Abstract
The afferent inputs from peripheral sensory receptors and efferent signals from the central nervous system that underlie intentional movement can contribute to kinesthetic perception. Previous studies have revealed that tendon vibration to wrist muscles elicits an excitatory response—known as the antagonist vibratory response—in muscles antagonistic to the vibrated muscles. Therefore, the present study aimed to further investigate the effect of tendon vibration combined with motor imagery on kinesthetic perception and muscular activation. Two vibrators were applied to the tendons of the left flexor carpi radialis and extensor carpi radialis. When the vibration frequency was the same between flexors and extensors, no participant perceived movement and no muscle activity was induced. When participants imagined flexing their wrists during tendon vibration, the velocity of perceptual flexion movement increased. Furthermore, muscle activity of the flexor increased only during motor imagery. These results demonstrate that kinesthetic perception can be induced during the combination of motor imagery and co-vibration, even with no experience of kinesthetic perception from an afferent input with co-vibration at the same frequency. Although motor responses were observed during combined co-vibration and motor imagery, no such motor responses were recorded during either co-vibration alone or motor imagery alone, suggesting that muscular responses during the combined condition are associated with kinesthetic perception. Thus, the present findings indicate that kinesthetic perception is influenced by the interaction between afferent input from muscle spindles and the efferent signals that underlie intentional movement. We propose that the physiological behavior resulting from kinesthetic perception affects the process of modifying agonist muscle activity, which will be investigated in a future study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kinesthesia is the sense that enables awareness of bodily position, weight, or movement. Kinesthetic perception is generated by afferent inputs from muscle spindles and skin, or by the efferent signals from the central nervous system that underlie intentional movement. For example, tendon vibration applied to a biceps brachii in an appropriate pattern can evoke a kinesthetic illusion of elbow extension without any overt movement (Goodwin et al. 1972). Tendon vibration mainly activates Ia-type afferents from the muscle spindle primary endings (Burke et al. 1976; Roll and Vedel 1982; Roll et al. 1989), and consequently, humans can experience vivid kinesthetic illusions of limb movement in the direction corresponding to the stretch of the vibrated muscle. Thus, it is hypothesized that proprioceptive inputs from the muscles contribute to generating kinesthetic perception.
Additionally, vibrating two antagonistic muscles at different frequencies can evoke a kinesthetic illusion of movement in the direction of the muscle vibrated at the higher frequency (Gilhodes et al. 1986). However, co-vibration of the two antagonistic muscles at the same frequency does not evoke kinesthetic illusion (Gilhodes et al. 1986). Primary endings respond one-to-one in relation to vibration frequencies (Roll et al. 1989), therefore, the firing rate of Ia-type afferents increases with increasing vibration frequency. Accordingly, Gilhodes et al. (1986) concluded that illusory perception of movement is elicited only when there is difference in proprioceptive signals between two antagonistic muscles. In contrast, we previously reported that imagined movement direction is perceived during motor imagery with co-vibration of two antagonistic muscles, even with no experience of kinesthetic illusion from co-vibration of two antagonistic muscles at the same frequency without imagery (Shibata and Kaneko 2013). However, in our previous report, we discussed kinesthetic perception on the basis of a psychophysical index—namely, a change in perceived movement velocity induced by combining proprioceptive input and motor imagery.
Tendon vibration can elicit an excitatory response in antagonist to the vibrated muscles, which is termed the antagonist vibratory response (AVR) (Calvin-Figuière et al. 1999). The AVR is congruent with illusory movement because it happens in the muscle groups normally contracted if the illusory movement had been performed (Calvin-Figuière et al. 1999). Additionally, the AVR is not evoked if the kinesthetic illusion does not occur, such as when the participant looks at his/her own limb during vibration (Feldman and Latash 1982). Therefore, the AVR may be associated with kinesthetic perception. According to functional magnetic resonance imaging (Kavounoudias et al. 2008; Romaiguère et al. 2003), magnetoencephalography (Casini et al. 2006, 2008), and positron emission tomography studies (Kitada et al. 2002; Naito et al. 1999, 2002; Naito and Ehrsson 2001), motor and parietal activation (e.g., premotor, sensorimotor, and parietal cortices) is related to kinesthetic perception induced by tendon vibration. It has been suggested that a neural network mainly involving the primary motor cortex is related to kinesthetic perception strength (Casini et al. 2006, 2008). Although the neural mechanisms underlying the AVR remain somewhat unclear, Calvin-Figuière et al. (1999) suggested that the AVR may result from perceptual-to-motor transformation occurring at the cortical level, rather than from spinal reflex mechanisms. Similarities between voluntary contraction and the AVR support this hypothesis, as does the observation that the AVR occurs only when a kinesthetic illusion is evoked. Therefore, we hypothesized that agonist muscle activity for perceived movement would increase when kinesthetic perception is generated by the combination of tendon vibration and motor imagery. The purpose of the present study was to elucidate the characteristics of muscle activity as a physiological parameter to confirm the appearance of kinesthetic perception induced by equilibrium inputs with tendon vibration during motor imagery.
Materials and methods
Twenty healthy young adults (15 men and 5 women; age 23.6 ± 4.7 years, mean ± SD) participated in this study. Each participant provided informed consent for participation in this study, consistent with the Declaration of Helsinki. This study was approved by the ethics committee of Sapporo Medical University.
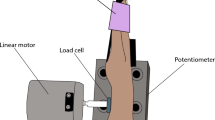
We investigated the velocity of the perceived movement and electromyogram (EMG) activity when two antagonistic muscles were vibrated at the same frequency during motor imagery. The participants sat in a comfortable chair and were asked to place their left forearms in a resting position on a desk (Fig. 1). The angle of the left wrist was measured using a three-dimensional small wireless motion-capture system (TECHNO CONCEPT, i4Motion) and displayed on a 20-inch monitor that was placed in front of the participants. The participants checked the monitor to maintain their wrist angle in the middle position before applying vibration during each experimental condition. After checking their wrist angle, the participants were instructed to relax, close their eyes, and focus on the perception of the movement of their left wrists during tendon vibration and motor imagery.
Experimental setup. The left hand was placed on a table. The two vibrators were applied to the distal tendons of the wrist extensor and flexor muscles. A wireless motion-capture system was fixed on a self-designed forearm stand, which was capable of movement in the direction of flexion/extension only
Procedures
We used eight conditions in our experiment, i.e., co-vibration at four pattern frequencies with motor imagery, or co-vibration at four pattern frequencies without motor imagery. Each experimental condition was randomly repeated three times, to avoid order effects. In addition, the stimulus interval was set to more than 30 s to avoid fatigue. A sound signal was projected for 150 ms before the vibrators were activated. The participants were instructed to imagine wrist flexion movements at the moment they perceived the sound signal; it was critical for tendon vibration to begin simultaneously with motor imagery. In addition, we instructed the participants to cease motor imagery when the vibration stopped.
Tendon vibration
Two vibrators (TECHNO CONCEPT, VB115) were applied to the tendons of the left flexor carpi radialis (FCR) and extensor carpi radialis (ECR). We used vibration to single muscle for inducing kinesthetic illusion and used antagonist co-vibration without inducing kinesthetic illusion. During pre-tests, we confirmed that all participants experienced vivid kinesthetic illusions such as wrist flexion or extension were evoked by vibrating ECR or FCR in advance. The vibration amplitude was 0.85 mm. The frequency, which was controlled by a software program (TECHNO CONCEPT, Pivot VB 115), was held at 40, 60, 80, and 100 Hz for 3 s. During the experiment, the left FCR and ECR were vibrated at same frequencies according to four co-vibration patterns (FCR × ECR; 40 Hz × 40 Hz, 60 Hz × 60 Hz, 80 Hz × 80 Hz, and 100 Hz × 100 Hz).
After each stimulus, the participants performed the wrist flexion movement in the ipsilateral hand at the velocity perceived during vibration. The velocity and direction of these reproduced movements were recorded to quantify the perceptual movement during each stimulus. We measured the track of the wrist joint angle. Angle data were sampled at 100 Hz, and low-pass filtered at 1 Hz. We did not share the experimental hypothesis with the participants before or during the experiment. The experimenter never actually moved the wrist joint of the participants; however, participants were told that there was a possibility that the left wrist joint would be passively moved during each stimulus without being touched by the experimenter.
Motor imagery
We evaluated the ability of each participant to generate motor imagery with Motor Imagery Questionnaire-Revised (Hall and Martin 1997). The participants performed left wrist flexion movement at a constant speed from the middle range to the maximum flexion range for 3 s. Then, they were instructed to image the kinesthetic sensation during the maximum wrist flexion. The imagined movement was executed after the imagery, and participants practiced repeatedly until they could perform five consecutive repetitions of the motor imagery task without muscle contraction. Background EMGs of the left FCR and ECR during motor imagery were displayed on a 19-inch monitor with a 20-μV scale per division (a total of five divisions for each muscle), and were checked for voluntary contraction by two experimenters (Aoyama and Kaneko 2011; Kaneko et al. 2007).
Surface EMG recording
Surface EMG activity was recorded from the left FCR and ECR using pairs of Ag–AgCl disk electrodes placed on the center of the muscle belly, with an 18-mm interelectrode distance. Prior to placing the electrodes, the skin was cleaned with alcohol and abraded with an abrasive skin-prepping gel. EMG signals were amplified (Nihon Kohden Co. Ltd., Neuropack MEB2200) and filtered (5–1000 Hz). The EMG signal was digitized and sampled at 2000 Hz using an A/D converter (Cambridge Electronic Design, Power 1401). EMG signals were recorded during each stimulus.
Data analysis
The velocity of the perceptual movement (°/s) was calculated from the onset of the imitative movement up to the offset, to index the degree of kinesthetic perception (Shibata and Kaneko 2013). The onset of the imitative movement angle was automatically determined at ±3 SD from the mean pre-stimulus level, and the offset was determined at ±3 SD from the mean post-stimulus level. After hand-tracking, participants were instructed to keep their wrist joint at the perceived angle when the vibration stopped, and this angle was recorded post-stimulus. An S-shaped curve was constructed and then differentiated, to calculate velocity. The means and SD across three trials were calculated for each condition. Statistical analyses were performed using IBM SPSS statistics. A two-way repeated measures analysis of variance (ANOVA) with a T test with the Bonferroni correction was used to evaluate the influence of ‘motor imagery’ (non-MI and MI) and ‘vibration frequency’ (40, 60, 80, and 100 Hz) on average velocities. Statistical significance was determined by p < 0.05. Furthermore, the correlations between the mean velocity and the EMG activities of FCR or ECR during motor imagery were analyzed using the Pearson’s correlation coefficient.
Surface EMG activities were recorded from FCR and ECR. A fast Fourier transform (FFT) analysis of the EMG signals recorded from the FCR and ECR clearly demonstrated that the spectrum power was greater at 50 Hz than it was at other frequencies, regardless of vibration frequencies. An alternating current of 50 Hz is supplied in eastern Japan, where we recorded the EMG data, and it is possible that this spectrum power at 50 Hz was power line frequency noise. For this reason, we first filtered this data using a notch filter targeting the power line frequency noise. The EMG value was then calculated as the root mean squared (RMS) value for each trial. The RMS value occurred an average of 1000–2000 ms after the onset of vibration for each muscle (Fig. 2). The mean and SD across three trials were then calculated for each condition. A three-way repeated measures ANOVA with the t test with the Bonferroni correction was used to evaluate the influence of ‘muscle’ (FCR and ECR), ‘motor imagery’ (non-MI and MI), and ‘vibration frequency’ (40, 60, 80, and 100 Hz) on the RMS value. Statistical significance was determined by p < 0.05. Furthermore, correlations between FCR and ECR activity were analyzed using the Pearson’s correlation coefficient.
Results
Perceived movement during motor imagery, with co-vibration at the same frequency
During pre-tests, all participants experienced a vivid illusory sensation that their wrist was flexing or extending, when tendon vibration was applied to the wrist extensor or flexor. When the difference in frequency between the wrist flexor and extensor was 0 Hz without motor imagery, the velocity of the perceived movement indicated that the illusory movements had very low values (Table 1). During the 40 Hz condition, two participants perceived movement (i.e., average velocity was greater than the mean ±3 SD). Similarly, one participant perceived movement during the 60 and 100 Hz conditions, while all participants did not perceived movement during 80 Hz condition. Conversely, during co-vibration with motor imagery, the velocity of the perceived movement was greater than that during co-vibration without motor imagery (Table 1). A two-way repeated measures ANOVA was used to evaluate the influence of ‘motor imagery’ (non-MI and MI) and ‘vibration frequency’ (40, 60, 80, and 100 Hz) on average velocities (p < 0.05). There were significant main effects of ‘motor imagery’ and ‘vibration frequency’ [imagery: F (1, 19) = 21.066, p < 0.0005; frequency: F (1.742, 33.096) = 5.616, p = 0.010]. The interaction between ‘vibration frequency’ and ‘motor imagery’ was also significant [F (1.287, 24.451) = 5.676, p = 0.019]. Post hoc comparisons using t test with the Bonferroni correction revealed that velocity significantly increased at 60 Hz during MI compared to non-MI, as well as at 80 Hz during MI compared to non-MI, and at 100 Hz during MI compared to non-MI. Moreover, velocity significantly increased at 80 and 100 Hz during MI compared to at 40 Hz during MI (Fig. 3).
Typical angle data for recorded movement in each condition (a–d). Dotted and solid lines show non-MI and MI conditions, respectively. Average velocity of perceived movement with four different frequencies (e). Empty and filled columns show the velocity of non-MI and MI conditions, respectively. Error bars represent SD. MI motor imagery. *p < 0.05, **p < 0.01
EMG activity during motor imagery with co-vibration at the same frequency
A two-way repeated measures ANOVA was used to evaluate the influence of ‘motor imagery’ (non-MI and MI), and ‘vibration frequency’ (40, 60, 80, and 100 Hz) on the RMS value (Fig. 4). There was a significant main effect of ‘motor imagery’ in FCR [F (1, 19) = 5.404, p = 0.031], whereas the main effect in ECR was not significant [F (1, 19) = 1.832, p = 0.192]. The main effect of ‘vibration frequency’ was significant in ECR only [FCR: F (3, 57) = 2.077, p = 0.113; ECR: F (1.651, 31.371) = 1.637, p = 0.213]. The interaction between ‘motor imagery’ and ‘vibration frequency’ was not significant in both muscles [FCR: F (1.853, 35.209) = 0.420, p = 0.645; ECR: F (1.749, 33.226) = 1.315, p = 0.279]. Furthermore, the correlation between FCR and ECR activities was analyzed using the Pearson’s correlation coefficient, and a significant negative correlation occurred during motor imagery [r = −0.269, t (18) = −1.616, p = 0.038; Fig. 5b].
Comparison between perceived movement and EMG activity during kinesthetic perception
The relationship between the average velocity and EMG activity of the FCR or ECR during motor imagery was analyzed using a Pearson’s correlation coefficient (Fig. 6), and non-significant findings were observed for both muscles during motor imagery [FCR: r = −0.004, t (18) = −0.017, p = 0.975; ECR: r = 0.129, t (18) = 0.552, p = 0.327].
Discussion
In the present study, the perceptual strength of kinesthetic illusion was based on reproduced movement velocity. We found that the flexion velocity of perceived movement was higher when participants imagined wrist flexion during co-vibration, despite that kinesthetic perception was not generated with co-vibration alone—that is, when the two antagonistic muscles were vibrated at the same frequency. The RMS values for ECR during co-vibration did not differ, regardless of motor imagery. Conversely, the RMS value for the FCR increased to a greater extent during co-vibration with motor imagery than during co-vibration without motor imagery. The AVR would be expected only if illusory movement was perceived. During co-vibration at the same frequencies alone, or motor imagery alone, kinesthetic perception and disequilibrium of muscle activity for two vibrated muscles did not occur. This change in FCR activity appeared only when participants perceived wrist flexion during the combination of motor imagery and co-vibration, which has not previously been reported.
In the present study, most participants did not perceive vivid movements when the difference in frequency between the wrist flexor and extensor was 0 Hz with co-vibration alone. Previous research has established that there is no perception of wrist movement when the wrist flexor and extensor muscles are vibrated at the same frequency (Calvin-Figuière et al. 1999). Therefore, the present results are consistent with previous findings (Calvin-Figuière et al. 1999; Gilhodes et al. 1986; Shibata and Kaneko 2013). The present study found a significant main effect of vibration frequency. During the 40 Hz condition in non-MI, a total of 18 participants perceived no movement (i.e., average velocity was lower than the mean ±3 SD). Similarly, 19 participants perceived no movement during the 60 Hz and 100 Hz condition, although all participants perceived no movement during the 80 Hz condition. There is an apparent contradiction between the ‘main effect of vibration frequency on illusion velocities’ and the fact that ‘most participants did not perceive vivid movements’ during co-vibration. However, the interaction between “vibration frequency” and “motor imagery” was significant. And, the result of post hoc tests revealed that the average velocities differed significantly depending on the vibration frequency in MI condition, whereas the velocity had very low values and did not differ among each frequency in non-MI condition. Therefore, we consider that the main effect of vibration frequency was affected by the differences in average velocity under MI condition, not by the differences under non-MI condition. Moreover, the velocity of perceived movement increased at 80 and 100 Hz compared to 40 Hz during motor imagery, although, the participants imagined a constant velocity movement during all motor imagery conditions. The velocity of perceived movement during single-vibration changes depending on the vibration frequency (Roll and Vedel 1982; Roll et al. 1989). Previous studies show that individuals do not perceive movement regardless of the vibration frequency applied to each muscle when the difference in the frequency between the two antagonistic muscles is 0 Hz (Calvin-Figuière et al. 1999; Gilhodes et al. 1986). Our results indicate that the combination of motor imagery and co-vibration at the same frequencies can evoke kinesthetic perception even when the difference in frequency between the two antagonistic muscles is 0 Hz. Furthermore, the velocity of this perceived movement can change depending on the sum of two antagonistic muscles, consistent with the results of our previous study (Shibata and Kaneko 2013). However, we cannot exclude the possibility that knowledge of tendon vibration-induced kinesthetic perception could have influenced the perception of movement. The participants recruited in the present study had not previously experienced tendon vibration-induced kinesthetic perception. Furthermore, we did not share our experimental hypothesis with the participants before or during the experiment. Thus, such an influence on the velocity of perceived movement should be minimal.
Tendon vibration can induce two types of muscle activity (Calvin-Figuière et al. 1999), the AVR and the tonic vibration reflex (TVR). The TVR is an excitatory response in the vibrated muscle, since this response results in part from high-frequency activation of the myotatic pathway. If the TVR occurred during co-vibration with motor imagery in the present study, the RMS values for FCR and ECR would be the same. Conversely, the AVR occurs in an agonist muscle of perceived movement only when kinesthetic perception is generated (Blanchard et al. 2013; Feldman and Latash 1982; Gilhodes et al. 1986). Colebatch et al. (1990) recorded neuronal discharges in the primary motor cortex of a monkey, which responded to passive wrist flexion with sustained bursts of impulses. Discharge frequency of the neuron responding to passive wrist flexion increased when the wrist extensor was vibrated at 100 Hz, while the wrist flexor was not vibrated. Furthermore, vibration applied to the wrist flexor produced only a small change in the background firing of this neuron, and no sustained response was seen. Their results indicate that the neurons in the primary motor cortex associated with perceived movement are activated by sensory inputs during tendon vibration. In the present study, the FCR exhibited AVR-like activity associated with kinesthetic perception of flexion movement, indicating that the use of motor imagery during co-vibration caused participants to perceive the movement as if it had actually occurred. Moreover, RMS values for the FCR and ECR were negatively correlated. This negative correlation indicates that the higher activity in the agonistic muscle of perceived movement is associated with lower activity in the antagonistic muscle. No significant correlation was observed between perceptual strength and the RMS value; however, the finding that the RMS value of the FCR increased only when movement was perceived suggests that FCR activity during co-vibration with motor imagery was associated with kinesthetic perception. We hypothesize that the transcortical long-loop reflex via the primary motor cortex by sensory inputs during tendon vibration is mediated kinesthetic perception induced by the combination of tendon vibration and motor imagery. In other words, the brain activity during motor imagery may produce disequilibrium of the neural activity in the corresponding brain areas to the co-vibration of the muscles, although the co-vibration simultaneously induces similar afferent inputs from the two antagonistic muscles.
Romaiguère et al. (2003) suggested that the primary motor and premotor areas are minimally or not activated during co-vibration to antagonistic muscles at the same frequency, while large cortical areas associated with the sensorimotor cortex are activated during tendon vibration regardless of the inducing kinesthetic perception. These authors suggested that the primary motor and premotor areas would be associated with kinesthetic perception induced by tendon vibration. For motor imagery, several studies have revealed that the excitability of both the motor-related area (Ehrsson et al. 2003; Hanakawa et al. 2008; Porro et al. 1996; Roland et al. 1980; Roth et al. 1996; Stephan et al. 1995) and the corticospinal tract (Fadiga et al. 1999; Hashimoto and Rothwell 1999; Kasai et al. 1997; Kiers et al. 1997; Yahagi et al. 1996) are facilitated during motor imagery. These large areas are also activated during kinesthetic illusion with tendon vibration, and these neural networks may participate in integrating proprioceptive inputs from muscles and efferent input from motor imagery. Based on these studies, we consider that applying motor imagery to co-vibration may have changed the activation of motor-related areas and generated kinesthetic perception.
The present study focused on the integration of proprioceptive inputs from muscle spindles and efferent inputs from the brain during motor imagery. It has previously been established that kinesthetic perception is involved in integration of motor imagery and kinesthetic illusion by tendon vibration when single muscles are vibrated during motor imagery (Kitada et al. 2002; Thyrion and Roll 2009). The novel results of the present study are: (1) kinesthetic perception is induced by the combination of motor imagery and co-vibration, even with no experience of kinesthetic perception from afferent input with co-vibration at the same frequency, and (2) the agonist muscle activity of perceived movement increased when participants perceived movement during the combination of motor imagery and co-vibration.
In conclusion, during the combination of motor imagery and co-vibration, muscle activity responses of the agonist muscle appear to be associated with kinesthetic perception existence. However, during co-vibration at the same frequencies alone, or motor imagery alone, kinesthetic perception or changes in muscle activity of the two muscles studied here were not induced. Therefore, this process of changing the agonist muscle activity may be affected by the physiological behavior resulting from kinesthetic perception. We propose that motor imagery produces disequilibrium of neural activity in brain areas corresponding to the co-vibration of muscles, even if the co-vibration simultaneously induces similar afferent inputs from the two antagonistic muscles.
References
Aoyama T, Kaneko F (2011) The effect of motor imagery on gain modulation of the spinal reflex. Brain Res 1372:41–48
Blanchard C, Roll R, Roll JP, Kavounoudias A (2013) Differential contributions of vision, touch and muscle proprioception to the coding of hand movements. PLoS One 8:e62475
Burke D, Hagbarth KE, Löfstedt L, Wallin BG (1976) The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261:673–693
Calvin-Figuière S, Romaiguère P, Gilhodes JC, Roll JP (1999) Antagonist motor responses correlate with kinesthetic illusions induced by tendon vibration. Exp Brain Res 124:342–350
Casini L, Romaiguère P, Ducorps A, Schwartz D, Anton JL, Roll JP (2006) Cortical correlates of illusory hand movement perception in humans: a MEG study. Brain Res 1121:200–206
Casini L, Roll JP, Romaiguère P (2008) Relationship between the velocity of illusory hand movement and strength of MEG signals in human primary motor cortex and left angular gyrus. Exp Brain Res 186:349–353
Colebatch JG, Sayer RJ, Porter R, White OB (1990) Responses of monkey precentral neurones to passive movements and phasic muscle stretch: relevance to man. Electroencephalogr Clin Neurophysiol 75:44–55
Ehrsson HH, Geyer S, Naito E (2003) Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 90:3304–3316
Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G (1999) Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia 37:147–158
Feldman AG, Latash ML (1982) Inversions of vibration-induced senso-motor events caused by supraspinal influences in man. Neurosci Lett 31:147–151
Gilhodes JC, Roll JP, Tardy-Gervet MF (1986) Perceptual and motor effects of agonist-antagonist muscle vibration in man. Exp Brain Res 61:395–402
Goodwin GM, McCloskey DI, Matthews PB (1972) The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95:705–748
Hall CR, Martin KA (1997) Measuring movement imagery abilities: a revision of the movement imagery questionnaire. J Mental Imag 21:143–154
Hanakawa T, Dimyan MA, Hallett M (2008) Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex 18:2775–2788
Hashimoto R, Rothwell JC (1999) Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res 125:75–81
Kaneko F, Yasojima T, Kizuka T (2007) Kinesthetic illusory feeling induced by a finger movement movie effects on corticomotor excitability. Neuroscience 149:976–984
Kasai T, Kawai S, Kawanishi M, Yahagi S (1997) Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res 744:147–150
Kavounoudias A, Roll JP, Anton JL, Nazarian B, Roth M, Roll R (2008) Proprio-tactile integration for kinesthetic perception: an fMRI study. Neuropsychologia 46:567–575
Kiers L, Fernando B, Tomkins D (1997) Facilitatory effect of thinking about movement on magnetic motor-evoked potentials. Electroencephalogr Clin Neurophysiol 105:262–268
Kitada R, Naito E, Matsumura M (2002) Perceptual changes in illusory wrist flexion angles resulting from motor imagery of the same wrist movements. Neuroscience 109:701–707
Naito E, Ehrsson HH (2001) Kinesthetic illusion of wrist movement activates motor-related areas. NeuroReport 12:3805–3809
Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999) Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19:6134–6144
Naito E, Kochiyama T, Kitada R, Nakamura S, Matsumura M, Yonekura Y, Sadato N (2002) Internally simulated movement sensations during motor imagery activate cortical motor areas and the cerebellum. J Neurosci 22:3683–3691
Porro CA, Francescato MP, Cettolo V, Diamond ME, Baraldi P, Zuiani C, Bazzocchi M, di Prampero PE (1996) Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci 16:7688–7698
Roland PE, Larsen B, Lassen NA, Skinhøj E (1980) Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 43:118–136
Roll JP, Vedel JP (1982) Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47:177–190
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76:213–222
Romaiguère P, Anton JL, Roth M, Casini L, Roll JP (2003) Motor and parietal cortical areas both underlie kinaesthesia. Brain Res Cogn Brain Res 16:74–82
Roth M, Decety J, Raybaudi M, Massarelli R, Delon-Martin C, Segebarth C, Morand S, Gemignani A, Décorps M, Jeannerod M (1996) Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. NeuroReport 7:1280–1284
Shibata E, Kaneko F (2013) Kinesthetic perception based on integration of motor imagery and afferent inputs from antagonistic muscles with tendon vibration. Neurosci Lett 541:24–28
Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, Frith CD, Frackowiak RS (1995) Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73:373–386
Thyrion C, Roll JP (2009) Perceptual integration of illusory and imagined kinesthetic images. J Neurosci 29:8483–8492
Yahagi S, Shimura K, Kasai T (1996) An increase in cortical excitability with no change in spinal excitability during motor imagery. Percept Mot Skills 83:288–290
Acknowledgements
This work was supported by the Sasakawa Scientific Research Grant from The Japan Science Society, a Grant-in-Aid for Young Scientists B (26750191), and Scientific Research B (26282157). We thank Prof. T. Nagamine, Prof. N. Kozuka, Prof. M. Nakamura, and Prof. H. Ota for their tremendous support and insightful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibata, E., Kaneko, F. & Katayose, M. Muscular responses appear to be associated with existence of kinesthetic perception during combination of tendon co-vibration and motor imagery. Exp Brain Res 235, 3417–3425 (2017). https://doi.org/10.1007/s00221-017-5057-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5057-6