Abstract
Tendon vibration is used extensively to assess the role of peripheral mechanoreceptors in motor control, specifically, the muscle spindles. Periodic tendon vibration is known to activate muscle spindles and induce a kinesthetic illusion that the vibrated muscle is longer than it actually is. Noisy tendon vibration has been used to assess the frequency characteristics of proprioceptive reflex pathways during standing; however, it is unknown if it induces the same kinesthetic illusions as periodic vibration. The purpose of the current study was to assess the effects of both periodic and noisy tendon vibration in a kinesthetic targeting task. Participants (N = 15) made wrist extension movements to a series of visual targets without vision of the limb, while their wrist flexors were either vibrated with periodic vibration (20, 40, 60, 80, and 100 Hz), or with noisy vibration which consisted of filtered white noise with power between ~ 20 and 100 Hz. Overall, our results indicate that both periodic and noisy vibration can induce robust targeting errors during a wrist targeting task. Specifically, the vibration resulted in an undershooting error when moving to the target. The findings from this study have important implications for the use of noisy tendon vibration to assess proprioceptive reflex pathways and should be considered when designing future studies using noisy vibration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Movement control requires the central nervous system (CNS) to have accurate information regarding the position of the limbs in both external space as well as in relation to each other. There are many receptors in the muscles, joints, and skin that could potentially signal the position of the limbs (Proske and Gandevia 2012). Muscle spindles, however, are thought to be the primary sensory receptors that contribute to our sense of position and movement of the limbs, commonly referred to as kinesthesia (Proske and Gandevia 2012, 2018). A common method to study the role of muscle spindles in kinesthesia is to artificially activate them with tendon vibration. Periodic vibration over a muscle tendon or belly will increase the firing rate of both the primary (Ia) and secondary (group II) muscle spindle afferents (Burke et al. 1976a, b). Additionally, if the vibrated muscle is contracting, the Ib afferents originating in the Golgi tendon organs may also respond to the vibration (Burke et al. 1976b; Fallon and Macefield 2007). When a muscle is vibrated a subpopulation of muscle spindles will become entrained to the stimulus, while others will become partially entrained or not respond at all (Burke et al. 1976a; Roll and Vedel 1982; Roll et al. 1989). Overall, with tendon vibration the population firing rate of the muscle spindles is increased and this increase is thought to induce kinesthetic illusions (Goodwin et al. 1972; Inglis and Frank 1990; Inglis et al. 1991; Cordo et al. 1995). Specifically, the CNS interprets the increase in afferent activity as the muscle being in a more lengthened position than it actually is (Proske and Gandevia 2012, 2018) and therefore, in a situation where someone is asked to move their limb to a target and the lengthening muscle is vibrated, the person will undershoot the target (Inglis and Frank 1990; Cordo et al. 1995). This is because the vibration has induced a kinesthetic error, as the person will feel like their limb is at the target, while it is actually short of the target by several degrees. One of the issues when examining the effects of tendon vibration, particularly in a dynamic task, is that tendon vibration induces changes in both the perceived position of the limb and the perceived velocity of the limb movement (Sittig et al. 1985). The position and velocity illusions also seem to depend on what the participant is focusing on. For example, when the task is focused on a position of the limb, there are clear changes in the perceived hand position, while when the task is focused on the velocity of the limb, there are clear changes in the perceived velocity of the limb movement (Sittig et al. 1985).
Tendon vibration also produces two other effects within the CNS that are important to consider when using tendon vibration to induce kinesthetic illusions. First, tendon vibration, and the subsequent increased Ia afferent feedback, produce a suppression of the excitability of the Ia afferent-α motoneuron synapse (Gail et al. 1966; Gillies et al. 1969; Eschelmuller et al. 2021). This change in gain of the Ia afferent mediated modulation of the α-motoneuron output, may disrupt a participant’s ability to properly complete a given motor task. For example, if a task invoked a stretch of the muscle, the muscle spindle mediated modulation of the α motoneuron would be blunted and therefore, may disrupt the normal corrective response. Second, if the vibration is long enough in duration a tonic vibration reflex (TVR) may be evoked (Gail et al. 1966; Eklund and Hagbarth 1966; Gillies et al. 1971). The TVR is typically seen as a slowly growing involuntary contraction of the vibrated muscle. Since the contraction is involuntary, it could invoke unwanted movement of the joint which would act in the opposite direction of the kinesthetic illusion. For example, in a matching task, when one limb is vibrated, and the participant is asked to match the position of the vibrated arm with the non-vibrated arm the participant may track the combination of the actual movement from the TVR and the perceived kinesthetic illusion (Goodwin et al. 1972). This makes it difficult to ascertain the real size of the kinesthetic illusion when a TVR develops. Both of these effects of tendon vibration should be considered when interpreting the tendon vibration evoked kinesthetic illusions, particularly if the measurement allows for movement of the vibrated limb.
Typically, periodic tendon vibration is used to activate the muscles spindles; however, a technique using noisy tendon vibration to assess the frequency characteristics of proprioceptive reflex pathways, found that noisy vibration during standing did not induce any noticeable changes in postural sway as reflected in the center of pressure (COP) movements (Mildren et al. 2017). During periodic vibration of the Achilles tendon during standing, there would be an illusion of forward sway and it would be expected that the participant would lean backwards to compensate (Hayashi et al. 1981; Kadri et al. 2020, 2023). This may indicate that the noisy tendon vibration does not induce the same kinesthetic illusion that periodic vibration does. Perhaps it is the periodic nature of tendon vibration that drives the kinesthetic illusions and not just the general increase in the population firing of the muscle spindle afferents. However, it is important to note that the kinesthetic effect of vibration was not the purpose of Mildren et al. (2017), and therefore, it is hard to make definitive conclusions on the kinesthetic effects of noisy tendon vibration. Therefore, the purpose of the current study was to investigate the effects of periodic and noisy tendon vibration in a kinesthetic targeting task. To test this, we investigated vibration-induced kinesthetic errors during a wrist targeting task, using both periodic and noisy tendon vibration. If it is the periodic nature of the tendon vibration that drives the kinesthetic error that the vibrated muscle is longer than it actually is, leading to an undershooting relative to the no-vibration condition, we would expect that the noisy vibration will not result in a kinesthetic bias. However, if the error is due to the general increase in muscle spindle afferent activity regardless of the nature of this input, then both periodic and noisy vibration will result in a kinesthetic error. It has been previously demonstrated that both periodic and noisy vibration induce similar amounts of suppression of the Ia afferent-α motoneuron synapse, indicating that this phenomenon at least is due to the general increase in muscle spindle input and not the periodic nature of that input (Eschelmuller et al. 2021).
Methods
Participants
15 participants (age: 21.1 ± 1.5; 8 female) free of any neurological or musculoskeletal disorders were recruited to participate in this study. Procedures were approved through the University of British Columbia behavioral research ethics board. Informed written consent was obtained from all participants. Participants were remunerated $10 for their participation.
Experimental setup
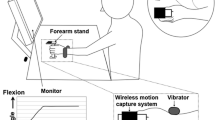
Participants sat in a height adjustable chair with their arm secured in an apparatus that allowed wrist flexion and extension but prevented other movements of the forearm and wrist (Fig. 1). The hand was secured to a board, which held the hand stable during the trials, and their wrist was aligned with the manipulandum’s axis of rotation. A potentiometer (Vishay Spectrol 157) was fitted inside the manipulandum to measure wrist angle. A load cell (Phidget, Button Load Cell) was fitted at the end of a linear motor (Ling Dynamics V203 Vibrator, Ling Electronics) which was used to deliver the vibration and was pushed into the participants flexor carpi radialis approximately 3–4 cm proximal to the wrist (Fig. 1). The linear motor was pushed against the tendon so that it was secure and provided constant contact but provided no discomfort for the participants as their wrist moved through the full range of motion. This resulted in a preload force of ~ 12 N. Force was monitored throughout the experiment to ensure it did not change between trials. Participants’ vision of the hand was obscured by a black poster board, which had three LED targets embedded into it.
Experimental procedures
Before any testing began, participants were given an opportunity to feel all the vibrations and move their wrist through the full range of motion as much as needed. Next participants were given a practice session to move to each of the targets (without feedback on accuracy) and were asked if they felt confident that they could line their hand up with each of the three targets. This was followed by the experimental block, which contained a total of 210 trials. Trials were broken up into 10 blocks, with each block containing each vibration—target pair. There were three targets, and seven vibration conditions, including a no-vibration, five periodic vibrations (20, 40, 60, 80, 100 Hz), and one noisy vibration (filtered white noise with power between ~ 20 and 100 Hz). The linear motor was programmed such that the standard deviation of the force exerted was constant across vibration conditions, which was determined when the motor was applied to a slightly cushioned surface. The order of the vibration-target pairs was randomized within each block. Participants’ start position was purposely varied by ± 2°–3° to avoid participants memorizing the movement distance to the target. The target distances were approximately 45°, 50°, and 55° from the start position. Each trial began with the three LED targets flashing simultaneously for 50 ms and the vibration turning on (when present), followed 500 ms later by one target turning on and staying on for 1.5 s. Participants were instructed that when the single target turned on, to make a smooth wrist extension movement to the target at ~ 40°–50°/s. Once they finished the movement, they were asked to leave their hand where it was, and an experimenter would move their hand back to the start position (flexed position). To reduce the effects of thixotropy and to remove any distance cues to the participant, the hand was moved through flexion and extension randomly multiple times before being brought back to the start position. Participants were informed that they could take a break at any time.
Analysis
To investigate the effects of the tendon vibration on the targeting task, the error score, calculated as the difference between the end point and the target, was calculated for each condition separately. This will be referred to as target error. The end position of each movement was calculated as the position when the velocity dropped and remained below 2°/s. As the purpose of the multiple targets was simply to reduce the possibility that participants would memorize the target distance, and not to examine effects of movement distance, all scores were collapsed across targets. Variable error was calculated as the standard deviation in target error. To estimate whether there was a vibration-induced kinesthetic illusion, the difference in target error between the vibration and no-vibration was calculated. This error will be referred to as vibration error. To examine if vibration affected participants’ movement speed, the mean velocity during the middle 50% of the movement was analyzed and compared between conditions. To check if there were any differences in force applied to the tendon between the programmed vibration signals, the standard deviations from each vibration condition were compared. We only investigated the time from vibration onset to movement onset (~ 500–700 ms) to avoid contamination of movement related changes in force against the tendon.
Statistical analysis
To determine if there was an effect of tendon vibration on target error, variable error, and movement speed, separate one-way repeated measures analysis of variance (rm-ANOVA) were run. When significant, the rm-ANOVA was followed up with Bonferroni corrected t-tests to determine how each vibration condition was different from the no-vibration condition. To determine if there were differences in the force applied to the tendon during the vibration, the standard deviation scores from the load cell were analyzed using a one-way rm-ANOVA. Statistical significance was set at p < 0.05 for all measurements and a Greenhouse–Geisser adjustment was applied when sphericity was violated. Uncorrected degrees of freedom are reported in the text.
Results
Overall, participants tended to undershoot the target in the no-vibration condition by ~ 5.4 degrees, which served as our baseline measurement of error. When examining the end point positions across conditions our results indicate that there was a statistically significant difference in the target error between the vibration conditions [F(6, 84) = 83.094, p < 0.001, \({\eta }_{\mathrm{p}}^{2}\) = 0.856] (Fig. 2). There was no effect of vibration condition on the variable error [F(6, 84) = 0.656, p = 0.685, \({\eta }_{\mathrm{p}}^{2}\) = 0.045] (Fig. 3). To decompose our end point position ANOVA, we compared each vibration condition to the no-vibration condition. First, to answer our primary question, our results indicate that the noisy vibration induced a vibration error of ~ 4.5° (p < 0.001). When examining the periodic vibration conditions, we found that all vibration conditions produced vibration errors (Table 1). Specifically, the 20 Hz vibration induced an average vibration error of ~ 1.5° (p = 0.012), the 60 and 80 Hz vibration resulted in a vibration error of ~ 7° (p < 0.001), and the 40 and 100 Hz produced vibration errors of ~ 6° (p < 0.001). Vibration additionally caused a statistically significant change in movement speed [F(6, 84) = 9.871, p < 0.01, \({\eta }_{\mathrm{p}}^{2}\) = 0.414], with each vibration condition having a significantly slower movement velocity compared to the no-vibration condition (p < 0.01), except for the 20 Hz condition (p = 0.054).To verify that our vibration was not producing different forces across conditions, as this could affect the size of the kinesthetic error, we analyzed the standard deviation of the force applied to the tendon. Our results indicate that there were no significant differences in the applied force between vibration conditions [F(5, 70) = 0.688, p = 0.503, \({\eta }_{\mathrm{p}}^{2}\) = 0.047], with a mean standard deviation of ~ 0.5 N (min: 0.478, max: 0.511). Table 1 provides a summary of our results.
Mean difference score between the vibration and no-vibration conditions. Negative values indicate an undershooting in the vibration condition relative to the no-vibration condition. Centre line in box plot indicates median value, and edges of box are the 25th and 75th quartile limits. Whiskers are the max and minimum values that do not contain an outlier. Outliers were calculated as values greater or less than 1.5 × interquartile range. Plot and calculations generated using the boxchart function in MATLAB 2022b
Variable error for all conditions. Centre line in box plot indicates median value, and edges of box are the 25th and 75th quartile limits. Whiskers are the max and minimum values that do not contain an outlier. Outliers were calculated as values greater or less than 1.5 × interquartile range. Plot and calculations generated using the boxchart function in MATLAB 2022b
Discussion
The main findings from this experiment were that both periodic and noisy vibration induced a vibration error during a wrist extension task with no effect on end point variability. This would indicate that it is not the periodic nature of the tendon vibration that induces the kinesthetic illusion but is instead the general increase in afferent input that is produced. That is, both periodic and noisy tendon vibration are likely biasing a subpopulation of muscle spindles to a higher firing rate than what would be expected in this task, and therefore, the population of muscle spindles are signaling to the CNS that the muscle is longer than it actually is. This is in contrast with previous work that suggested that there are no changes in COP movements when using a noisy tendon vibration stimulus to assess the frequency characteristics of proprioceptive reflexes (Mildren et al. 2017). Previous work has demonstrated that during standing, periodic vibration of the Achilles tendon produces robust postural responses as the participant will lean backwards in response to the vibration-induced illusion that the triceps surae are being lengthened (Hayashi et al. 1981; Kadri et al. 2020, 2023). It is important to note that in the previous study by Mildren et al. (2017), the vibration was only applied to one leg and the participants were standing with eyes open, which could explain the lack of vibratory illusion. Visual feedback is known to modulate the size of the vibration-induced kinesthetic illusion (Lackner and Taublieb 1984; Hagura et al. 2007; Seizova-Cajic and Azzi 2011; Proske and Gandevia 2018). For example, when there is a conflict between the kinesthetic signal from the vibrated arm and the visual feedback, the size of the resulting illusion is attenuated, but not abolished completely (Izumizaki et al. 2010; Tsuge et al. 2012). Interestingly, if the visual feedback is in line with the expected direction of illusion, the size of the vibratory illusion is increased (Tsuge et al. 2012). In the previous work of Mildren et al. (2017) the participants were maintaining standing balance, which requires the integration of multiple sensory signals (Peterka 2002; Maurer et al. 2005), and it is thought that sensory signals are integrated in a statistically optimal way to minimize the final state estimate’s variance (Ernst and Banks 2002). Specifically, the weights for the sensory estimates are based on their normalized reciprocal variances, and therefore, a signal with higher levels of noise will have a lower weight in the final estimate (Beers et al. 1999; Ernst and Banks 2002). For example, during standing when there is conflict between the visual and proprioceptive input, the proprioceptive input is down weighted, which is thought to be because the proprioceptive signal is less reliable and therefore is weighted less than the visual signal (Kabbaligere et al. 2017). Perhaps in the case of the Mildren et al. (2017) standing experiment the normal visual feedback, combined with normal vestibular and proprioceptive feedback from the non-vibrated limb was enough to abolish the potential illusory effects of the vibration through a down-weighting process of the proprioceptive feedback from the vibrated limb. Future studies using the technique should investigate the integration of vision and vibration during standing to fully understand these interactions. Another important distinction between the findings of Mildren et al. (2017) is that this was a continuous task in which the goal was to maintain standing balance, which likely relies primarily on feedback mechanisms, while in the current study participants were making discrete reaches to a target which would rely on both feedback and feedforward mechanisms. Regardless, in the current study, when no visual feedback was present, noisy tendon vibration induced robust kinesthetic illusions during the wrist targeting task. Our findings in the periodic conditions are in line with previous literature investigating the effects of periodic tendon vibration in a kinesthetic targeting task (Inglis and Frank 1990; Cordo et al. 1995).
The size of the vibratory illusion is dependent on how much the vibration biases the muscle spindle population. The population response is a combination of the reafferent muscle spindle feedback due to the movement and the exafferent vibration-induced muscle spindle feedback. As increased vibration force would presumably entrain more muscle spindles to the vibration stimulus, resulting in a larger illusion, it would be interesting to examine if the illusion generated with noisy vibration scales with vibration force. Additionally, it is known that the size of the illusion also depends on the speed of movement, with the typical result that the size of the illusion decreases with increasing speed (Cordo et al. 1995). Due to the faster movement, the difference between expected feedback and the actual feedback is smaller, and therefore, a smaller illusion is produced. This is because the actual feedback is more biased by the reafferent movement-related feedback compared to the exafferent vibration-induced feedback, and therefore, there is less final error. Future studies should systematically vary the force of vibration and speed of movement with both periodic and noisy vibration to fully understand if there are any major differences.
We additionally found that all vibration conditions except for the 20 Hz condition elicited a change in velocity. Specifically, participants tended to move slower during tendon vibration compared to the no-vibration condition, as during the vibration they may have perceived that their limb was moving faster than it actually was. This finding is not surprising, as velocity illusions associated with tendon vibration have been demonstrated previously (Sittig et al. 1985, 1987). Our finding that the 20 Hz vibration produced an undershooting relative to the no-vibration condition without any change in velocity is in line with previous work suggesting that 20 Hz vibration can be used to induce positional errors without velocity errors (McCloskey 1973). The physiological explanation of this finding is that higher frequency vibrations preferentially activate the muscle spindle primary endings, which are thought to signal muscle movement and position, while lower frequency vibration (e.g., 20 Hz) will also strongly activate secondary endings which primarily signal muscle position (McCloskey 1973; Burke et al. 1976a; Proske and Gandevia 2018). Therefore, in the current study it is likely that the 20 Hz vibration strongly activated the secondary endings resulting in a position illusion without the associated movement illusion.
It is also important to consider how the other consequences of tendon vibration may have influenced our results, most importantly the suppression of the Ia afferent-α motoneuron synapse and the generation of a TVR. In our task, the movements were relatively slow and did not require any rapid corrections. Therefore, the stretch response in the wrist flexor muscles was likely not critical to completing the task, so the change in gain of this response with tendon vibration likely did not influence our results. The TVR could have generated a contraction of the wrist flexor muscles during the movement, which would have resisted the extension effort the participants were making and could have biased our end point in the same direction as the kinesthetic illusion (undershooting the target). However, typically, the TVR takes multiple seconds to build up (Gail et al. 1966; Eklund and Hagbarth 1966; Arcangel et al. 1971), while in our study, the trial only lasted between 1 and 2 s, which likely did not give enough time for a sufficient TVR to accumulate. One limitation of the current study is that we did not record electromyography of the wrist flexors, and therefore, cannot determine whether a TVR developed or not. To definitively answer this question, a different protocol could be used that can measure a TVR if it develops, such as the arm matching task used in Goodwin et al. (1972).
With the growing use of noisy tendon vibration as a method to assess proprioceptive reflex pathways (Mildren et al. 2017, 2019, 2020, 2021; Eschelmuller et al. 2020; Hodgson et al. 2023), it is critical to ensure we understand the differences and similarities between noisy and periodic vibration. It has already been demonstrated that both noisy and periodic vibration suppress the monosynaptic Ia afferent-α motoneuron synapse (Eschelmuller et al. 2021). The results from the current study have clearly shown that both noisy and periodic vibration can also disrupt kinesthesia. Specifically, both periodic and noisy vibrations induce the illusion that the vibrated muscle is longer than it actually is and leads participants to undershoot relative to their no-vibration controls. These findings should be taken into consideration when designing studies using noisy tendon vibration to assess proprioceptive reflex pathways. It is still unclear if noisy and periodic vibration-induced illusions show the same modulation with changes in movement speed and force exerted by the vibrator. Therefore, future work is needed to fully understand the effects of periodic and noisy vibration on kinesthesia.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Arcangel CS, Johnston R, Bishop B (1971) The Achilles tendon reflex and the H-response during and after tendon vibration. Phys Ther 51:889–905. https://doi.org/10.1093/ptj/51.8.889
Burke D, Hagbarth KE, Löfstedt L, Wallin BG (1976a) The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiology 261:673–693. https://doi.org/10.1113/jphysiol.1976.sp011580
Burke D, Hagbarth KE, Löfstedt L, Wallin BG (1976b) The responses of human muscle spindle endings to vibration during isometric contraction. J Physiology 261:695–711. https://doi.org/10.1113/jphysiol.1976.sp011581
Cordo P, Gurfinkel VS, Bevan L, Kerr GK (1995) Proprioceptive consequences of tendon vibration during movement. J Neurophysiol 74:1675–1688. https://doi.org/10.1152/jn.1995.74.4.1675
Eklund G, Hagbarth KE (1966) Normal variability of tonic vibration reflexes in man. Exp Neurol 16:80–92. https://doi.org/10.1016/0014-4886(66)90088-4
Ernst MO, Banks MS (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415:429–433. https://doi.org/10.1038/415429a
Eschelmuller G, Mildren RL, Blouin J-S et al (2020) Frequency characteristics of heteronymous responses evoked by Achilles tendon vibration during quiet stance. Neurosci Lett 736:135290. https://doi.org/10.1016/j.neulet.2020.135290
Eschelmuller G, Chua R, Carpenter MG, Inglis JT (2021) The acute effects of periodic and noisy tendon vibration on wrist muscle stretch responses. Neurosci Lett 764:136279. https://doi.org/10.1016/j.neulet.2021.136279
Fallon JB, Macefield VG (2007) Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve 36:21–29. https://doi.org/10.1002/mus.20796
Gail PD, Lance JW, Neilson PD (1966) Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry 29:1–11. https://doi.org/10.1136/jnnp.29.1.1
Gillies JD, Lance JW, Neilson PD, Tassinari CA (1969) Presynaptic inhibition of the monosynaptic reflex by vibration. J Physiology 205:329–339. https://doi.org/10.1113/jphysiol.1969.sp008968
Gillies JD, Burke DJ, Lance JW (1971) Supraspinal control of tonic vibration reflex. J Neurophysiol 34:302–309. https://doi.org/10.1152/jn.1971.34.2.302
Goodwin GM, McCloskey DI, Matthews PBC (1972) Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science 175:1382–1384. https://doi.org/10.1126/science.175.4028.1382
Hagura N, Takei T, Hirose S et al (2007) Activity in the posterior parietal cortex mediates visual dominance over kinesthesia. J Neurosci 27:7047–7053. https://doi.org/10.1523/jneurosci.0970-07.2007
Hayashi R, Miyake A, Jijiwa H, Watanabe S (1981) Postural readjustment to body sway induced by vibration in man. Exp Brain Res 43:217–225. https://doi.org/10.1007/bf00237767
Hodgson DD, King JA, Darici O et al (2023) Visual feedback-dependent modulation of arousal, postural control, and muscle stretch reflexes assessed in real and virtual environments. Front Hum Neurosci 17:1128548. https://doi.org/10.3389/fnhum.2023.1128548
Inglis JT, Frank JS (1990) The effect of agonist/antagonist muscle vibration on human position sense. Exp Brain Res 81:573–580. https://doi.org/10.1007/bf02423506
Inglis JT, Frank JS, Inglis B (1991) The effect of muscle vibration on human position sense during movements controlled by lengthening muscle contraction. Exp Brain Res 84:631–634. https://doi.org/10.1007/bf00230975
Izumizaki M, Tsuge M, Akai L et al (2010) The illusion of changed position and movement from vibrating one arm is altered by vision or movement of the other arm. J Physiol 588:2789–2800. https://doi.org/10.1113/jphysiol.2010.192336
Kabbaligere R, Lee B-C, Layne CS (2017) Balancing sensory inputs: Sensory reweighting of ankle proprioception and vision during a bipedal posture task. Gait Posture 52:244–250. https://doi.org/10.1016/j.gaitpost.2016.12.009
Kadri MA, Chevalier G, Mecheri H et al (2020) Time course and variability of tendinous vibration-induced postural reactions in forward and backward directions. J Electromyogr Kinesiol 51:102386. https://doi.org/10.1016/j.jelekin.2020.102386
Kadri MA, Bouchard E, Lauzier L et al (2023) Distinctive phases and variability of vibration-induced postural reactions highlighted by center of pressure analysis. PLoS ONE 18:e0280835. https://doi.org/10.1371/journal.pone.0280835
Lackner JR, Taublieb AB (1984) Influence of vision on vibration-induced illusions of limb movement. Exp Neurol 85:97–106. https://doi.org/10.1016/0014-4886(84)90164-x
Maurer C, Mergner T, Peterka RJ (2005) Multisensory control of human upright stance. Exp Brain Res 171:231. https://doi.org/10.1007/s00221-005-0256-y
McCloskey DI (1973) Differences between the senses of movement and position shown by the effects of loading and vibration of muscles in man. Brain Res 61:119–131. https://doi.org/10.1016/0006-8993(73)90521-0
Mildren RL, Peters RM, Hill AJ et al (2017) Frequency characteristics of human muscle and cortical responses evoked by noisy Achilles tendon vibration. J Appl Physiol 122:1134–1144. https://doi.org/10.1152/japplphysiol.00908.2016
Mildren RL, Peters RM, Carpenter MG et al (2019) Soleus single motor units show stronger coherence with Achilles tendon vibration across a broad bandwidth relative to medial gastrocnemius units while standing. J Neurophysiol 122:2119–2129. https://doi.org/10.1152/jn.00352.2019
Mildren RL, Schmidt ME, Eschelmuller G et al (2020) Influence of age on the frequency characteristics of the soleus muscle response to Achilles tendon vibration during standing. J Physiology 598:5231–5243. https://doi.org/10.1113/jp280324
Mildren RL, Peters RM, Carpenter MG et al (2021) Soleus responses to Achilles tendon stimuli are suppressed by heel and enhanced by metatarsal cutaneous stimuli during standing. J Physiol 599:3611–3625. https://doi.org/10.1113/jp281744
Peterka RJ (2002) Sensorimotor integration in human postural control. J Neurophysiol 88:1097–1118. https://doi.org/10.1152/jn.2002.88.3.1097
Proske U, Gandevia SC (2012) The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev 92:1651–1697. https://doi.org/10.1152/physrev.00048.2011
Proske U, Gandevia SC (2018) Kinesthetic senses. Compr Physiol 8:1157–1183. https://doi.org/10.1002/cphy.c170036
Roll JP, Vedel JP (1982) Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47:177–190. https://doi.org/10.1007/bf00239377
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76:213–222. https://doi.org/10.1007/bf00253639
Seizova-Cajic T, Azzi R (2011) Conflict with vision diminishes proprioceptive adaptation to muscle vibration. Exp Brain Res 211:169–175. https://doi.org/10.1007/s00221-011-2663-6
Sittig AC, van der Gon JJD, Gielen CCAM (1985) Separate control of arm position and velocity demonstrated by vibration of muscle tendon in man. Exp Brain Res 60:445–453. https://doi.org/10.1007/bf00236930
Sittig AC, van der Gon Denier JJ, Gielen CCAM (1987) The contribution of afferent information on position and velocity to the control of slow and fast human forearm movements. Exp Brain Res 67:33–40. https://doi.org/10.1007/bf00269450
Tsuge M, Izumizaki M, Kigawa K et al (2012) Interaction between vibration-evoked proprioceptive illusions and mirror-evoked visual illusions in an arm-matching task. Exp Brain Res 223:541–551. https://doi.org/10.1007/s00221-012-3281-7
van Beers RJ, Sittig AC, van der Gon JJD (1999) Integration of proprioceptive and visual position-information: an experimentally supported model. J Neurophysiol 81:1355–1364. https://doi.org/10.1152/jn.1999.81.3.1355
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Sciences and Engineering Research Council to JTI [Grant number: 2017-04504] and RC [Grant number: 2019-04513] and a graduate research award to GE.
Author information
Authors and Affiliations
Contributions
GE: conceptualization, methodology, formal analysis, investigation, visualization, and writing—original draft. AS: investigation, writing—review and editing. BG: investigation, writing—review and editing. RC: conceptualization, methodology, writing—review and editing, supervision, and funding acquisition. JTI: conceptualization, methodology, writing—review and editing, supervision, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
All procedures used in this study were approved by the behaviour research ethics board at the University of British Columbia. Freely given informed written consent was obtained from all participants prior to participation in any study procedures.
Consent for publication
All participants consented for their de-identified data to be published.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eschelmuller, G., Szarka, A., Gandossi, B. et al. The effects of periodic and noisy tendon vibration on a kinesthetic targeting task. Exp Brain Res 242, 59–66 (2024). https://doi.org/10.1007/s00221-023-06727-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-023-06727-1