relative speed was determined as the time interval from heel contact (HC) to the next HC divided by the interval duration and the treadmill speed.
Cycle duration (s), stance, and swing time (s) were defined as the time intervals from HC to ipsilateral HC, HC to ipsilateral toe-off (TO) and from TO to ipsilateral HC. Swing length (cm) was calculated as the anteroposterior distance of heel positions from TO to ipsilateral HC. Step length and width (cm) was measured as anteroposterior and mediolateral distances, respectively, between right and left heel positions during successive foot contacts. In addition, to determine if the gait characteristics were restored, the average durations of the first and the second four post-perturbation cycles were compared.
Testing hypothesis 2
The raw EMG signals were initially filtered to remove very low and high frequencies and background noise. Then, the rectified EMG values of each leg muscle were normalized to 1, i.e., to maximal EMG burst amplitude in each trial. Onset time and frequency of occurrence of EMG minima were determined by computing the max EMG curve (St-Onge and Feldman 2004; Feldman et al. 2011; Chan-Viquez et al. 2020):
where \({a}_{i}\left(t\right)(i=1, \dots , m)\) denotes the rectified and normalized EMG value at time t; m is the total number of muscles (m = 8). Note that, for each muscle: \(0\le \mathrm{max}EMG\le 1\). EMG minima were identified as the times at which the values of the max EMG did not exceed 15% of the maximal EMG amplitude. Criteria for identification of the depth of EMG minima are different in different studies. In the study of jeté movements of ballet dancers (Lepelley et al. 2006) and in the present study, a 15% threshold was used. However, a threshold of 5% was used in a study of overground walking (St-Onge and Feldman 2004) and a 10% threshold was used in determining EMG minima during head movements in monkeys (Lestienne et al. 2000). The threshold in our study (15%) was based on the level of coactivation of agonist and antagonist muscles during walking when vision was blocked.
Since EMG signals consist of single motor unit spikes, there was a probability of short-duration EMG minima between spikes. To avoid the possibility of overestimating the number of minima, only EMG minima with a duration of > 50 ms were considered. The number of EMG minima per cycle and each condition (pre-, during-, and post-perturbation cycles) was determined (Mullick et al. 2018). To study the distribution of minima within the gait cycle in different perturbation conditions, each gait cycle was divided into three sub-phases: 0–29%, 30–59%, and 60–90%.
Testing hypothesis 3
To test if the rate of shift in the referent body configuration resembled the walking speed, the two values were compared in pre-, during-, and post-perturbation phases. The rate r of shift in the referent body configuration (Fig. 1) was calculated as the speed of the shift in the position of EMG minima and the walking speed was taken as the treadmill speed (Hypothesis 3).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.1. Data distributions were evaluated for normality by Shapiro–Wilk tests. We used a one-way repeated-measure ANOVA followed by the uncorrected Fisher’s LSD test for transient and permanent (phase resetting) responses for each limb separately. To test the global phase shift, we used a one-way repeated-measure ANOVA (with limb as the within factor). Paired t tests were used to compare the first and second four post-perturbation cycles for both transient and long-lasting (persistent) responses (Hypothesis 1). The distribution of EMG minima was tested with Friedman tests followed by the uncorrected Dunn’s tests (Hypothesis 2).
To evaluate the similarity of the rates of the referent body shift and the treadmill speed at different gait phases (Hypothesis 3), a paired equivalence test for median differences were performed (Rogers et al. 1993). Equivalence was defined as the difference between the median values that remained within the bounds of ± 50% magnitude of the maximal 95% confidence interval of the two values being compared. The null hypothesis is that the minimal difference between two sets of data stays outside these bounds, meaning that the sets are not equivalent (Rogers et al. 1993). The equivalency of the median values of two variables is confirmed if p < 0.05.
Results
Phase resetting in response blocked vision (testing hypothesis 1)
The effects of the perturbation on the arm and leg motion are shown in Fig. 3 for a representative subject. There was a significant effect of pre-, projected, and during-perturbation compared to projected and post-perturbation) on the phase shift values (right leg: F(2, 18) = 10.41, p = 0.001; left leg: F(2, 18) = 10.16, p = 0.001; right arm: F(2, 18) = 8.39, p = 0.003; left arm: F(2, 18) = 13.51, p = 0.000). For all four limbs, there were significant differences between pre- and projected-during-perturbation (p < 0.001) and pre- and projected-post-perturbation (p < 0.05) phase shifts. However, there were no differences in phase shifts between projected-during and projected-post-perturbation phases (p > 0.05). In addition, there were no differences between phase shifts of the first and second four post-perturbation cycles for all four limbs (p > 0.05), i.e., there was long-lasting (persistent) phase resetting, which was maintained for as least three cycles after perturbation.
There was no limb effect on the long-lasting phase shift (F(3, 27) = 1.685, p = 0.196, η2 = 0.157). In other words, the gait pattern was equally shifted in time for all four limbs, i.e., the effect of visual perturbation was global.
Figure 4A–C shows changes in kinematic variables in pre-, during-, and post-perturbation cycles. Body configurations are shown by stick diagrams in Fig. 4A. Figure 4B and C show the COM and heel positions, respectively. EMG MAX curves from 8 muscles of one leg are illustrated in Fig. 4D and E. The vertical lines indicated the points at which EMG minima occurred for two consecutive gait cycles.
EMG minima and respective body configurations during two consecutive gait cycles. Vertical red lines show the phases of gait at which EMG activity of eight muscles was minimized. A Body configuration, B COM trajectory, C Heel trajectory, D EMGMax curve, and E EMG activity from 8 muscles. ES erector spinae, GM gluteus maximus; BF biceps femoris; RF rectus femoris; TFL tensor fascia lata; VM vastus medialis; MG medial gastrocnemius; TA tibialis anterior
Most of the spatio-temporal gait characteristics transiently changed in response to visual perturbation and their values were restored three or four cycles after the perturbation. There was no change in relative gait speed in all perturbation conditions. There were no significant differences between pre-, during-, and post-perturbation cycle durations, p > 0.05). Also, there were no significant differences between the first and second post-perturbation cycles for most variables. Stance time and length were restored later than other kinematic variables. Their values remained elevated during the second four post-perturbation cycles. Compared to mechanical perturbations (Feldman et al. 2011), the effects of visual perturbation on gait were sluggish and gradual, becoming apparent only after three-to-four cycles (Fig. 4).
Presence and location of EMG minima (hypothesis 2)
The number of EMG minima was significantly different between pre-, during-, and post-perturbation cycles (χ2 = 15.8, p = 0.000, Fig. 5). The number of EMG minima decreased in response to perturbation and was then restored. The number was also smaller during-perturbation compared to pre-perturbation (p < 0.001) and post-perturbation (p = 0.003), while there were no differences between pre- and post-perturbation cycles (p > 0.05).
The distribution of minima across the gait cycle differed in pre-, during-, and post-perturbation cycles (Fig. 6). In the first bin (0–29%), the number of EMG minima decreased during-perturbation compared to pre-perturbation cycles. However, there was no difference between the number of EMG minima in post-perturbation cycles compared to pre-perturbation cycles, suggesting that the number of EMG minima was restored after perturbation. In both the second (30–59%) and the third (60–90%) bins, the number of EMG minima was similar in the pre- and post-perturbation conditions, while this number decreased during-perturbation. These observations are consistent with Fig. 1, showing that EMG minimization during transition from the stance to the swing phase could be more distinctive (sharp) than during the transition from the swing to the stance phase of each leg, that could be initiated at various points and spread across the swing phase.
Testing hypothesis 3
The rates of shifts in referent body configuration and the walking/treadmill speed were similar in all walking phases (paired equivalence test: pre: p = 0.005; during: p = 0.023; post: p = 0.003).
Discussion
Basic findings
In response to visual occlusion, there was long-lasting phase resetting, thus supporting Hypothesis 1. We also observed minimisation of multi-muscle activity of both legs at two specific phases of motion before and after visual occlusion, thus supporting Hypothesis 2. Finally, we found that the speed of shifts in the referent body configuration at which EMG minimisations occurred were similar to changes in the walking speed (Hypothesis 3), implying that shifts in the referent body configuration may underlie the transfer of body balance and stability in the environment as a primary cause of locomotion with subsequent rhythmic generation of multi-muscle activity by the CPG. These hypotheses have previously been supported using mechanical perturbations during the swing phase of the gait (Feldman et al. 2011) and the present study shows that visual perturbation can elicit similar effects. The effects of mechanical perturbation on gait were comparatively rapid and were compensated within one gait cycle (Feldman et al. 2011). In contrast, the effects of visual perturbation on gait were sluggish and gradual, becoming apparent only after three-to-four cycles. This can be explained by the much shorter latency of proprioceptive feedback from the ankle (i.e., 65–75 ms, Dietz et al. 1991) compared to visual feedback (i.e., 1.2–2.2 s, Lestienne et al. 1977) acting on lower limb responses.
It is possible that the sudden perturbation of gait employed in the study of Feldman et al. (2011) was more effective in challenging gait and balance stability, requiring a more rapid response to prevent falling compared to the visual perturbation. Although subjects can walk for some time in the absence of vision, the role of vision is different during walking on a treadmill compared to overground walking (Feldman et al. 2021). In the first case, it is necessary to stabilize the retinal image of the environment, thus providing visual constancy to minimize changes in the body position on the treadmill that usually occur as soon as vision is blocked. In contrast, during overground walking, subjects need to perceive their own motion in the environment (Gibson 1966). The ability to control walking speed in response to mechanical and visual perturbations is essential in both cases. Thereby, response strategies to perturbation of gait can vary: subjects may change the referent body configuration, R, and, as a consequence, the actual body configuration Q, thus preventing a fall without a change in the rate of shift in the R. Another response would be to slow down the rate of shifts in R, thus accelerating the transition of the leg from stance to swing. Both strategies were observed in response to mechanical perturbations. The observation of long-lasting and global phase resetting manifests a response to perturbation at neural levels that regulate gait speed (Feldman et al. 2011). Arm displacements presented on a phase (velocity versus position) plane (Fig. 3B and D) also show that responses to visual perturbation can vary from trial to trial.
Taken together, our results are consistent with the suggestion that the control of human bipedal locomotion involves two neural levels as described in the introduction. One level is responsible for producing a non-rhythmical process of the progressive translation of stable body balance in the environment. This process may have resulted from shifts in the referent body configuration in the environment (Fig. 1) The second level, subordinate to the first, converts this monotonic process into rhythmic activation of multiple muscles of the body by the CPG. In other words, the primary, “motive force” underlying human locomotion is the monotonic translation of stable body balance in the environment, whereas rhythmic muscle contractions of body muscles (CPG) emerge due to this translation combined with the interaction of the legs with the ground.
Indeed, the suggested two-level scheme for the control of locomotion is an alternative to the conventional view that practically all aspects of locomotion are accomplished by central and reflex regulation of basic characteristics of the CPG, including its frequency (e.g., Grillner 1975). In contrast, our data suggest that shifts in the referent body configuration (R) are a primary, global control factor responsible for balance and stability of the entire body, without the need to focus on motion of individual limbs or activity of each muscle.
Chan-Viquez et al. (2020) investigated locomotor maturation in the framework of referent control. Their result showed that young children had fewer EMG minima compared to young adults for vertical and forward jumping tasks, and suggested that young children likely had not completely learned how to specify the referent shifts for these two jumping tasks. In contrast, for the task of walking, the number of EMG minima was similar between children and young adults, since both groups had achieved a similar level of walking maturation. This suggests that the probability of EMG minima occurring decreases when the task is new or incompletely learned, which was also evidenced by an increase in variability in the body position at which EMG minima occurred in Chan-Viquez et al. (2020).
Based on the referent control concept, Günther and Ruder (2003) modeled two-dimensional human walking involving 11 segments, 14 muscles per leg, and 3 segments per leg resulting in physiologically feasible EMG patterns, robust walking, and trunk stabilization. The model produced rhythmical feedforward shifts between two referent body configurations, each of which was composed of a set of threshold muscle lengths, λs. Consistent with the notion of referent control, the timing of the movement and EMG activity was not pre-set by a CPG but emerged from the interaction of the musculoskeletal system with the ground. Although this model is an important illustration of the physiological feasibility of referent control of human locomotion, it failed to illustrate how gait speed can be changed. To address this issue, it is important to note that the referent leg configuration does not only change in time but is also shifted spatially in the direction of locomotion (Figs. 1, 3).
Shifts in the location of the referent body configuration in the environment are responsible for transferring stable equilibrium in space during locomotion. By changing the rate of referent shifts, r, the nervous system can influence gait speed (Fig. 1) resulting in a transformation of walking to running and jumping forward. This assumption can be tested by implementing the proposed mechanism (“place resetting”) in the model of Günther and Ruder (2003). It can also be verified experimentally by testing the prediction of phase resetting to external mechanical perturbations.
As previously illustrated, during unperturbed gait, there is no danger of falling when the COM leaves the previous BOS and moves toward the next, referent BOS (Fig. 1). However, when a perturbation occurs that temporarily impedes leg swing, or stumbling occurs, a situation arises in which COM motion toward the referent BOS is disrupted or delayed. The system has a set of responses to prevent falling in these cases. One response would be to temporarily decrease the rate of shift in the referent body location to allow the perturbed leg time to reach the referent BOS, while propelling the other leg into swing. Following the delay in the referent body displacement, the whole gait pattern will be transferred and remain transferred in time until the other gait characteristics (speed, cycle duration, and swing/stance duration ratio) are restored. This phase resetting will involve all four limbs (i.e., be global, since the gait pattern was equally shifted in time for all four limbs, see Results) and be long-lasting. This type of phase resetting may be a principal response to certain perturbations unlike transient phase resetting, during which all changes in the gait pattern including changes in the phase of the gait cycle, completely disappear after the perturbed or subsequent cycle (cf. Rybak et al. 2006). Long-lasting phase resetting in response to perturbation of human gait has been observed in healthy subjects by Feldman et al. (2011) as well as in post-stroke and elderly subjects by Krasovsky et al. (2013).
Neurophysiological studies implying a two-level structure for the control of gait
There are several neurophysiological studies that are consistent with the notion that the primary cause of locomotion is non-rhythmical, but instead, a monotonic translation of stable body balance in the environment. The monotonic shift in stable body balance and stability in the environment might be accomplished by a ramp-shaped propagation of excitation at spinal and/or supraspinal levels of the CNS. In other words, this process may result in continuous relocation of the referent body configuration in external space and would elicit the subsequent generation of rhythmical activity of multiple muscles of the body by a CPG.
Several neurophysiological studies are consistent with the hypothesis of the existence of the two-level structure of gait control. It is worth noting that Mark Shik was not only a co-discoverer of the mesencephalic locomotor area in cats (Shik et al. 1969) but also showed with his colleagues that there is a strip of neurons along the entire spinal cord in cats, such that walking can be elicited by stimulating neurons practically at any point of this strip (e.g., Selionov and Shik 1984). A similar strip of spinal neurons was more recently found in rats (Gerasimenko et al. 2010). Percutaneous stimulation of the strip can improve locomotion after spinal cord injuries in both rats and humans (Gerasimenko et al. 2010). Theoretically, the propagation of excitation along the strip might underlie shifts in the referent body configuration, and by modifying the intensity of the strip activation, the system can change the gait speed. There is also evidence that, rather than directly changing muscle activation, corticospinal, reticulospinal, and vestibulospinal pathways control motor actions indirectly by shifting the spatial thresholds or muscle lengths at which muscles begin to be activated (Feldman and Orlovsky 1972; Raptis et al. 2010), thus influencing shifts in the referent body configuration. In future studies, one can investigate whether descending pathways can initiate human locomotion by monotonically shifting stable balance in the environment. This suggestion is also consistent with the observations that propagation of excitation can be observed at the level of the primary motor cortex (Hatsopoulos and Amit 2011). Effects of visual perturbation can also be mediated by controlling the propagation of excitation in the superior colliculus that projects to MNs of limb muscles (Feldman et al. 2021; Zhang et al. 2022). The involvement of different spinal and supraspinal areas in shifting of stable body balance as primary cause of locomotion can be addressed in future studies in humans and animals.
Reactions to perturbations of different nature can be similar in the context of the two-level locomotor control
Qualitatively different, long-lasting phase resetting in response to visual occlusion and to mechanical perturbations observed in the study by Feldman et al. (2011) was similar. EMG minimization of multiple muscle activity of each leg was observed not only in the presence or absence of vision during walking on a treadmill in the present study but also during overground forward and backward walking in the presence of vision (Feldman et al. 2011). This can be anticipated if reactions to perturbations and other changes in locomotion start at the same, first level of locomotor control responsible for the rate and direction of shifts in the referent body configuration in the environment. These reactions are likely related to the speed of propagation of neural excitation along the spinal strip discovered by Selionov and Shik 1984) and Gerasimenko et al. (2010). If so, different types of perturbations (i.e., visual, mechanical, acoustical, proprioceptive, and painful stimuli, and internal, mental distractions) can elicit similar effects: such equivalency of effects of different perturbations can be tested in future studies.
Referent control of locomotion in complex terrains with integration of visual information from distant objects
In Fig. 1, we considered the referent leg-foot configuration to explain how ground reaction forces and single steps are produced. To step over obstacles of varying heights, vision of distant objects during locomotion in complex terrains can be used (Matthis and Fajen 2014; Matthis et al. 2017) to flex the referent leg configuration while providing a sufficient clearance above each obstacle. This can be done without stumbling and interruption of locomotion, as occurs for example during hurdle sport competition. Thus, the two-level referent control scheme can be adapted to explain corrective stumbling reactions in human and animals during walking on complex terrains, which may require a visual-dependent modulation of referent control. This is applicable especially to terrains in which subjects should jump from one place on the ground to another. Indeed, this study tested only limited suggestions of how visual information can be used in referent control of locomotion. Further details of visually guided referent control of different aspects of locomotion (rhythm, speed, step size, direction, etc.) can be elucidated in future studies, with respective details of the two-level referent control of locomotion.
Conclusions
Human locomotion is likely guided by feedforward shifts in the referent body location, eliciting rhythmical changes in the activity of multiple muscles by the CPG. The speed of locomotion likely depends on the rate of referent shifts affected by sensory reflexes and descending central pathways. Walking can be transformed into running and jumping forward by increasing the rate of referent shifts. The two-level scheme for referent control can be adapted to consider locomotion on complex terrains with the integration of visual information about distant objects.
Data availability
The original data are available from the corresponding author at a reasonable request.
Change history
22 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00221-023-06642-5
References
Andronov AA, Khajkin SE (1949) Theory of oscillations. Princeton, NJ (Original work in Russian, published in 1937): Princeton University Press.
Asatryan DG, Feldman AG (1965) Functional tuning of the nervous system with control of movement or maintenance of a steady posture: I. Mechanographic analysis of the work of the limb on execution of a postural task. Biophysics 10:925–935
Baghdadi G, Nasrabadi AM (2012) Comparison of different EEG features in estimation of hypnosis susceptibility level. Comput Biol Med 42:590–597
Chan-Viquez D, Hasanbarani F, Zhang L, Anaby D, Turpin NA, Lamontagne A, Feldman AG, Levin MF (2020) Development of vertical and forward jumping skills in typically developing children in the context of referent control of motor actions. Dev Psychobiol 62(6):711–722. https://doi.org/10.1002/dev.21949
Costa T, Rognoni E, Galati D (2006) EEG phase synchronization during emotional response to positive and negative film stimuli. Neurosci Lett 406:159–164
Day BL, Bancroft MJ (2018) Voluntary steps and gait initiation. Handb Clin Neurol 159:107–118. https://doi.org/10.1016/B978-0-444-63916-5.00006-9
Dietz V, Trippel M, Horstmann GA (1991) Significance of proprioceptive and vestibulo-spinal reflexes in the control of stance and gait. Adv Psychol 78:37–52. https://doi.org/10.1016/S0166-4115(08)60737-2
Dubreucq L, Mereu A, Blanc G, Filiatrault J, Duclos C (2017) Introducing a psychological postural threat alters gait and balance parameters among young participants but not among most older participants. Exp Brain Res 235:1429–1438
Feldman AG (1986) Once more on the equilibrium-point hypothesis (λ model) for motor control. J Mot Behav 18:17–54
Feldman AG (2015) Referent control of action and perception: Challenging conventional theories in behavioral neuroscience. Springer, New York
Feldman AG, Orlovsky GN (1972) The influence of different descending systems on the tonic stretch reflex in the cat. Exp Neurol 27(3):481–494. https://doi.org/10.1016/0014-4886(72)90091-x
Feldman AG, Zhang L (2020) Eye and head movements and vestibulo-ocular reflex in the context of indirect, referent control of motor actions. J Neurophysiol 124:115–133
Feldman AG, Krasovsky T, Baniña MC, Lamontagne A, Levin MF (2011) Changes in the referent body location and configuration may underlie human gait, as confirmed by findings of multi-muscle activity minimizations and phase resetting. Exp Brain Res 210:91–115
Feldman AG, Levin MF, Garofolini A, Piscitelli D, Zhang L (2021) Central pattern generator and human locomotion in the context of referent control of motor actions. Clin Neurophysiol 132:2870–2889
Gerasimenko Y, Gorodnichev R, Machueva E, Pivovarova E, Semyenov D, Savochin A, Roy RR, Edgerton VR (2010) Novel and direct access to the human locomotor spinal circuitry. J Neurosci 30(10):3700–3708
Gibson JJ (1966) The senses considered as perceptual systems. Houghton Mifflin, Boston
Glansdorff P, Prigogine I (1971) Thermodynamic theory of structure, stability and fluctuations. Wiley-Interscience, p 306
Grillner S (1975) Locomotion in vertebrates: central mechanisms and reflex interation. Physiol Rev 55(2):2470–3304. https://doi.org/10.1152/physrev.1975.55.2.247
Günther M, Ruder H (2003) Synthesis of two-dimensional human walking: a test of the λ-model. Biol Cybern 89:89–106
Hatsopoulos NG, Amit Y (2011) Synthesizing complex movement fragment representations from motor cortical ensembles. J Physiol (paris) 106(3–4):112–119. https://doi.org/10.1016/j.jphysparis.2011.09.003
Ilmane N, Sangani S, Feldman AG (2013) Corticospinal control strategies underlying voluntary and involuntary wrist movements. Behav Brain Res 236(1):350–358. https://doi.org/10.1016/j.bbr.2012.09.008
Ivanenko YP, Poppele RE, Lacquaniti F (2006) Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol 95:602–618
Krasovsky T, Baniña MC, Hacmon R, Feldman AG, Lamontagne A, Levin MF (2012) Stability of gait and interlimb coordination in older adults. J Neurophysiol 109(1):77–88. https://doi.org/10.1152/jn.00552.2012
Krasovsky T, Lamontagne A, Feldman AG, Levin MF (2013) Reduced gait stability in high-functioning poststroke individuals. J Neurophysiol 109(1):77–88. https://doi.org/10.1152/jn.00552.2012
Krasovsky T, Lamontagne A, Feldman AG, Levin MF (2014) Effects of walking speed on gait stability and interlimb coordination in younger and older adults. Gait Posture 39(1):378–385. https://doi.org/10.1016/j.gaitpost.2013.08.011
Kubo M, Wagenaar RC, Saltzman E, Holt KG (2004) Biomechanical mechanism for transitions in phase and frequency of arm and leg swing during walking. Biol Cybern 91:91–98
Lepelley MC, Thullier F, Koral J, Lestienne FG (2006) Muscle coordination in complex movements during Jeté in skilled ballet dancers. Exp Brain Res 175:321–331
Lestienne F, Soechting J, Berthoz A (1977) Postural readjustments induced by linear motion of visual scenes. Exp Brain Res 28:363–384. https://doi.org/10.1007/BF00235717
Lestienne FG, Thullier F, Archambault P, Levin MF, Feldman AG (2000) Multi-muscle control of head movements in monkeys: the referent configuration hypothesis. Neurosci Lett 283:65–68
Loeb GE (1995) Control implications of musculoskeletal mechanics. In: annual international conference of the ieee engineering in medicine and biology - proceedings, pp 1393–1394.
MacKinnon CD, Winter DA (1993) Control of whole body balance in the frontal plane during human walking. J Biomech 26:633–644
Matthis JS, Fajen BR (2014) Visual control of foot placement when walking over complex terrain. J Exp Psychol Hum Percept Perform 40(1):106–115
Matthis JS, Barton SL, Fajen BR (2017) The critical phase for visual control of human walking over complex terrain. Proc Natl Acad Sci U S A 114(32):E6720–E6729
Mullick AA, Turpin NA, Hsu SC, Subramanian SK, Feldman AG, Levin MF (2018) Referent control of the orientation of posture and movement in the gravitational field. Exp Brain Res 236:381–398
Patla A (2011) Adaptability of human gait: implications for the control of locomotion. North-Holland, Amsterdam
Pilon JF, Feldman AG (2006) Threshold control of motor actions prevents destabilizing effects of proprioceptive delays. Exp Brain Res 174:229–239
Raptis H, Burtet L, Forget FAG (2010) Control of wrist position and muscle relaxation by shifting spatial frames of reference for motoneuronal recruitment: possible involvement of corticospinal pathways. J Physiol 588(Pt 9):1551–1570. https://doi.org/10.1113/jphysiol.2009.186858
Rogers JL, Howard KI, Vessey JT (1993) Using significance tests to evaluate equivalence between two experimental groups. Psychol Bull 113(3):553–565. https://doi.org/10.1037/0033-2909.113.3.553
Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA (2006) Modelling spinal circuitry involved in locomotor pattern generation: Insights from deletions during fictive locomotion. J Physiol 577(Pt 2):617–639. https://doi.org/10.1113/jphysiol.2006.118703
Schelter B, Winterhalder M, Maiwald T, Brandt A, Schad A, Schulze-Bonhage A, Timmer J (2006) Testing statistical significance of multivariate time series analysis techniques for epileptic seizure prediction. Chaos 16(1):013108. https://doi.org/10.1063/1.2137623
Selionov VA, Shik ML (1984) Medullary locomotor strip and column in the cat. Neuroscience 13(4):1257–1278. https://doi.org/10.1016/0306-4522(84)90297-5
Shik ML, Severin FV, Orlovksy GN (1969) Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr Clin Neurophysiol 26(5):549
Shoja O, Farsi A, Towhidkhah F, Feldman AG, Abdoli B, Bahramian A (2020) Visual deprivation is met with active changes in ground reaction forces to minimize worsening balance and stability during walking. Exp Brain Res 238(2):369–379. https://doi.org/10.1007/s00221-020-05722-0
St-Onge N, Feldman AG (2004) Referent configuration of the body: a global factor in the control of multiple skeletal muscles. Exp Brain Res 155:291–300
Turvey MT (2019) Lectures on perception: an ecological perspective. Routledge, New York
Winter DA (2009) Biomechanics and motor control of human movement, 4th edn. Wiley, New Jersey
Zatsiorsky VM, Duarte M (2000) Rambling and trembling in quiet standing. Mot Control 4:185–200
Zhang L, Guberman S, Feldman AG (2022) Shifts in the eye-centered frame of reference may underlie saccades, visual perception, and eye-hand coordination. J Neurophysiol 128(4):1025–2039. https://doi.org/10.1152/jn.00531.2021
Acknowledgements
We thank Philippe Gourdou for help in data collection and analysis.
Funding
This study was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) under Grant 121473-2012RGPIN (to A.G.F.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shoja, O., Towhidkhah, F., Hassanlouei, H. et al. Reaction of human walking to transient block of vision: analysis in the context of indirect, referent control of motor actions. Exp Brain Res 241, 1353–1365 (2023). https://doi.org/10.1007/s00221-023-06593-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06593-x