Abstract
A startling auditory stimulus delivered during preparation for execution of a ballistic movement in a simple reaction time task experiment induces two effects: a startle response and a reaction time shortening (the StartReact effect). We investigated whether prepulse inhibition of the startle response is effective in suppressing either one of these effects during motor preparation. Twelve healthy volunteers were presented with seven different experimental conditions in random order: while at rest, subjects received a low intensity electrical shock on the middle finger of the left hand (Prep), a loud auditory stimulus (Start), or a combination of these two (PrepStart). While engaged in preparation for a visual simple reaction time task, they were presented with the imperative signal for execution of the reaction (React), or with any of the combinations PrepReact, StartReact, or PrepStartReact. We recorded the EMG activity from the orbicularis oculi and the sternocleidomastoid muscles to assess the startle response, and from the wrist extensor muscles to assess reaction time. The startle response was markedly reduced when Prep was presented 100 ms before Start regardless of whether the subjects were at rest or preparing for the reaction. Reaction time shortened significantly in StartReact trials with respect to React trials, and the percentage shortening was not different in trials in which Prep preceded StartReact and inhibited the startle response. The fact that prepulse inhibition of the startle response is not accompanied by modification of the StartReact effect indicates that there are separate physiological mechanisms for the two effects, an observation that has implications for further understanding of the processes underlying motor preparation for a ballistic reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presentation of a startling auditory stimulus together with the imperative signal in a reaction time task paradigm induces a significant acceleration of the reaction time (Valls-Solé et al. 1995, 1999a; Siegmund et al. 2001; Carlsen et al. 2004). The physiological mechanisms accounting for this phenomenon, termed the StartReact effect (Valldeoriola et al. 1998), are not fully understood. While Valls-Solé et al. (1999a) attributed the effect to the release of the motor program from fully prepared subcortical motor structures directly activated by the startling stimulus, Siegmund et al. (2001) suggested a temporal and spatial summation of startle response and voluntary action. Apart from its effect on reaction time, a startling stimulus applied while subjects are prepared to react, leads to larger startle responses with reduced habituation in comparison to those elicited in conditions with no motor preparation (Valls-Solé et al. 1995, 1997).
Another feature of the startle response is its susceptibility to be inhibited by a preceding weak sensory stimulus that does not elicit any reflex response by itself, an effect known as prepulse inhibition (Graham 1975; Blumenthal and Gescheider 1987; Ison et al. 1990; Swerdlow et al. 1995; Blumenthal 1999; Valls-Solé et al. 1999b). Even though the relationship between prepulse and startle response has been thoroughly studied in normal human subjects, there remain some interesting points to explore. In this study, we investigated whether the inhibitory effects of the prepulse on the startle response were still present during motor preparation, and examined whether or not the prepulse inhibition of the startle response was associated with inhibition of the StartReact effect. We aimed at expanding the current knowledge on the physiology of the startle response and prepulse effects and, more specifically, at investigating the possibility of suppression of the startle component in trials combining reaction time and a startle response.
Materials and methods
Subjects
The study was carried out in 12 healthy volunteers, seven men and five women, 23–52 years of age, who gave their consent for the study after being fully informed about the nature of the experiments. The study protocol was approved by the local Ethical Committee.
Recording apparatus
All subjects wore pairs of surface recording electrodes over the right orbicularis oculi and sternocleidomastoid muscles, to record the EMG activity related to the startle response, and over the right wrist extensor muscles, to record the EMG activity related to voluntary muscle activation. A piezoelectric accelerometer (model 348720; Bionic Ibérica S.A., Barcelona, Spain) was placed on the dorsum of the right hand to record hand movement. The bandpass frequency filter was 20–1,000 Hz for EMG recordings, and 0.5–10 Hz for the accelerometer signal. All recordings were done with a conventional electromyograph (Mystro5Plus; Oxford Instruments, Inc., SA, London). Subjects were sitting on a comfortable chair, with their hands resting on armrests, facing a blank computer screen placed at a distance of 1 m from the subject’s eyes.
Stimuli used for prepulse inhibition and for startle responses
The stimulus used as a prepulse was a weak electrical shock delivered to the third finger of the left hand through a pair of ring electrodes. Stimulus intensity was set between 1.5 and 2.5 times the perception threshold, and we made sure that this stimulus did not elicit any reflex response of its own. The startling auditory stimulus was produced by the discharge of the coil of a magnetic stimulator on top of a metallic platform. This method permits the delivery of loud stimuli of 130 dB as measured with a Brüel and Kjaer impulse precision sound level meter type 2204 and a condenser microphone Cartridge type 4145 at a distance of 1 m from the source of the noise. This acoustic stimulus is capable of inducing consistent startle responses (Valls-Solé et al. 1999a). In all experimental conditions containing a prepulse (see below), the prepulse stimulus was applied 100 ms before the startling stimulus (Valls-Solé et al. 1999b).
Setup for reaction time task
Out of the subject’s view, the experimenter pressed a computer key which triggered the presentation of the forewarning stimulus, a small cross at the center of an otherwise blank monitor screen. The imperative signal, a 5×5 cm white square, followed always the forewarning after a fixed foreperiod of 2000 ms. Subjects were required to react as fast as possible by hitting with their right hand a clearly marked switch placed at a distance of about 20 cm. One second after the appearance of the forewarning (i.e. 1000 ms before the appearance of the imperative signal), a pulse was generated by the computer triggering the electromyograph for response recordings.
Experimental procedure
Subjects were informed about all types of stimuli involved in the experiment, such as visual cues, electrical stimuli on the finger, and auditory stimuli. They were also instructed to pay attention to the monitor and to be prepared to react to the imperative signal whenever the forewarning stimulus appeared. The experiment was composed of seven different conditions, with trials of each condition presented in random order and an interval of 10–15 s between two consecutive trials. In three conditions, the stimuli were presented with no forewarning: (1) prepulse alone (Prep), in which subjects were presented with the prepulse stimulus alone, (2) startle alone (Start), in which subjects were presented with the startling auditory stimulus alone, (3) prepulse and startle (PrepStart), in which subjects were presented with the prepulse stimulus 100 ms prior to the startling auditory stimulus. In the remaining four conditions, the stimuli were presented in the context of motor preparation after the forewarning signal: (4) reaction alone (React), in which subjects were presented with the imperative signal only, (5) startle plus reaction (StartReact), in which subjects were presented with the imperative signal together with the startling auditory stimulus at the same time, (6) prepulse and reaction (PrepReact), in which subjects were presented with the prepulse stimulus preceding the imperative signal by an interval of 100 ms, and (7) prepulse, startle, and reaction (PrepStartReact), in which the prepulse stimulus was delivered 100 ms before the simultaneous presentation of the imperative signal and the startling auditory stimulus.
We collected ten trials for each condition, except for React. This was considered the default condition, applied randomly among all other conditions, in order to interrupt possible expectancy of a prepulse or startling stimulus. As a result, the total number of trials collected in the condition React was considerably larger than in the other conditions, producing a minimum of 40 trials per subject. The mean results obtained in trials of the condition React were used as reference values for all other trials involving reaction time tasks.
Data reduction and analysis
Trials that furnished an incomplete set of data because of the subject’s failure to move, interrupted movement execution, or inattention, were counted for each condition, rejected on-line, and repeated, to obtain the projected number of trials per condition. All data measurements were made off-line, on thermosensitive paper printouts.
In trials containing a startling auditory stimulus (Start, PrepStart, StartReact, and PrepStartReact), we determined whether there was a burst of EMG activity in the orbicularis oculi and sternocleidomastoid muscles, at the expected latency from the stimulus (40–80 ms), in accordance with previously published data (Brown et al. 1991a; Chokroverty et al. 1992; Kofler et al. 2001). When a response was present, we measured its onset latency from the startling auditory stimulus, its duration and its peak-to-peak amplitude. We then calculated the size of each startle response by multiplying amplitude in μV times duration in ms, and expressed the results as percentages of the mean size of the startle response in Start trials, which was considered the individual’s baseline startle burst size. Habituation was evaluated, independently for each condition, by expressing the differences in size between the first and the last response as a percentage of the size of the first response. We also measured the probability of a startle response in orbicularis oculi and sternocleidomastoid muscles, as the number of responses expressed in percentage of the total number of stimuli.
In trials involving a reaction time task (React, PrepReact, StartReact, and PrepStartReact), we measured three time-related variables: the onset of EMG activity in wrist extensor muscles (WE-EMG), the onset of accelerometric displacement of the hand (MOV), and the moment of pressing the switch (TASK), relative to the imperative signal. We calculated the means and SDs for each subject and condition. The baseline reaction time was determined using the first 40 trials collected per subject in the condition React, and the values obtained in other conditions were expressed as percentages of the baseline values. We considered the time relationship between WE-EMG, MOV, and TASK in every trial as a measure of task consistency, to determine the possibility of incomplete execution of the task, or performance of movements other than those requested.
We calculated the grand mean among all individuals for all parameters measured in all trials of the same condition. All statistical group analysis for comparison of data among trial types was carried out using analysis of variance (ANOVA). We used the one-factor ANOVA to evaluate the effect of conditions Start, PrepStart, StartReact, and PrepStartReact on the size of the startle response and the two-way ANOVA to determine the effects of startle and prepulse on reaction time in conditions React, PrepReact, StartReact, and PrepStartReact. We also determined the influence of motor preparation on the effects of the prepulse and of the startling auditory stimulus by comparing the size of the startle response and the degree of prepulse inhibition observed in conditions involving no preparation (Prep, Start, and PrepStart) with those observed in conditions requiring motor preparation (PrepReact, StartReact, and PrepStartReact). Post-hoc comparisons between specific trial types were made using the Bonferroni’s test. Statistical significance was set at P<0.05.
Results
All subjects endured the experiment with no signs of boredom or fatigue. Some subjects made spontaneous comments in trials containing either the prepulse or the startling auditory stimulus. Although we did not analyze these comments systematically, we mention them here because of their possible interest for the interpretation of results. In reaction time trials containing the prepulse or the startling auditory stimulus, a few subjects reported spontaneously that some external force made them react even though they were not yet completely ready. In some PrepReact or PrepStartReact trials, a few subjects said that they did not feel any electrical stimulus at all.
The number of rejected trials was less than 5% in any subject, with no particular accumulation in any condition. However, there was an unexpected finding in some trials of the PrepReact and PrepStartReact conditions: We observed that the onset of the EMG activity occasionally coincided with or even preceded the presentation of the imperative signal. We considered the possibility that, in these trials, subjects reacted to the presentation of the prepulse rather than to the imperative signal. Therefore, we decided to exclude trials in which reaction time was shorter than 65 ms from the main statistical analysis. This figure was chosen on the basis of results from previous studies, in which the delivery of a startling auditory stimulus together with the imperative signal never induced reaction times shorter than 65 ms (Valls-Solé et al. 1999a). As a consequence, we rejected 41 trials (34.2%) in the PrepReact condition, and 39 trials (32.5%) in the PrepStartReact condition.
Size of startle response and amount of prepulse inhibition: Prep, Start, PrepStart, StartReact and PrepStartReact trials
No evident responses were observed in any subject in the condition Prep. A generalized startle response was observed in all subjects in the first trials of the condition Start. In spite of the fact that the size of the generalized response decreased in subsequent trials, responses were present in the orbicularis oculi in almost all Start trials and in the sternocleidomastoid in most of them. Table 1 shows the mean values of reflex response probability, response size, and habituation, in orbicularis oculi and sternocleidomastoid muscles for all trials containing a startling auditory stimulus. Statistical comparison of the means showed significant differences in startle response probability [ANOVA; F(3,44)=571.0; P<0.001 for the orbicularis oculi, and F(3,44)=738; P<0.001 for the sternocleidomastoid) as well as in startle response size [ANOVA; F(3,44)=178.2; P<0.001 for the orbicularis oculi, and F(3,44)=39.6; P<0.001 for the sternocleidomastoid]. Post hoc analysis showed that the probability of the startle response was significantly higher in the StartReact condition than in the Start condition for the sternocleidomastoid (P<0.001) but not for the orbicularis oculi (P=0.09). The probability of the startle response was lower in conditions PrepStart and PrepStartReact than in the Start condition (P<0.001 for both conditions in both muscles). Similarly, the startle responses were significantly larger in the StartReact condition in comparison to all other conditions (P<0.0001 for all comparisons), and significantly smaller in PrepStart and PrepStartReact conditions than in the other conditions (P<0.0001 for all comparisons in both conditions). No differences were found between PrepReact and PrepStartReact conditions (P=0.7). The percentage habituation was significantly reduced in StartReact trials with respect to Start trials (ANOVA; F[1,22]=18.9; P<0.001). It was not possible to calculate habituation in trials containing Prep because of frequently absent startle responses in the first trials. No statistically significant differences were observed between conditions regarding the latency of the orbicularis oculi or sternocleidomastoid responses [ANOVA; F(3,44)=0.04; P>0.05 for the orbicularis oculi muscle, and F(3,44)=0.11; P>0.05 for the sternocleidomastoid muscle].
Effects of startling stimulus on reaction time: React, PrepReact, StartReact and PrepStartReact trials
Mean values and 1 SD of the reaction times in the React, PrepReact, StartReact and PrepStartReact conditions are shown in Table 2. As expected, reaction time was markedly shorter in the StartReact than in the React condition (Fig. 1a,b). Single trial reaction time values for the EMG activity ranged from 130 to 416 ms in the React condition, and from 67 to 168 ms in the StartReact condition. Figure 2 shows a plot of all mean values for WE-EMG, MOV, and TASK in all conditions for each of the subjects of the study. The similarity in the degree of dispersion of the data and the consistent relationship between WE-EMG, MOV, and TASK in all conditions is notable. The mean percentage latency change observed in a given condition was similar for WE-EMG, MOV, and TASK (Fig. 3).
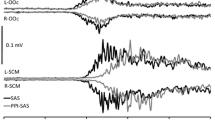
Examples of a reaction time response in (a) React and (b) StartReact. From top to bottom, the traces represent the hand movement and the EMG activity in the agonist muscle, the sternocleidomastoid muscle and the orbicularis oculi muscle. Note the shortening of the reaction time, and the elicitation of startle responses in the sternocleidomastoid and orbicularis oculi muscles, in StartReact in comparison to React
Distribution of the mean data on reaction time in ms for each subject in the conditions React (1), StartReact (2), PrepReact (3), and PrepStartReact (4). Black squares represent onset of wrist extensors EMG activity, white triangles represent onset of movement, and black circles represent the moment in which the task was accomplished
Histograms showing the mean percentage (and 1 SD) of the wrist extensors EMG, onset of movement, and task performance, in trials of StartReact, PrepReact, and PrepStartReact, with respect to the values of the condition React, considered to be 100% for each parameter. Note the similarities in the percentage change of all parameters in all conditions
Statistical comparisons of the subject’s mean reaction time were calculated after exclusion of trials in which subjects were suspected to have reacted to the prepulse stimulus. The two-way ANOVA showed a significant difference in reaction time between trials containing a startling auditory stimulus (StartReact and PrepStartReact) vs those that did not contain it (React and PrepReact) (F=82.9; P<0.001). On the contrary, there was no difference in reaction time between trials containing prepulse (PrepReact and PrepStartReact) versus those that did not contain it (React and StartReact) (F=0.5; P=0.5), and there was no interaction between startle and prepulse (F=1.2; P=0.3).
We also calculated the mean reaction time in trials in which we considered that the subjects reacted to the prepulse stimulus rather than to the imperative signal (trials excluded from the analysis described in the previous paragraph). The mean reaction time, taken from the prepulse ranged between 72 and 173 ms for the PrepReact condition and between 68 and 157 ms in the PrepStartReact condition. These values were not different from those obtained for the reaction time in StartReact trials (67–168 ms).
Effects of prepulse on startle response and on startle-induced reaction time shortening—PrepStartReact trials
The startle-induced reaction time shortening observed in PrepStartReact trials was not different from that observed in StartReact trials (Table 2, Fig. 4). However, there were differences between the two conditions with regard to the size of the startle response recorded in the orbicularis oculi and the sternocleidomastoid muscles (Table 1, Fig. 4). While the bursts recorded in the orbicularis oculi and sternocleidomastoid muscles were evident in StartReact trials, they were markedly reduced or even absent in the PrepStartReact condition. Furthermore, the size of the startle bursts recorded in the orbicularis oculi and sternocleidomastoid muscles did not differ in conditions PrepStart and PrepStartReact.
Examples taken from the same subject as Fig. 1, showing reaction time trials in the conditions StartReact (a) and PrepStartReact (b). Note the marked decrease of the startle responses in the sternocleidomastoid and the orbicularis oculi, with no concomitant modification of the reaction time shortening, in PrepStartReact in comparison to StartReact
Discussion
The results of our study confirm and expand previous findings regarding the physiology of prepulse inhibition and the StartReact phenomenon, and bring new insight into the physiology of voluntary movement and its relation to the startle pathways. The most important conclusion from our study is that prepulse stimuli maintain their inhibitory effects over the startle response even during motor preparation for execution of a motor task, while it does not act on the startle-induced reaction time acceleration, or StartReact phenomenon. This suggests that a startling stimulus is capable of inducing two different effects, the startle response and the acceleration of the reaction time, and both seem to be mediated by different neuronal circuits.
The physiology of the startle response is well known in experimental animals, and all data gathered so far permit suggesting similar physiological mechanisms in humans (Davis et al. 1982; Lingenhöhl and Friauf 1994; Koch 1999). The physiology of the StartReact phenomenon is more complex. In a previous experiment in which healthy subjects performing ballistic wrist movements were trained to produce a triphasic agonist-antagonist-agonist muscle activation pattern, Valls-Solé et al. (1999a) showed that the entire triphasic pattern was moved to a latency as short as that of the startle response when the subject was subjected to an auditory stimulus concomitantly with the imperative visual signal. The triphasic pattern of a ballistic movement is supposed to be a package of motor commands generated in the central nervous system (Hallett 1975; Berardelli et al. 1996). However, in the experiments of Valls-Solé et al (1999a), the StartReact effect involved no change in the configuration of the triphasic pattern other than the significant latency shortening. Similarly, in the experiments presented here, we found that the time relationship between WE-EMG, MOV, and TASK was not altered in StartReact trials, in agreement with the observation of unaltered movement kinematics in a similar experiment, as reported by Carlsen et al. (2004). These observations are consistent with the absence of collision between the startle-induced and any other simultaneous descending volley in the motor pathway, and suggests that the tract conveying the StartReact phenomenon, presumably the reticulospinal tract (Davis et al. 1982; Valls-Solé et al. 1999a), holds a trace of excitability representing the pattern of the intended ballistic movement. In simple reaction time task experiments, such excitability would be enhanced in relation to motor preparation, and external activation of the reticulospinal tract by the auditory stimulus will lead to the execution of the whole “pre-programmed” motor task (Carlsen et al. 2004). Additional activation of other tracts seems unlikely to converge simultaneously with the startle response at the motoneuronal level. If this were the case, one would expect some distortion in the configuration of the EMG pattern or in the time relationship between EMG, MOV, and TASK. Therefore, we suggest that the pathways conveying the startle response contribute significantly to the fast execution of centrally programmed ballistic movements, after being specifically modulated by the voluntary commands. An alternative explanation has been suggested by Siegmund et al. (2001), who examined the StartReact phenomenon using the sternocleidomastoid muscle as the prime mover. They found enough modification of the response in StartReact trials to suggest summation of the startle response and an accelerated execution of the voluntary commands. The sternocleidomastoid muscle is a more active muscle in a startle response than wrist extensor and flexor muscles. However, if the results of the study presented here hold true for the neck muscles, prepulse inhibition would suppress the startle component in the activity of the sternocleidomastoid muscle during the StartReact phenomenon.
Preparation to perform a certain motor task requires not only the activation of specific circuits but also inhibition of activity in other, unnecessary, motor circuits (Shadmehr and Holcomb 1999; Hummel et al. 2002). The movement requested from our subjects was wrist extension. The trace of growing excitability in subcortical motor circuits during motor preparation would involve these muscles and others, such as those engaged in postural adjustments. Hence, in the StartReact paradigm, activity is released not only in the muscles involved in the task but also in the orbicularis oculi and the sternocleidomastoid muscles. Our assumption is that the startling stimulus triggers the prepared action together with an indiscriminate startle response, which incidentally is often larger than the one elicited by the same stimulus at rest. The action triggered by the startling stimulus may be considered incomplete because of the lack of inhibition of unwanted contraction in other muscles. We postulate that this inhibitory aspect of the voluntary movement cannot actually be built in at the subcortical level, where the startling stimulus triggers the circuit. The necessary inhibition of unwanted movements, which is an intrinsic aspect of a motor program for performing accurate movements, may come into play only when there is participation of more rostral structures in movement execution. So far, the presence and role of the inhibitory functions integrated in voluntary commands have been investigated at the cortical level only (Ziemann et al. 1996; Jackson et al. 1999; Shadmehr and Holcomb 1999; Hummel et al. 2002).
According to our results, a prepulse stimulus may trigger commands destined to inhibit the unnecessary startle-related motor activity. Interestingly, patients with disorders presenting with excess motor activity, such as dystonia (Berardelli et al. 1998) or attention deficit hyperactivity disorder (Barkley 1997), may exhibit at the same time a failure of inhibition at the cortical level and a defective prepulse inhibition (Castellanos et al. 1996; Gómez-Wong et al. 1998). Even though prepulse inhibition has received considerable attention regarding both, the definition of its circuits in animals (Koch et al. 1993; Swerdlow and Geyer 1999) and its physiological characteristics and clinical applications in humans (Swerdlow et al. 1995; Valls-Solé et al. 1999b), we are still far from knowing the exact significance of prepulse inhibition, and the extent with which prepulse effects contribute to human behavior. In any case, the fact that the prepulse inhibits startle-related activity but not the StartReact effect is another piece of information to be elaborated in future studies.
As would be expected, the prepulse stimulus, a weak and innocuous electrical stimulus at the third finger, did not trigger any motor response of its own in conditions without preparation. However, in our subjects, the same stimulus triggered the prepared response in about 30% of trials during motor preparation. These observations suggest that there are different effects of a sensory signal on the motor system depending on the degree of motor preparation (Brunia 1993). Another example is the reduced habituation of the startle response in conditions of motor preparation, which is significantly different from the rapid habituation occurring in other conditions (Valls-Solé et al. 1997). Interestingly, there were no differences in reaction times between trials in which the subjects reacted to the prepulse and StartReact trials. This is compatible with the prepulse stimulus being able to trigger the entire motor program, as occurred in the StartReact trials. As this effect occurred with stimuli presented 100 ms before the actual imperative signal, some subjects may have developed a sufficient amount of motor preparation by that time for a barely perceptible prepulse stimulus to trigger the execution of the motor task. In a simple reaction time task paradigm, in which the subject knows exactly what to do, motor programs should be ready for execution as soon as the imperative signal is detected. In conditions of extreme preparedness, the time involved in processing and decoding the sensory signal may be reduced to zero but, even in these instances, performing a voluntary open-loop ballistic movement will require time for perception and execution (Henderson and Dittrich 1998). Full preparedness implies an enhanced excitability of the motor system. Such an increase in excitability has been demonstrated by an enhanced amplitude of the motor evoked potential in the agonist muscle following transcranial stimulation (Starr et al. 1988; Pascual-Leone et al. 1992), and of the agonist muscle H reflex (Michie et al. 1976; Schieppati et al. 1986), before the onset of any voluntary EMG activity in the target muscle. We think that the enhanced excitability of the motor system involves also the startle pathway, although we do not know yet when exactly the enhancement of excitability in this pathway begins in the process of preparation for execution of a motor task. Nevertheless, this should necessarily be some time before the presentation of the imperative signal, since a startling auditory stimulus is indeed able to trigger the whole ballistic movement at intervals of up to 300 ms before the visual signal used as a cue (Valls-Solé et al. 2002). We suggest that external stimuli impinging on highly excitable motor structures at a sufficiently high level in the hierarchy of the motor pathway should be able to trigger the whole set of motor commands for execution of the fully prepared voluntary ballistic reaction.
In the sensory system, little processing is probably required of the inputs generated by the imperative signal in a simple reaction time task. In the absence of any uncertainty about the cue, simple detection of a sensory stimulus would be enough to trigger the motor commands (Henderson and Dittrich 1998) due to the high degree of motor preparedness. In these instances, prepulse stimuli may have escaped conscious perception but still been able to carry out their effects at a subcortical level. The presence of a more powerful stimulus soon afterwards might have contributed to mask the prepulse and explain why some subjects reported no sensation from the electrical stimulus (Taylor and McCloskey 1990). It is relevant, however, to point out that prepulse inhibition was powerful enough to overcome the potentiation of the startle response as a consequence of its presentation during motor preparation.
The dichotomy of the prepulse effects in our subjects suggests that the startle response and the effects of a startling stimulus on reaction time are two separate phenomena, responding differently to the presence of a prepulse. Evidence for dissociation between these two effects of a startling stimulus has been also found regarding habituation: While habituation of the startle response is prominent (Brown et al. 1991a; Chokroverty et al. 1992; Kofler et al. 2001), the StartReact phenomenon shows remarkable consistency during a series of experiments (Valldeoriola et al. 1998). The startle response itself shows reduced habituation when elicited during motor preparation compared to rest (Valls-Solé et al. 1997). This may be the consequence of the progressive enhancement of excitability in the reticulospinal tract that takes place during movement preparation. Such excitability enhancement is priming the muscles implied in the execution of a motor program, including agonist and postural muscles alike (Brown et al. 1991b; Valls-Solé et al. 1999a). In contrast, other muscles may retain the reactivity to startle and prepulse stimuli characteristic of resting conditions and, therefore, sustain the effects of the prepulse without being primed by the preparation-related excitability enhancement. Further studies on the relationship between voluntary and reflex actions are required to fully understand the role of subcortical motor circuits in the organization and execution of human motor acts.
References
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65–94
Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD (1996) Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain 119:661–674
Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M (1998) The pathophysiology of dystonia. Brain 121:1195–1212
Blumenthal TD (1999) Short lead interval startle modification. In: Dawson ME, Schell AM, Böhmelt AH (eds) Startle modification: implications for cognitive science, clinical science, and neuroscience. Cambridge University Press, Cambridge, pp 51–71
Blumenthal TD, Gescheider GA (1987) Modification of the acoustic startle reflex by a tactile prepulse: the effects of stimulus onset asynchrony and prepulse intensity. Psychophysiology 24:320–327
Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD (1991a) New observations on the normal auditory startle reflex in man. Brain 114:1891–1902
Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD (1991b) The effect of posture on the normal and pathological auditory startle reflex. J Neurol Neurosurg Psychiatry 54:892–897
Brunia CHM (1993) Waiting in readiness: gating in attention and motor preparation. Psychophysiology 30:327–339
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004) Prepared movements are elicited early by startle. J Mot Behav 36:253–264
Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M (1996) Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry 39:33–41
Chokroverty S, Walczak T, Hening W (1992) Human startle reflex: technique and criteria for abnormal response. Electroencephalogr Clin Neurophysiol 85:236–242
Davis M, Gendelman DS, Tischler MD, Gendelman PM (1982) A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 2:791–805
Gómez-Wong E, Martí MJ, Tolosa E, Valls-Sole J (1998) Sensory modulation of the blink reflex in patients with blepharospasm. Arch Neurol 55:1233–1237
Graham F (1975) The more or less startling effect of a weak prestimulation. Psychophysiology 12:238–248
Hallett M, Shahani BT, Young RR (1975) EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry 38:1154–1162
Henderson L, Dittrich WH (1998) Preparing to react in the absence of uncertainty: I. New perspectives on simple reaction time. Br J Psychol 89:531–554
Hummel F, Andres F, Altenmuller E, Dichgans J, Gerloff C (2002) Inhibitory control of acquired motor programmes in the human brain. Brain 125:404–420
Ison JR, Sanes JN, Foss JA, Pinckney LA (1990) Facilitation and inhibition of the human startle blink reflexes by stimulus anticipation. Behav Neurosci 104:418–429
Jackson SR, Jackson GM, Roberts M (1999) The selection and suppresion of action: ERP correlates of executive control in humans. Neuroreport 10:861–865
Koch M (1999) The neurobiology of startle. Prog Neurobiol 59:107–128
Koch M, Kungel M, Herbert H (1993) Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res 97:71–82
Kofler M, Müller J, Reggiani L, Valls-Solé J (2001) Influence of gender on auditory startle responses. Brain Res 921:206–210
Lingenhöhl K, Friauff E (1994) Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? J Neurosci 14:1176–1194
Michie PT, Clarke AM, Sinden JD, Glue LCT (1976) Reaction time and spinal excitability in a simple reaction time task. Physiol Behav 16:311–315
Pascual-Leone A, Valls-Solé J, Wassermann EM, Brasil-Neto JP, Cohen LG, Hallett M (1992) Effects of focal transcranial magnetic stimulation on simple reaction time to acoustic, visual, and somatosensory stimuli. Brain 115:1045–1059
Schieppati M, Nardone A, Musazzi M (1986) Modulation of the Hoffmann reflex by rapid muscle contraction or release. Hum Neurobiol 5:59–66
Shadmehr R, Holcomb HH (1999) Inhibitory control of competing motor memories. Exp Brain Res 126:235–251
Siegmund GP, Inglis JT, Sanderson DJ (2001) Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535:289–300
Starr A, Caramia M, Zarola F, Rossini PM (1988) Enhancement of motor cortical excitability in humans by non-invasive electrical stimulation appears prior to voluntary movement. Electroencephalogr Clin Neurophysiol 70:26–32
Swerdlow NR, Geyer MA (1999) Neurophysiology and neuropharmacology of short lead interval modification. In: Dawson ME, Schell AM, Böhmelt AH (eds) Startle modification: implications for neuroscience, cognitive science, and clinical science. Cambridge University Press, Cambridge, pp 114–133
Swerdlow NR, Paulsen J, Braff DL, Butters N, Geyer MA, Swenson MR (1995) Impaired prepulse inhibition of acoustic and tactile startle response in patients with Huntington’s disease. J Neurol Neurosurg Psychiatry 58:192–200
Taylor JL, McCloskey DI (1990) Triggering of preprogrammed movements as reactions to masked stimuli. J Neurophysiol 63:439–446
Valldeoriola F, Valls-Solé J, Tolosa E, Ventura PJ, Nobbe FA, Martí MJ (1998) The effects of a startling acoustic stimulus on reaction time in patients with different Parkinsonian syndromes. Neurology 51:1315–1320
Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, González LE, Tolosa ES (1995) Reaction time and acoustic startle in normal human subjects. Neurosci Lett 195:97–100
Valls-Solé J, Valldeoriola F, Tolosa E, Nobbe F (1997) Habituation of the auditory startle reaction is reduced during preparation for execution of a motor task in normal human subjects. Brain Res 751:155–159
Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz JE (1999a) Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516:931–938
Valls-Solé J, Valldeoriola F, Molinuevo JL, Cossu G, Nobbe F (1999b) Prepulse modulation of the startle reaction and the blink reflex in normal human subjects. Exp Brain Res 129:49–56
Valls-Solé J, Summerfield C, Monells J, Kumru H, Sanegre MT, Contreras R (2002) The startle reaction as a probe for the preparation of subcortical motor pathways before movement execution. Clin Neurophysiol 113, S11 [Abstract]
Ziemann U, Rothwell JC, Ridding MC (1996) Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol 496:873–881
Acknowledgements
This work was accomplished in part thanks to grants number V-2003-REDC06H–O and P1040970 from Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valls-Solé, J., Kofler, M., Kumru, H. et al. Startle-induced reaction time shortening is not modified by prepulse inhibition. Exp Brain Res 165, 541–548 (2005). https://doi.org/10.1007/s00221-005-2332-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-2332-8