Abstract
We studied the relationship between the velocity of movement illusion and the activity level of primary motor area (M1) and of the left angular gyrus (AG) in humans. To induce illusory movement perception, we applied co-vibration at different frequencies on tendons of antagonistic muscle groups. Since it is well established that the velocity of illusory movement is related to the difference in vibration frequency applied to two antagonistic muscles, we compared magnetoencephalography (MEG) signals recorded in two conditions of co-vibration: in the “fast illusion” condition a frequency difference of 80 Hz was applied on the tendons of the right wrist extensor and flexor muscle groups, whereas in the “slow illusion” condition a frequency difference of 40 Hz was applied on the same muscle groups. The dipole strength, reflecting the activity level of structures, was measured over M1 and the left AG in two different time-periods: 0–400 and 400–800 ms in each condition. Our results showed that the activity level of the AG was similar in both conditions whatever the time-period, whereas the activity level of M1 was higher in the “fast illusion” condition compared to the “slow illusion” condition from 400 ms after the vibration onset only. The data suggest that the two structures differently contributed to the perception of illusory movements. Our hypothesis is that M1 would be involved in the coding of cinematic parameters of the illusory movement but not the AG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When vibrations at about 80 Hz are applied to the tendon of flexor muscles of the wrist, one gets the illusion that the wrist stretches despite it remains immobile. This kinaesthetic illusion is produced because the vibration activates the muscle spindles in a similar way to when the muscle actually stretches (Goodwin et al. 1972; Burke et al. 1976; Roll and Vedel 1982; Roll et al. 1989). Over the last decade, several studies employing imaging techniques aimed to determine which cortical areas are activated when subjects experience such kinaesthetic illusion. In a positron emission tomography (PET) study, Naito et al. (1999) were the first to investigate brain areas involved in perception of kinaesthetic illusions. Their results suggested that activated cortical areas were all motor areas, but a few years later, studies using functional magnetic resonance imagery (FMRI) technique showed that the perception of kinaesthetic illusion elicited by vibrating the tendons of a limb was associated with activation of both motor and parietal areas (Radovanovic et al. 2002; Romaiguère et al. 2003).
To more thoroughly investigate this question, we performed a magnetoencephalography (MEG) study aimed first to confirm the role of both motor and parietal areas in perception of movement illusions with a different brain imaging technique and second to study the dynamics of cortical activities associated to the perception of kinaesthetic illusions. Indeed, the main advantage of MEG is its excellent temporal resolution in the order of millisecond. MEG measures magnetic fields produced by the small currents of neuronal activity over time and sophisticated analysis models allow localisation of neuronal activity from the measures with high reliability and good spatial resolution (Hari 1991; Hamalainen 1992). In the MEG study, we measured MEG responses in three conditions of stimulation similar to those used in the FMRI study (Romaiguère et al. 2003): “fast illusion”, “slow illusion” and “no illusion”. In each condition, vibrations were simultaneously applied to the tendons of wrist extensor and flexor muscles. It is known that co-vibration of the tendons of antagonistic muscles at the same frequency does not induce movement illusions while co-vibration of these tendons at different frequencies does, and moreover, that the velocity of the illusory movement depends on the difference in vibration frequencies applied to two antagonistic muscle groups (Calvin-Figuière et al. 1999; Gilhodes et al. 1986). Therefore, in the “no illusion” condition, co-vibration was delivered at the same frequency on the tendons of both muscle groups and no movement was perceived. In the “fast illusion” condition, co-vibration with a frequency difference of 80 Hz was applied on the wrist extensor and flexor muscle groups, whereas in the “slow illusion” condition a frequency difference of 40 Hz was applied on the antagonistic muscle groups of the wrist.
Data comparing the “no illusion” and “fast illusion” conditions have been reported in a previous paper (Casini et al. 2006). Two results were of particular interest. First, by identifying equivalent current dipoles (ECD), we could determine a set of active structures for each condition over the first 900 ms of recording. In all subjects some areas were found active in both conditions and others were more particularly involved in the “fast illusion” condition only. Dipole locations were found in the postcentral gyrus (S1), in the superior parietal gyrus, in the supramarginal gyrus and in the right angular gyrus (AG) in both conditions. The left AG, the left supplementary motor area and the precentral gyrus (M1) were activated in the “fast illusion” condition only. We, thus, confirmed that the arising of kinaesthetic sensations was related to the activation of the posterior parietal areas, precisely the left AG, as well as of motor areas (M1 and the left supplementary motor area). Second, the temporal analysis of the cortical activities has shown a significant increase in M1 activity level from 400 ms after the vibration onset and a trend of increasing activity level with time in the left AG. Because some anatomic data have shown connections between AG and motor areas (Nieuwenhuys et al. 1988; Iacoboni et al. 1999), we proposed that the increase in M1 activity level could be the result of some projections arriving from the left AG and that the conjoined activity of these two structures would support the arising of kinesthetic illusion in subjects.
From these results we decided to further analyse MEG signals concerning the “slow illusion” and the “fast illusion” conditions with the aim to study whether or not M1 and the left AG could be functionally dissociated. Therefore, in the present paper we focused on the comparison of activities recorded over these two structures between both conditions. The dipole moments, which indicate the strength of the activity, were measured over M1 and over the AG in the “slow illusion” and “fast illusion” conditions to identify whether or not these regions changed their neural activity in relation to the velocity of movement illusion. From results obtained in the previous study, two time-windows of interest were chosen: 0–400 and 400–800 ms.
Experimental procedure
Participants
Ten healthy right-handed subjects (5 women and 5 men, 24–38 years old) participated in this study. They were chosen for their ability to feel clear vibration–induced movement illusions. They were paid for their participation. All the participants gave informed consent to the experimental procedure as required by the Helsinki Declaration (1964).
Stimulation paradigms
Participants were seated in a magnetically shielded room with their heads placed inside of the neuromagnetometer. Vibration was delivered by two pneumatic devices independently driven by a software program that permits synchronization between the vibration trains and the MEG acquisition. Vibration was applied perpendicularly to the distal tendons of the right wrist extensor and flexor muscle groups. The vibrators were held on the wrist of the subjects by rubber straps. The hand was maintained in a constant position by a light plastic splint attached to the hand and forearm of the subjects by adjustable straps.
Participants were told not to blink during stimulus presentation and were required to keep immobile and to fixate a cross on a screen placed in front of them. Co-vibration of extensor and flexor muscle groups were applied following three different patterns:
-
In the “Fast illusion” condition, co-vibration was applied at 30 Hz on wrist extensor tendons and at 110 Hz on wrist flexor tendons (frequency difference 80 Hz).
-
In the “Slow illusion” condition, co-vibration was applied at 50 Hz on wrist extensor tendons and at 90 Hz on wrist flexor tendons (frequency difference 40 Hz).
-
In the “No illusion” condition, co-vibration was applied at 70 Hz on both extensor and flexor muscle tendons (frequency difference 0 Hz).
The perceptual effects of the three conditions of stimulation were carefully controlled just before beginning the MEG recordings. All of the subjects clearly reported fast movement illusions in the condition where the frequency difference was of 80 Hz, slow movement illusions in the condition where the frequency difference was of 40 Hz, and no movement illusion when the vibration frequency was the same on both tendons.
Each trial consisted of a 5 s vibration train followed by 4 s of inter-stimulus interval. There were 102 repetitions of each condition presented in a pseudo-random order that differed for each subject.
Magnetic response measurement and anatomical magnetic resonance imaging (MRI)
The magnetic fields were measured over the entire head using a 151-DC-SQUID whole-head type MEG system (Omega, 151, CTF Systems, Port Coquitlam, BC, Canada). The averaging epochs lasted from 1 s before stimulus onset up to 7 s thereafter. Epochs contaminated by eye blinks were rejected and trials with MEG changes exceeding 0.5 pT were discarded. Data were low-pass filtered at 20 Hz.
High-resolution 3D T1-weighted structural images were acquired for each subject using a 3-T whole-body imager Medspec 30/80 Avance (Brucker, Ettlingen, Germany) for anatomical localisation (inversion-recovery 3D sequence, 1× 0.75 ×1.22 mm). MRI visible markers were placed at the locations of the head-positioning coils used during MEG registration (nasion and two periauricular points). The MEG sensor coordinate system was later aligned with the MRI images using these three head markers which enable to localise each dipole in the own brain of each subject by using the anatomical images obtained by MRI.
Source modeling from MEG data
Neuronal sources were modeled as equivalent current dipoles (ECD) and CTF’s DipoleFit software package was used for the computation of source generators. Spatiotemporal modeling of sources was performed using a spherical head model whose parameters were fitted to the MRI of each subject.
The analysis of data was made in two steps. First, we used the same method as the one described in Casini et al. (2006) to identify and localise the set of dipoles active in the “slow illusion” condition. And in a second step, to answer the question raised in this paper, the more precisely as possible, source modeling was performed by using the same starting parameters of fit for both conditions, “slow illusion” and “fast illusion”, in each time-window: 0–400 and 400–800 ms. The residual variance was kept below 15% for all dipoles. Then, CTF’s MRI viewer software package was used to localise each dipole in the own brain of each subject by using the anatomical images obtained by MRI, and brain structures corresponding to each dipole location were identified with an anatomical atlas (Duvernoy 1991). Finally, moments of dipoles located in M1 and in the left AG were measured for each time-window in each condition. Values were then normalised between conditions.
Results
First, the localisation of activity sources in the “slow illusion” condition has revealed that dipoles were located in the same structures as in the “fast illusion” condition: in the left supplementary motor area, bilateral M1, bilateral S1, bilateral superior parietal gyrus, bilateral supramarginal gyrus and in the left AG. These results confirmed that the structures activated when subjects were experiencing movement illusion were the left supplementary motor area, bilateral M1 and the left AG.
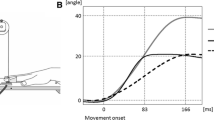
Second, to study whether or not M1 and the AG changed their neural activity in relation to the velocity of movement illusion, we carried out two separate ANOVAs including the Time-window (2) and Condition (2) factors, for each structure. Results are illustrated in Fig. 1 which shows the activity level of AG and M1 in both conditions and in the two time-windows. Concerning the AG, the ANOVA revealed neither significant main effects (condition: F(1, 7) = 1.67, P = 0.23; time-window: F(1, 7) = 3.77, P = 0.09) nor a significant interaction (condition × time-window : F(1, 7) = 0.912). Indeed, as seen in Fig. 1a, the activity level did not significantly differ between conditions whatever the time period.
Left: Activity level of the angular gyrus (a) and of primary motor area (b) in the two experimental conditions: “slow illusion” (in white) and “fast illusion” (in dark) and in two time-windows: 0–400 and 400–800 ms. The values presented correspond to the normalised moment dipole (averaged data, 10 subjects). Error bars reflect 95% confidence level intervals around each mean. Right: Dipole locations (white circles) on MR images (in an axial plane) in a representative subject in the angular gyrus (a) and in primary motor area (b). Central sulcus is overlined in black. The top of the figure corresponds to the frontal lobe, the bottom of the figure corresponds to the occipital lobe
Concerning M1, the ANOVA revealed significant effects of both factors (condition: F(1, 8) = 9.96, P < 0.01, time-window: F(1, 8) = 26.38, P < 0.001) and a significant interaction (condition × time-window: F(1, 8) = 5.34, P < 0.05). The significant interaction between factors is illustrated in Fig. 1b where we can observe that the activity level of M1 did not differ between “slow illusion” and “fast illusion” conditions (t = 1.23 no significant) in the 0–400 ms time-window, whereas in the 400–800 ms time-window, M1 activity level was significantly higher in the “fast illusion” condition than in the “slow illusion” condition (t = 2.42, P < 0.05).
We can also notice that the activity level of M1 was significantly higher in the 400–800 ms time-window compared to the 0–400 ms time–window for “slow illusion” as for “fast illusion” condition (slow condition t = 2.14, P < 0.05; fast condition t = 3.85, P < 0.01).
Discussion
Our results showed that the activity level of the AG was similar in both conditions while the activity level of M1 was higher in the “fast illusion” condition compared to the “slow illusion” condition from 400 ms after the vibration onset. This suggests that these two structures probably contribute differently to the perception of illusory movement.
Concerning M1, the difference in activity level observed between the two conditions occurred after 400 ms of vibration. If we remember that subjects generally experienced movement illusion about 400–500 ms after the beginning of the vibration onset (Gilhodes et al. 1986), the fact that the difference in activity levels between conditions is restricted only to the period of time when the subjects are feeling the illusion of movement, once more, highlights the role of M1 in the generation of kinaesthetic illusion. The first difference between experimental conditions was that a large difference of vibration frequencies was applied to the two antagonistic muscle groups in the “fast illusion” condition whereas the difference of vibration was small in the “slow illusion” condition. We thus could be tempted to relate this larger imbalance to the higher level of activity recorded in the “fast illusion” condition, but this difference in frequency vibration existed from the very beginning of the vibration train, that is also during the (0–400 ms) time–window, when dipole strength did not differ between conditions. Thus the larger imbalance in the fusorial afferences reaching M1 in the “fast illusion” condition can not be alone associated to the higher activity level of M1. This higher activity level can rather be related to the difference in the velocity of perceived movement, all the more so since the difference in perceived movement velocity is concomitant with the increase of M1 activity level since the movement perception only occurs 400–500 ms after the beginning of the vibration. Therefore, these data suggest that the role of M1 in the perception of illusory movement could be associated to cinematic parameterisation of the illusory movement. This hypothesis is supported by several PET and FMRI studies on real movements which have shown a relationship between movement rate and motor cortex activity in which higher rates of finger tapping are associated with stronger motor cortex signals in terms of both volume and strength of activity (VanMeter et al. 1995; Blinkenberg et al. 1996; Schlaug et al. 1996; Rao et al. 1996; Jäncke et al. 1998). Electrophysiological data have also demonstrated that movement rate is reflected in discharge rates. Humphrey et al. (1970) used a multiple linear regression to correlate the discharge activity of several neurons in M1 with the position, velocity, and force in a wrist flexion and extension task. More recently, Ashe and Georgopoulos (1994) found that a large fraction of neurons in M1 had activity correlated significantly with position, velocity, and acceleration parameters. Considering all of these data, it seems reasonable to suggest that cortical neurons of M1 could be involved in the specification of cinematic parameters of illusory movement as it is the case for real movements.
As shown in several papers (Radovanovic et al. 2002; Romaiguère et al. 2003; Casini et al. 2006), the perception of illusory movement is not supported only by the activation of motor areas but rather emerges from the conjoined pattern of activity of motor and parietal areas.
From the results exposed in Casini et al. (2006), it seems that the left AG also plays a predominant role in the generation of illusory movement. Since in the present study there was no relationship between the velocity of the illusory movement and the activity level of the AG, this structure was probably not involved in the cinematic parameterisation of movement. Our data did not enable us to precise the kind of processes which could be supported by the left AG, but data found in the literature report that this structure is activated in tasks where motor program has to be changed or updated (De Jong et al. 1999, for review, see Rushworth et al. 2003). If we try to relate the results presented in the present paper and in Casini et al.’s (2006) to the literature, we can propose that the left AG could be part of a parietal network, including the superior parietal gyrus and the supramarginal gyrus (Romaiguère et al. 2003; Casini et al. 2006), involved in the elaboration of motor representation. Through its connections with motor and premotor areas (Nieuwenhuys et al. 1988; Iacoboni et al. 1999), it could act as a link between this parietal network and motor frontal areas. If we hypothesise that some projections from the left AG to M1 are indispensable to the illusion to emerge, we could, at once, explain the increase in M1 activity level after 400 ms and the delay necessary for the subjects to experience sensation of movement.
To conclude, the conjoined activity of several cortical areas is necessary in the arising of kinesthetic illusion. Our study has focused on functional differences between the left AG and M1. If we can propose that M1 would be rather involved in the coding of cinematic parameters, the specific contribution of the left AG remains to be further investigated, as well as the nature of the interaction between these two structures.
References
Ashe J, Georgopoulos AP (1994) Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex 4:590–600
Burke D, Hagbarth KE, Lofstedt L, Wallin BG (1976) The response of human muscle spindle endings to vibration during isometric contraction. J Physiol 261:695–711
Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, Law I (1996) Rate dependence of regional cerebral activation during performance of a repetitive motor task: a PET study. J Cereb Blood Flow Metab 16:794–803
Calvin-Figuière S, Romaiguère P, Gilhodes JC, Roll JP (1999) Antagonist motor responses correlate with kinesthetic illusions induced by tendon vibration. Exp Brain Res 124:342–350
Casini L, Romaiguère P, Ducorps A, Schwartz D, Anton JL, Roll JP (2006) Cortical correlates of illusory hand movement perception in humans: a MEG study. Brain Res 1121:200–206
De Jong BM, Willemsen ATM, Paans AMJ (1999) Brain activation related to the change between bimanual motor programs. NeuroImage 9:290–297
Duvernoy HM (1991) The human brain surface, blood supply, and the three dimensional sectional anatomy. Springer, Wien NewYork
Gilhodes JC, Roll JP, Tardy-Gervet MF (1986) Perceptual and motor effects of ago-antagonist muscle vibration in man. Exp Brain Res 61:395–402
Goodwin GM, McCloskey DI, Matthews PBC (1972) The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95:705–748
Hamalainen MS (1992) Magnetoencephalography: a tool for functional brain mapping. Brain Topogr 5:95–102
Hari R (1991) On brain’s magnetic responses to sensory stimuli. J Clin Neurophysiol 8:157–169
Humphrey DR, Schmidt EM, Thompson WD (1970) Predicting measures of motor performance from multiple cortical spike trains. Science 178:758–762
Iacoboni M, Woods R, Brass M, Bekkering H, Mazziota J, Rizzolatti G (1999) Cortical mechanisms of human imitation. Science 286:2526–2528
Jäncke L, Peters M, Schlaug G, Posse S, Steinmetz H, Müller-Gärtner HW (1998) Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Cog Br Res 6:279–284
Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999) Illusory hand movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19:6134–6144
Nieuwenhuys R, Voogd J, Van Huijzen C (1988) The human central nervous system: a synopsis and atlas. Springer, Berlin
Radovanovic S, Korotkov A, Ljubisavljevic M, Lyskov E, Thunberg J, Kataeva G, Danko S, Roudas M, Pakhomov S, Medvedev S, Johansson H (2002) Comparison of brain activity during different types of proprioceptive inputs: a positron emission tomography study. Exp Brain Res 144:276–285
Rao SM, Bandetinni PA, Binder JR, Bobholz JA, Hammeke TA, Stein EA, Hyde JS (1996) Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab 16:1250–1254
Roll JP, Vedel JP (1982) Kinesthetic role of muscle afferents in man studied by tendon vibration and microneurography. Exp Brain Res 47:177–190
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76:213–222
Romaiguère P, Anton JL, Roth M, Casini L, Roll JP (2003) Motor and parietal cortical areas both underlie kinaesthesia. Cog Brain Res 16:74–82
Rushworth MFS, Johansen-Berg H, Göbel SM, Devlin JT (2003) The left parietal and premotor cortices: motor attention and selection. NeuroImage 20:89–100
Schlaug G, Sanes JN, Thangaraj V, Edelman RR, Warach S (1996) Cerebral activation covaries with movement rate. Neuroreport 7:879–883
VanMeter JW, Maisog JM, Zeffiro TA, Hallett M, Herscovitch P, Rapoport SI (1995) Parametric analysis of functional neuroimages: application to variable-rate motor task. Neuroimage 2:273–283
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casini, L., Roll, JP. & Romaiguère, P. Relationship between the velocity of illusory hand movement and strength of MEG signals in human primary motor cortex and left angular gyrus. Exp Brain Res 186, 349–353 (2008). https://doi.org/10.1007/s00221-008-1325-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1325-9