Abstract
During a simple reaction time (RT) task, movements can be initiated early and involuntarily through presentation of a loud startling acoustic stimulus (SAS), a phenomenon termed the StartReact effect. In order to infer that activity in startle-related structures led to the early response triggering, it is important to observe a concurrent startle reflex in sternocleidomastoid. It is generally accepted that to consistently elicit a startle reflex, the SAS must be both intense and unpredictable. However, it remains unclear what effect explicit foreknowledge of an impending SAS has on the effectiveness of a SAS to elicit a startle reflex when preparing a motor response. To test this, participants completed two separate blocks of a simple RT task (counterbalanced order), where the control auditory go-signal was replaced with a SAS on 20 % of trials. In an unwarned block, knowledge of the trial type (SAS vs. control) was not provided in advance, while in a warned block, the trial type was forewarned. Results revealed that while foreknowledge of an impending SAS reduced the magnitude of the startle reflex, it did not affect the proportion of startle reflexes elicited or the magnitude of the StartReact effect. An increase in control trial RT was observed during the unwarned block, but only when it was performed first. These results indicate that preparation of a motor response leads to sufficiently increased activation in startle-related neural structures such that even with explicit knowledge of an upcoming SAS, participants are unable to proactively gate the upcoming sensory input.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many real-life activities, upcoming actions can be prepared in advance if the required action to be performed is known beforehand. For example, when a sprinter is waiting in the ready position for the starter’s pistol to fire, or when one is expecting a traffic signal to change, the response can prepared or “programmed” (Summers and Anson 2009) in advance of the go-signal leading to a shorter reaction time (RT). Several seminal studies have used instructed-delay simple RT tasks to show that preparation of a known action could be completed in advance of the imperative go-signal (Donders 1969; Klapp et al. 1974; Wadman et al. 1979). Some more recent studies have also provided electrophysiological evidence for movement-related preparatory activity. For example, electroencephalography (EEG) has been used to show neural activity build-up occurs in motor cortical areas in advance of the go-signal during both self-initiated (Shibasaki and Hallett 2006) as well as instructed-delay paradigms (Leuthold et al. 2004). Additionally, TMS has been used to show that motor preparatory activity increases early following a warning signal and is held relatively consistent until the onset of the go-signal (Kennefick et al. 2014). Recent data suggest that the initiation of a response is a separate neural process and in some cases may act independently of response preparation as it was shown that response initiation could be forced even when the response was not fully prepared (Haith et al. 2016). In this way, initiation has traditionally been modelled as a sensory evidence accumulator (Carpenter and Williams 1995). In contrast, urgency-gating models (Cisek et al. 2009) suggest that sensory signal combined with temporally-dependent urgency signal results in a given state of the initiation-related activation. Indeed, it has been argued that the total level of neural activity may also be dependent on the underlying preparatory state of the system (Weinberg 2016). However, in most simple RT tasks where the response is prepared in advance, it is difficult to distinguish between these possibilities; but importantly, further increases in motor cortical activity are associated with the process of initiating and executing a prepared response (Kennefick et al. 2014; Maslovat et al. 2015a).
Several studies over the past two decades have shown that preplanned movements that are ready for initiation or execution can be involuntarily triggered through presentation of a loud acoustic stimulus that is also capable of eliciting a classical startle reflex (Carlsen et al. 2004b, 2011; Valls-Solé et al. 1995, 1999). Specifically, in simple RT tasks it has been shown that presenting a startling acoustic stimulus (SAS) results in the early and involuntary initiation of the planned response (Carlsen et al. 2004b; Marinovic and Tresilian 2016; Valls-Solé et al. 1999), a phenomenon that has been termed the “StartReact” effect. However, the neural mechanism and pathways underlying this effect are currently a matter of debate (Carlsen et al. 2012; Marinovic and Tresilian 2016; Nonnekes et al. 2014). For example, Marinovic and Tresilian (2016) suggested that the RT speeding seen in StartReact responses simply represents a particularly strong instance of the well-known impact of a more intense stimulus on RT (Kohfeld 1971; Luce 1986) and involves the same neural pathways used for volitional responses. Alternatively, others have argued that due to the substantially faster reactions seen following a SAS (typically <80 ms), coupled with the similar latency of these responses to that of the startle reflex itself, that StartReact responses may represent the involuntary triggering of a motor plan that is stored subcortically in structures also associated with the startle reflex—such as reticular formation (Carlsen et al. 2004a; Nonnekes et al. 2014; Valls-Solé et al. 1999). Finally, some studies have indicated that StartReact responses are due to the early release of a cortically stored motor plan, triggered via ascending brainstem activation associated with the startle reflex, bypassing the normal volitional cortical response initiation pathway (Alibiglou and MacKinnon 2012; Carlsen et al. 2012). In the two latter cases, because the response in inextricably associated with activity in centres associated with the startle reflex, it has been argued that startle reflex-related activity should be observed in order to infer this alternate pathway (Carlsen et al. 2007; Carlsen 2015). Irrespective of the putative neural mechanism involved, what does appear consistent across experiments is that responses are only involuntarily triggered by the SAS if they are sufficiently prepared (Carlsen and MacKinnon 2010), allowing the StartReact effect to be used as a behavioural index of response preparation. Consequently, a SAS has been used to assess motor preparatory circuits in a variety of tasks and populations, from targeted upper limb movements and saccades in healthy participants (Carlsen et al. 2004b; Castellote et al. 2004), to gait initiation and limb movement in motor disordered populations such as patients with Parkinson’s disease (Alibiglou et al. 2012; Carlsen et al. 2013) and hereditary spastic paraplegia (Nonnekes et al. 2014).
As noted above, due to its similar short latency, the StartReact effect is typically associated with the appearance of an overt startle reflex. Although several studies have been published showing no RT differences between SAS trials with or without an overt startle reflex (e.g., MacKinnon et al. 2007; Marinovic et al. 2014; Maslovat et al. 2012; Nonnekes et al. 2014; Reynolds and Day 2007; Valls-Solé et al. 2005), these null effects should be carefully considered, particularly if a brainstem-mediated response triggering mechanism is being inferred. In order to infer strong activity in brainstem startle-related structures (i.e., pontomedullary reticular formation), the startle reflex is typically evidenced by a short latency burst of activity in sternocleidomastoid (SCM) (Brown et al. 1991). Indeed, several studies have shown that faster RTs are observed in the presence of an overt classical startle reflex in SCM compared high intensity SAS trials where no startle response is evoked (Carlsen et al. 2007; Carlsen 2015; Honeycutt and Perreault 2012; Maslovat et al. 2015b; Tresch et al. 2014). Requiring the elicitation of a startle reflex in order to imply a StartReact response limits it use in certain situations, as the startle reflex has been shown to habituate in participants after as few as 2–5 repeated presentations of the unexpected and intense stimulus when sitting relaxed in a chair (Brown et al. 1991). However, this habituation appears to be greatly attenuated if the participant is actively preparing a voluntary motor response. This attenuation is believed to be the result of increased excitability in both the cortical and subcortical parts of the response pathway (Carlsen et al. 2003; Siegmund et al. 2001; Valls-Solé et al. 1997).
It is generally accepted that in order to reliably elicit a startle reflex in most individuals, the startling stimulus must be unpredictable, in addition to being sufficiently intense (Brown et al. 1991; Landis et al. 1939). Therefore, to ensure the highest possible proportion of startle trials where a startle reflex is observed, most studies only present the SAS on a subset (e.g., 20 %) of total trials (Carlsen et al. 2011). SAS trials are presented randomly, and thus unpredictably (Carlsen et al. 2011), which is assumed to increase the probability of eliciting a startle reflex. However, it remains unclear what effect explicit foreknowledge of an impending SAS has on the effectiveness of a SAS to elicit a startle reflex, particularly when the participant is engaged in a motor task. Modulation of the startle reflex has been shown when a SAS is preceded by an antecedent prepulse stimulus at short latency (30–500 ms), resulting in an absence or minimization of the startle reflex (Abel et al. 1998; Acocella and Blumenthal 1990; Blumenthal 1996; Ison and Hammond 1971). This “short latency prepulse inhibition” of the startle reflex is thought to be the result of involuntary sensory gating of the startle stimulus caused by the preceding auditory stimulus (Abel et al. 1998). Of interest was whether a visual warning stimulus that provides explicit foreknowledge of an impending SAS might lead to similar gating of the startle stimulus when participants were engaged in a RT task. Therefore, the purpose of the current study was to investigate whether providing a warning (i.e., foreknowledge) of an upcoming SAS would affect the proportion and magnitude of startle reflex responses elicited, and whether this warning would result in RT differences in control and/or startle trials. It was hypothesized that when given explicit foreknowledge of stimulus type (i.e., control or SAS), the participant may be able to proactively gate the incoming stimulus, leading to a diminished startle reflex and reduced StartReact effect.
Methods
Participants
Twenty-one volunteers (13M/8F; mean age = 25.0 years, SD = 8.2) with no known sensory or motor dysfunctions participated in the study after being fully briefed and signing an informed consent. All participants self-reported to be right handed or ambidextrous. As the primary research question related to the proportion of startle responses observed between two conditions for the same participants, a power calculation was carried out using G*Power (ver. 3.1.9.2) for two dependent means. Previous studies have indicated that the mean probability of observing a startle reflex is typically >.7 (SD ~ .1) for unwarned startle stimuli in simple RT tasks (Carlsen and MacKinnon 2010; Carlsen et al. 2011); thus, the power calculation showed that in order to detect a difference in startle reflex probability of greater than .1 between conditions, a sample size of 10 participants would be required. Furthermore, it was expected that data from approximately 20 % of participants would be discarded due to an unreliable SCM startle reflex (Brown et al. 1991). Data from five participants were discarded due to an unreliable startle reflex in response to a SAS (see Data reduction section); thus, the primary data set presented here is from 16 participants (9M/7F; mean age = 24.9 years, SD = 7.2) which provides adequate statistical power. This research was approved by the Research Ethics Board at the University of Ottawa and conformed to the latest revision of the Declaration of Helsinki.
Apparatus and task
Participants sat in a height adjustable chair with the right shoulder abducted and flexed ~30 deg. The right forearm rested parallel to the floor in a custom-made aluminium manipulandum that fixed the forearm in place while enabling free flexion/extension movement of the wrist in the horizontal plane. The arm was secured in a semi-prone position using Velcro straps, with the palm facing inward. A 24″ computer monitor was placed at eye level 1.5 m in front of the participant. Participants performed a simple RT task requiring a rapid 20 deg extension of the right wrist from the neutral home position (wrist neither flexed nor extended) in response to an auditory go-signal.
Procedure
Participants performed 20 familiarization RT trials, followed by two blocks (counterbalanced order) of testing RT trials. In one block of trials, the trial type (SAS vs. control) was not indicated by the precue (unwarned), while in the other block, the trial type was provided via the precue (warned). During the unwarned block, the precue consisted of the words “Get Ready” presented on the computer screen, while during the warned block, either “Get Ready for Control” or “Get Ready for Startle” was presented depending on upcoming trial type. The precue was shown for 1 s, followed by a variable foreperiod (2000–2500 ms) and then presentation of the imperative go-signal. In control trials, the imperative go-signal was a “beep” sound (82 dB, 1000 Hz, 40 ms), whereas in SAS trials, the go-signal was a broadband noise pulse (120 dB, white noise, 25 ms). Both stimulus types were generated using digital to analog hardware (PCIe-6321, National Instruments Inc.), amplified, and presented via a loudspeaker (M54-H, MG Electronics Inc.) placed 30 cm directly behind the participant’s head. Stimulus intensity was confirmed using a data logging sound level meter (Cirrus Research Optimus, CR: 162C, A-weighted, impulse setting) which was placed alongside a Styrofoam head form at a location corresponding to the position of the right ear of the participant during testing.
In the unwarned block, participants performed 50 RT trials consisting of 40 control trials and 10 SAS trials. In the warned block, 75 trials were completed consisting of 60 control trials and 12 SAS trials, as well as 3 trials where a SAS was warned but the control go-signal was presented (invalid warning). These trials were included to assess whether simply providing a warning of an upcoming SAS would have an impact on RT. In both blocks, the SAS trials occurred pseudorandomly such that no two consecutive trials included a SAS and a SAS did not occur in the first three trials of a block. Participants were instructed to make a 20 deg wrist extension movement as quickly and accurately as possible following the presentation of the acoustic stimulus. Following a 2 s response window, feedback providing RT as well as movement accuracy with respect to the target was presented on the computer screen for 3.5 s. A points system was used whereby points were awarded for achieving a displacement RT faster than 140 ms on any given trial; a running total was provided along with RT and accuracy feedback on each trial. These points had no monetary value and were simply used to increase participant motivation and to encourage fast RTs.
Recording equipment
Surface EMG was collected from the muscle bellies of the right extensor carpi radialis (ECR) and the flexor carpi radialis longus (FCR) as well as from the left sternocleidomastoid (SCM, to indicate the presence of a startle reflex) using bipolar preamplified electrodes (DE-2.1, Delsys Inc.) connected to an external amplifier (Delsys Bagnoli 8). Prior to electrode attachment, skin sites were lightly cleaned using an abrasive gel (Nuprep) and alcohol wipes. EMG Electrodes were oriented parallel to the length of the muscle fibres and attached to the skin surface using double-sided adhesive tape. A reference electrode (Dermatrode HE-R) was placed over the right lateral epicondyle. Wrist position was monitored using a linear potentiometer, powered by a low-noise 5 VDC source, attached to the pivot of the manipulandum where the voltage change corresponded to 0.0246 V/deg providing an angular resolution of .01 deg. Raw band-passed (20–450 Hz) EMG as well as raw position data was sampled at 4000 Hz for 3 s using an analog to digital converter (PCIe-6321 via BNC-2090A, National Instruments Inc.). Data collection was initiated 1 s prior to the go-signal in each trial using a custom LabVIEW program (National Instruments, Austin, TX) and stored for offline analysis.
Data reduction
Kinematic variables were calculated for displacement onset, peak velocity, peak displacement, time to peak displacement, and final position. Onset of angular displacement was determined as the first time point at which displacement changed more than 0.2 deg following the go-signal. Peak velocity was the maximum angular velocity achieved prior to reaching peak displacement. Peak displacement was the maximum angular displacement observed between movement onset and movement final position, and time to peak displacement was the time between displacement onset and this point. The final position of the movement was defined as the first point at which angular velocity remained below 8 deg/s for at least 150 ms.
EMG variables included time of EMG burst onsets in each muscle measured, as well as integrated EMG amplitudes. EMG signals were rectified and filtered using a 25 Hz low-pass elliptic filter and displayed on a computer monitor using a customized LabVIEW program. A custom computer program placed markers indicating EMG burst onsets, positioned at the first point at which filtered EMG activity first reached a value two standard deviations above baseline (calculated as the mean of 100 ms of EMG activity preceding the go-signal). EMG offset markers were positioned where EMG activity first fell below 80 % of peak activity and remained below this value for at least 25 ms. Activity between EMG onset and offset was defined as a distinct burst. EMG markers were visually inspected and manually adjusted if necessary to allow for correction of errors due to the strictness of the computer algorithm (Hodges and Bui 1996). Premotor RT was defined as the time between the go-signal and EMG onset in the wrist extensor. Integrated EMG (iEMG) was used to quantify EMG burst magnitude by numerically integrating the raw rectified EMG for 30 and 100 ms following burst onset. A startle reflex was noted if a burst of EMG occurred in SCM within 50–120 ms following the SAS. Example raw data showing EMG bursts and associated kinematics in control and SAS trials for similar tasks have been previously published (see Carlsen et al. 2009, 2011).
Five of the original 21 participants did not exhibit a reliable startle reflex, defined as a short latency burst of EMG in SCM in at least 40 % of SAS trials for at least one of the blocks. Data from these low-responders were used in the analysis of startle reflex incidence, but the data were discarded from all further analyses, with two participants removed due to low incidence of a startle reflex in the warned block, one due to low response in the unwarned block, and two due to low response in both blocks. In the remaining participants, a startle reflex in SCM was not observed on eight total trials (4.5 % of all SAS trials) and these trials were discarded from further analysis after calculation of startle reflex incidence. Finally, trials were discarded if premotor RT was <50 ms (anticipation) or greater than 350 ms (distraction). This led to the removal of 58 trials (2.9 % of total) and 38 trials (1.9 % of total), respectively. Thus, a total of 1895 of 2000 total trials were included in the analysis (94.8 % inclusion rate).
Statistical analysis
Data were screened for normality using Shapiro–Wilk tests. If a data set was found to be significantly non-normal, the data were subjected to a Log10 transformation and normality was confirmed. This procedure led to the use of transformed data for wrist extensor iEMG, SCM iEMG, peak velocity, peak displacement, and final position. Proportion variables were subjected to an arcsine square root transform prior to analysis (Howell 2010). Untransformed means are presented along with 95 % CIs, corrected for within-subject comparisons where appropriate (Cai et al. 2011; Morey 2008). Dependent measures were analysed using mixed-model analysis of variance (ANOVA) where BlockOrder (unwarned block first vs. warned block first) was an independent factor and all other factors were repeated. EMG and kinematic measures were analysed using 2 BlockOrder × 2 BlockType (unwarned block vs. warned block) × 2 Stimulus (control vs. SAS) mixed-model ANOVAs. For premotor RT, a secondary analysis investigated any potential order or learning effects by separating control trials within each block type into quarters (i.e., first 25 % of control trials in order, second, third, and fourth) and comparing these using a 2 BlockOrder × 2 BlockType × 4 BlockQuarter mixed-model ANOVA. Incidence of startle was analysed using a 2 BlockOrder × 2 BlockType mixed ANOVA. Finally, any effect of the warning was analysed using a 2 BlockOrder × 2 WarningValidity (valid warning signal vs. invalid warning) mixed-model ANOVA. Greenhouse-Geisser corrected degrees of freedom were used to correct for violations of the assumption of sphericity if necessary. Differences with a probability <.05 were considered to be significant, and Tukey’s honestly significant difference post hoc tests were administered to determine the locus of any significant differences. Effect sizes (r) are reported for any main effects comparing only two means or other direct contrasts.
Results
Startle reflex
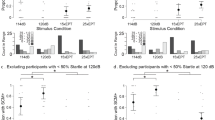
Analysis of the proportion of trials where a burst of EMG was detected in SCM showed no significant main effects of BlockType (warned vs. unwarned), p = .662, r = .010, or BlockOrder (warned vs. unwarned block first), p = .976, r < .001, and no interaction between the factors, p = .467. Figure 1 shows the startle reflex data collapsed across BlockOrder groups, including proportion of SCM responses observed in SAS trials (A), and SCM integrated EMG over 30 and 100 ms (B). SCM EMG integrated over 100 ms showed no main effects of BlockType, p = .601, r = .020, or BlockOrder, p = .199, r = .115, and no interaction between the factors, p = .696. In contrast, when SCM EMG was integrated over the first 30 ms of the burst (Fig. 1b, white bars), there was a significant effect of BlockType, F(1,14) = 7.375, p = .017, r = .345, indicating that the EMG burst was larger for the first 30 ms in the unwarned block compared to the warned block (Fig. 1b). However, there was no significant main effect of BlockOrder, p = .059, r = .232, and no significant interaction between the factors, p = .137.
Mean proportion of startling acoustic stimulus trials in which a startle reflex was observed in the sternocleidomastoid (SCM) (a), and mean integrated EMG of the first 30 ms (white bars) or 30–100 ms (grey bars) of SCM startle reflex activity (b) as a function of block (unwarned or warned). Error bars represent 95 % CIs correct for within-subject comparisons where applicable. *Significant difference between unwarned and warned blocks
Response latency
Premotor RT is presented in Fig. 2, with means separated based on block order and stimulus. Analysis of premotor RT showed a significant main effect of BlockType, F(1,14) = 9.664, p = .008, r = .408, as well as a main effect of Stimulus, F(1,14) = 95.513, p < .001, r = .873, but these were superseded by an interaction between BlockType and BlockOrder, F(1,14) = 5.621, p = .033, as well as an interaction between BlockType and Stimulus, F(1,14) = 9.800, p = .007. Post hoc analysis of the BlockType × BlockOrder interaction showed that irrespective of Stimulus type (control vs. SAS), premotor RT was significantly shorter in the warned blocks, but only for the group that performed the unwarned block first (control, 136.2 vs. 113.8 ms; startle, 81.4 vs. 76.0 ms; Fig. 2 black symbols) as opposed to the group that performed the warned block first (control, 120.6 vs. 116.4 ms; startle, 83.2 vs. 83.7 ms; Fig. 2 grey symbols) (p < .05). Post hoc analysis of the BlockType × Stimulus interaction showed that irrespective of BlockOrder (unwarned first vs. warned first), premotor RT was significantly shorter in SAS trials compared to control trials whether they were warned or not (see Fig. 2 control vs. startle). In addition, for the control trials RT was significantly shorter in the warned blocks (113.8, 116.4 ms, Fig. 2, control squares) compared to the unwarned blocks (136.2, 120.6 ms, Fig. 2, control circles) (p < .05). The three-way interaction between the factors was not significant, p = .096. The main effect of BlockOrder was not significant, p = .885, r = .002, and there were no other significant interaction effects.
Mean premotor reaction time (RT) during control and startle trial types as function of block (unwarned or warned) and block order (unwarned or warned first). Circles represent data from the unwarned block, whereas squares represent the warned block. Data shaded in black indicate that the unwarned block was completed first, whereas grey indicate that the warned block was first. Error bars represent 95 % CIs correct for within-subject comparisons where applicable
A secondary analysis was performed on control trials to determine whether any RT differences between blocks may attributable to a learning effect. As in the previous analysis, a significant main effect of BlockType was found, F(1,14) = 14.225, p = .002, r = .504, as well as a significant BlockType by BlockOrder interaction, F(1,14) = 6.650, p = .022. This indicates that RT during unwarned control trials was longer compared to warned control trials, but only for the group performing the unwarned block first (see above). However, there was no main effect of BlockQuarter, F(3,42) = .963, p = .378, no main effect of BlockOrder, F(1,14) = .478, p = .500, r = .033, and no interactions involving BlockQuarter or BlockOrder. All other main effects and interactions were not significant.
A final analysis was performed on premotor RT in the warned block comparing control trials (valid warning) to trials where a SAS was warned, but the control stimulus was presented (invalid warning). It should be noted that the opposite situation did not occur where a control trial was warned but an unexpected SAS was presented. Analysis showed that there was no main effect of WarningValidity, p = .865, r = .002, no main effect of BlockOrder, p = .298, r = .077, and no significant interaction between the factors.
Analysis of the integrated value from the initial 100 ms of EMG burst (iEMG) from the wrist extensors showed a significant main effect of Stimulus, F(1,14) = 19.050, p = .001, r = .576, indicating that the iEMG value was significantly larger in SAS trials [14.5 mV ms, 95 % CI (13.9, 15.2)] compared to control [12.3 mV ms, 95 % CI (11.7, 13.0)].
Response kinematics
Mean values for kinematic variables are presented in Fig. 3. For all of the kinematic variables, no significant main effect of BlockOrder was found (all p values >.160), and no significant interactions between BlockOrder and any other factors were found (all p values >.164); thus, data were collapsed across BlockOrder groups for graphical presentation. In all kinematic variables, a main effect of Stimulus was found (all F ratios >15.092, all p values <.002). However, this was superseded by a significant interaction between BlockType and Stimulus for peak velocity (Fig. 3a), F(1,14) = 8.937, p = .010, peak displacement (Fig. 3b), F(1,14) = 8.090, p = .013, and final position (Fig. 3d), F(1,14) = 8.697, p = .011. No other main effects or interactions were found to be significant for any of the kinematic variables. For peak velocity (Fig. 3a), post hoc analysis showed that in both the warned and unwarned blocks, peak velocity was significantly larger in SAS trials (p < .05). In addition, peak velocity was significantly larger in the warned block for control trials, but not for SAS trials. Peak displacement (Fig. 3b) was also larger in SAS trials for both the warned and unwarned blocks (p < .05), but there were no significant differences in peak displacement between warned and unwarned blocks in response to either the control stimulus or the SAS. A follow-up Student’s t test found a significant difference in the change in peak displacement between control and startle trials for the warned and unwarned blocks such that the increase in peak displacement was significantly larger, t(15) = 2.58, p = .021, r = .554, for the unwarned blocks [Δ peak displacement = 6.42 deg, 95 % CI (5.66, 7.19)] compared to the warned blocks [Δ peak displacement = 3.63 deg, 95 % CI (2.86, 4.40)]. Finally, post hoc analysis showed that final position (Fig. 3d) was significantly larger in SAS trials for the unwarned blocks (p < .05) but not for the warned blocks; furthermore, final position was significantly larger in the warned block for control trials (p < .05), but not for SAS trials.
Mean peak velocity (deg/s) (a), peak displacement (deg) (b), time to peak displacement (ms) (c) and final position (deg) (d) during control and startle trial types as function of block (unwarned or warned). Black circles represent data from the unwarned block, and grey squares represent data from the warned block. Error bars represent 95 % CIs correct for within-subject comparisons where applicable. †Significant difference between warned and unwarned, *significant difference between control and startle, ‡significantly different change from control to startle between BlockTypes, §main effect of Stimulus
Discussion
The purpose of the current study was to investigate whether providing a warning (i.e., foreknowledge) of an upcoming SAS during a simple RT task would affect the proportion of startle reflexes elicited or the magnitude of the startle reflex. Additionally, the study aimed to determine whether warning participants of stimulus type would result in RT differences in control or startle trials. Contrary to our hypothesis, warning participants of the impending SAS did not affect the proportion of startle reflexes elicited compared to when the SAS was unwarned. Warning participants did however result in a small but significant reduction in the magnitude of the startle reflex observed in SCM within 30 ms of onset (iEMG30). In addition, RT results indicated that providing foreknowledge of trial type had no influence on the RT speeding effect of startle (i.e., StartReact effect; Fig. 2), despite a small decrease in startle reflex magnitude. Together, the SAS results suggest that participants did not proactively gate the upcoming startle stimulus when warned. In contrast, control trial RT was modulated by providing knowledge of the upcoming type of trial. However, this modulation was dependent on the order in which the warned and unwarned blocks were completed. Specifically, premotor RT was significantly slower on control trials in the unwarned block, but only when the unwarned block was completed first, and thus prior to the warned block (Fig. 2).
The requirement to maximize the probability of eliciting a startle reflex in experiments employing a SAS has led researchers to present the SAS randomly and unexpectedly during the task. However, in the present study, warning the participant of an impending SAS, thereby making the stimulus much less unpredictable, did not reduce the probability of eliciting a startle reflex or alter the latency of “voluntary” responses triggered early by the SAS (i.e., StartReact effect). Nevertheless, when a warning was not provided, the magnitude of observed startle reflex in the first 30 ms was moderately larger (Fig. 1b). It has been suggested that the continued surprise resulting from random startle stimuli can result in a fear-potentiated startle reflex, which is likely mediated by input from the amygdala (Lefebvre et al. 2012). The startle reflex has been shown to be potentiated in numerous fear-related situations; in the presence of threat (e.g., actual or possible shock) (Bradley et al. 2008; Grillon et al. 1991), when viewing arousing unpleasant pictures (e.g., scenes of human threat, animal attack) (Lang 1995), and when viewing expressive faces (e.g., angry faces) (Dunning et al. 2010; Hess et al. 2007; Springer et al. 2007). In addition to fear, the unpredictable nature of the SAS may also potentiate the startle reflex via increased anxiety. For example, when participants were placed in a room where they received either unpredictable electric shock, predictable shock, or no shocks at all, results showed that anxiety ratings were highest when in the room with unpredictable shocks (Grillon et al. 1991). Importantly however, the baseline startle reflex was also largest in the unpredictable condition, providing evidence for fear- and anxiety-mediated potentiation of the startle reflex (Grillon et al. 1991). This is corroborated by reports that participants can become “annoyed” and/or “upset” by unexpected startle stimuli (Lin et al. 2012). Therefore, the exhibited decrease in startle reflex magnitude in the current experiment suggests that providing explicit foreknowledge of an impending SAS may have led to a reduction in the fear and anxiety related to the startling stimulus.
Even though the magnitude of the startle reflex was somewhat reduced, providing foreknowledge of trial type had no effect on the probability of eliciting a startle reflex in SCM. Specifically, even though participants received 22 startling stimuli within the 1-h duration experiment, a startle reflex was observed on 77 % of SAS trials irrespective of whether or not the SAS was forewarned (Fig. 1a). Though the startle reflex has been shown to habituate within 2–6 presentations in participants sitting quietly (Brown et al. 1991), it has been shown numerous times that a startle reflex can be elicited indefinitely when participants are engaged in a task requiring motor preparation (Carlsen et al. 2003, 2011; Valls-Solé et al. 1997). Accordingly, it is likely that in the present experiment, any diminishment of the startle reflex excitability resulting from foreknowledge of the stimulus was superseded by the additional activation provided to the startle reflex circuits due to motor preparatory activity (Carlsen et al. 2003). Although it has been suggested that the StartReact effect may be only indirectly related to the presence of a startle reflex (Marinovic and Tresilian 2016), no differences were seen in the amount of RT facilitation following a SAS that were associated with SCM burst magnitude. That is, RT was facilitated to a similar extent (Fig. 2) and kinematics were similarly exaggerated (Fig. 3) whenever a startle reflex was observed in SCM irrespective of its magnitude, a previously reported phenomenon (e.g., Carlsen et al. 2003; Maslovat et al. 2014, 2015a).
One final result of interest was the difference observed in control trial RT as a function of block order (warned first vs. unwarned first). Specifically, control trial RT was significantly longer when participants were not warned of stimulus type; however, this delay in RT was only observed in participants who completed the unwarned block first (Fig. 2). Although the present experiment was not designed to investigate the mechanism of this effect, it may be speculated that differences in anxiety associated with knowledge of whether or not a startling stimulus was upcoming may have played a role in these RT differences. Given that increased control RT was only observed in the unwarned block when it was performed first, an increased level of fear and anxiety associated with the SAS may have resulted in a delay in voluntary response initiation to the control stimulus, similar to the effect seen on the startle reflex itself. Even though faster RTs are often observed in response to heightened arousal or anxiety (Coombes et al. 2007; Ruegg and Eichenberger 1984; Welford 1980) it has also been previously shown that the induction of an anxious state can lead to increases in RT (Jones and Hardy 1988; Pacheco-Unguetti et al. 2010). In particular, increased anxiety is thought to impact attentional focus and cognitive performance in tasks requiring advance alerting and orienting (Pacheco-Unguetti et al. 2010). Performing the unwarned block first in the current experiment (prior to any experience with the SAS) may have led to a similarly anxious state that affected RT only in that block of trials, although this explanation is speculative since anxiety was not measured. In contrast, SAS RT was not affected by block order or foreknowledge. We suggest that this result provides further evidence that the neural circuits involved in the normal voluntary response pathway (which can be affected by anxiety levels) were bypassed when the response was triggered by startle. Similar effects were seen in response kinematics, such that when knowledge of trial type was provided, an increased “vigor” of response was seen between block types, as evidenced by differences in peak displacement.
Conclusion
In summary, providing explicit foreknowledge of an impending SAS did not affect the proportion of startle reflexes elicited compared to unwarned SAS trials, nor did it affect the latency of responses triggered early by the SAS. Consequently, in contrast to what is generally accepted, it appears that a startling stimulus does not have to be unpredictable in order to reliably elicit a startle reflex when preparing a motor response. As such, foreknowledge of an impending SAS does not affect the latency of the StartReact responses. Unexpectedly, control RT was increased in the unwarned block, but only when it was performed first. We argue that it is plausible that a lack of experience with the SAS gave rise to an anxious state, which led to delays in voluntary response initiation processes.
References
Abel K, Waikar M, Pedro B, Hemsley D, Geyer M (1998) Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. J Psychopharmacol 12:330–337
Acocella CM, Blumenthal TD (1990) Directed attention influences the modification of startle reflex probability. Psychol Rep 66:275–285
Alibiglou L, MacKinnon CD (2012) The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex. J Physiol 590:919–936
Alibiglou L, Marlin C, Videnovic A, Planetta PJ, Vailancourt DE, MacKinnon CD (2012) Gait initiation in rem seep behavior disorder and parkinson’s disease with freezing of gait. Mov Disord 27:792
Blumenthal TD (1996) Inhibition of the human startle response is affected by both prepulse intensity and eliciting stimulus intensity. Biol Psychol 44:85–104
Bradley MM, Silakowski T, Lang PJ (2008) Fear of pain and defensive activation. Pain 137:156–163
Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD (1991) New observations on the normal auditory startle reflex in man. Brain 114:1891–1902
Cai WD, Oldenkamp CL, Aron AR (2011) A proactive mechanism for selective suppression of response tendencies. J Neurosci 31:5965–5969
Carlsen AN (2015) A broadband acoustic stimulus is more likely than a pure tone to elicit a startle reflex and prepared movements. Physiol Rep 3:e12509
Carlsen AN, MacKinnon CD (2010) Motor preparation is modulated by the resolution of the response timing information. Brain Res 1322:38–49
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2003) Startle response is dishabituated during a reaction time task. Exp Brain Res 152:510–518
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004a) Can prepared responses be stored subcortically? Exp Brain Res 159:301–309
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004b) Prepared movements are elicited early by startle. J Motor Behav 36:253–264
Carlsen AN, Dakin CJ, Chua R, Franks IM (2007) Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res 176:199–205
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2009) Differential effects of startle on reaction time for finger and arm movements. J Neurophysiol 101:306–314
Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM (2011) Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35:366–376
Carlsen AN, Maslovat D, Franks IM (2012) Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol 123:21–33
Carlsen AN, Almeida QJ, Franks IM (2013) Using a startling acoustic stimulus to investigate underlying mechanisms of bradykinesia in parkinson’s disease. Neuropsychologia 51:392–399
Carpenter RHS, Williams MLL (1995) Neural computation of log likelihood in control of saccadic eye-movements. Nature 377:59–62
Castellote JM, Valls-Solé J, Sanegre MT (2004) Ballistic reactions under different motor sets. Exp Brain Res 158:35–42
Cisek P, Puskas GA, El-Murr S (2009) Decisions in changing conditions: the urgency-gating model. J Neurosci 29:11560–11571
Coombes SA, Cauraugh JH, Janelle CM (2007) Emotional state and initiating cue alter central and peripheral motor processes. Emotion 7:275–284
Donders FC (1969) On the speed of mental processes. Acta Psychol (Amst) 30:412–431
Dunning JP, Auriemmo A, Castille C, Hajcak G (2010) In the face of anger: startle modulation to graded facial expressions. Psychophysiol 47:874–878
Grillon C, Ameli R, Woods SW, Merikangas K, Davis M (1991) Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiol 28:588–595
Haith AM, Pakpoor J, Krakauer JW (2016) Independence of movement preparation and movement initiation. J Neurosci 36:3007–3015
Hess U, Sabourin G, Kleck RE (2007) Postauricular and eyeblink startle responses to facial expressions. Psychophysiol 44:431–435
Hodges PW, Bui BH (1996) A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr Clin Neurophysiol 101:511–519
Honeycutt CF, Perreault EJ (2012) Planning of ballistic movement following stroke: Insights from the startle reflex. PLoS One 7:e43097
Howell DC (2010) Statistical methods for psychology. Thomson Wadsworth, Belmont
Ison JR, Hammond GR (1971) Modification of the startle reflex in the rat by changes in the auditory and visual environments. J Comp Physiol Psychol 75:435–452
Jones JG, Hardy L (1988) The effects of anxiety upon psychomotor performance. J Sports Sci 6:59–67
Kennefick M, Maslovat D, Carlsen AN (2014) The time course of corticospinal excitability during a simple reaction time task. PLoS One 9:e113563
Klapp ST, Wyatt EP, Lingo WM (1974) Response programming in simple and choice reactions. J Motor Behav 6:263–271
Kohfeld DL (1971) Simple reaction time as a function of stimulus intensity in decibels of light and sound. J Exp Psychol 88:251–257
Landis C, Hunt WA, Strauss H (1939) The startle pattern. Farrar & Rinehart, New York
Lang PJ (1995) The emotion probe: studies of motivation and attention. Am Psychol 50:372–385
Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y (2012) Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front Hum Neurosci 6:343
Leuthold H, Sommer W, Ulrich R (2004) Preparing for action: inferences from CNV and LRP. J Psychophysiol 18:77–88
Lin CH, Chiang MC, Wu AD, Iacoboni M, Udompholkul P, Yazdanshenas O, Knowlton BJ (2012) Age related differences in the neural substrates of motor sequence learning after interleaved and repetitive practice. Neuroimage 62:2007–2020
Luce RD (1986) Response times: their role in inferring elementary mental organization. Oxford University Press, New York
MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW (2007) Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol 97:4368–4379
Marinovic W, Tresilian JR (2016) Triggering prepared actions by sudden sounds: reassessing the evidence for a single mechanism. Acta Physiol 217:13–32
Marinovic W, de Rugy A, Riek S, Tresilian JR (2014) The early release of actions by loud sounds in muscles with distinct connectivity. Exp Brain Res 232:3797–3802
Maslovat D, Kennedy PM, Forgaard CJ, Chua R, Franks IM (2012) The effects of prepulse inhibition timing on the startle reflex and reaction time. Neurosci Lett 513:243–247
Maslovat D, Carter MJ, Kennefick M, Carlsen AN (2014) Startle neural activity is additive with normal cortical initiation-related activation. Neurosci Lett 558:164–168
Maslovat D, Drummond NM, Carter MJ, Carlsen AN (2015a) Startle activation is additive with voluntary cortical activation irrespective of stimulus modality. Neurosci Lett 606:151–155
Maslovat D, Franks IM, Leguerrier A, Carlsen AN (2015b) Responses to startling acoustic stimuli indicate that movement-related activation is constant prior to action: a replication with an alternate interpretation. Physiol Rep 3:e12300
Morey RD (2008) Confidence intervals from normalized data: a correction to Cousineau (2005). Tutor Quant Methods Psychol 4:61–64
Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, Bloem BR, Weerdesteyn V, Geurts AC (2014) Startreact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci 34:275–281
Pacheco-Unguetti AP, Acosta A, Callejas A, Lupianez J (2010) Attention and anxiety: different attentional functioning under state and trait anxiety. Psychol Sci 21:298–304
Reynolds RF, Day BL (2007) Fast visuomotor processing made faster by sound. J Physiol 583:1107–1115
Ruegg DG, Eichenberger A (1984) Effects of electrical stimulation of low-threshold muscle afferents on visual reaction time. Electroencephalogr Clin Neurophysiol 57:184–187
Shibasaki H, Hallett M (2006) What is the bereitschaftspotential? Clin Neurophysiol 117:2341–2356
Siegmund GP, Inglis JT, Sanderson DJ (2001) Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535:289–300
Springer US, Rosas A, McGetrick J, Bowers D (2007) Differences in startle reactivity during the perception of angry and fearful faces. Emotion 7:516–525
Summers JJ, Anson JG (2009) Current status of the motor program: revisited. Hum Mov Sci 28:566–577
Tresch UA, Perreault EJ, Honeycutt CF (2014) Startle evoked movement is delayed in older adults: implications for brainstem processing in the elderly. Physiol Rep 2:e02125
Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES (1995) Reaction time and acoustic startle in normal human subjects. Neurosci Lett 195:97–100
Valls-Solé J, Valldeoriola F, Tolosa E, Nobbe F (1997) Habituation of the auditory startle reaction is reduced during preparation for execution of a motor task in normal human subjects. Brain Res 751:155–159
Valls-Solé J, Rothwell JC, Goulart FR, Cossu G (1999) Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516:931–938
Valls-Solé J, Kofler M, Kumru H, Castellote JM, Sanegre MT (2005) Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res 165:541–548
Wadman WJ, Denier Van der Gon JJ, Geuze RH, Mol CR (1979) Control of fast goal-directed arm movements. J Hum Mov Stud 5:3–17
Weinberg I (2016) Are movement preparation and movement initiation truly independent? J Neurosci 36:7076–7078
Welford AT (1980) Choice reaction time: basic concepts. In: Welford AT (ed) Reaction times. Academic Press, London, pp 73–128
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN 418361-2012) awarded to Anthony N. Carlsen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drummond, N.M., Leguerrier, A. & Carlsen, A.N. Foreknowledge of an impending startling stimulus does not affect the proportion of startle reflexes or latency of StartReact responses. Exp Brain Res 235, 379–388 (2017). https://doi.org/10.1007/s00221-016-4795-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4795-1