Abstract
Recent experiments pairing a startling stimulus with a simple reaction time (RT) task have shown that when participants are startled, a prepared movement may be triggered earlier in comparison to voluntary initiation (Carlsen et al. 2003, in press; Siegmund et al. 2001; Valls-Solé et al. 1999). The use of this paradigm in experiments may provide new insights into processes that control rapid voluntary actions. However, because the startle response habituates with repeated exposure to the startling stimulus, its use in experiments may be limited. Previously Brown et al. (1991) and later Siegmund et al. (2001) noted that individuals habituate to a startling stimulus at different rates depending on the required activity level of the participant in the task. The present experiment was designed to determine the rate at which participants habituate to a startle during the completion of a RT task. Participants completed 100 trials in which an active wrist extension to a target was performed as fast as possible following an auditory tone. An unexpected 124 dB auditory startle stimulus accompanied the imperative stimulus in 20 of these trials. For the duration of the experiment, startle response electromyographic (EMG) activity continued to be produced in the sternocleidomastoid muscle (SCM) indicating that habituation was not complete after 20 startle trials. Furthermore RT in the startle condition was significantly shorter than control RT. However, findings indicate that when a measurable EMG burst in the SCM was present, RT was significantly shorter than when no SCM burst was present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habituation has been described as one of the most basic forms of nonassociative learning, which involves a decrease in behavioural response to a repeated stimulus (Kupfermann 1991). Startle response habituation has been observed in most studies involving the use of a startling stimulus (Abel et al. 1998; Davis 1984; Davis and Heninger 1972; Leaton et al. 1985; Schicatano and Blumenthal 1998; Valls-Solé et al. 1997), and while a decrease in EMG response amplitude was observed, response latency was unaffected (Schicatano and Blumenthal 1998). Experimental results indicated that electromyographic (EMG) activity in the sternocleidomastoid (SCM) muscle was most consistent and among the last to disappear when participants were repeatedly exposed to the startling stimulus, making it the most important independent indicator of a startle response (Brown et al. 1991). Although activity in the orbicularis oculi (OOc) is also associated with the startle response and has been used extensively as an indicator of startle, it is also responsible for the physiologically separate blink response, making it an ambiguous indicator of startle (Brown et al. 1991)
Response reductions observed during habituation are thought to be caused by depressed synaptic transmission in the involved neural circuit (Kandel 1991). Although the neural mechanism of startle habituation is not well understood (Jordan et al. 2000), it is thought to be independent of processes underlying habituation of other systems. Two theories regarding the processes underlying habituation have been forefront in the literature (Rimpel et al. 1982). The first theory involves a reduction in the effectiveness of synaptic transmission. Repeated stimulation of the neuron itself leads to decreased neurotransmitter production and release resulting in diminished post-synaptic potentials (Rimpel et al. 1982). The other mechanism involves the build-up of activity in an inhibitory side chain. This theory holds that repeated stimulation acts not only on the neuron that habituates, but also on a side chain that may exert progressively increasing presynaptic or postsynaptic inhibition on the habituating neuron resulting in a depressed response (Wickelgren 1967).
An unexpected, loud acoustic stimulus (124 dB) has also been shown to elicit a startle response in participants preparing to react to a "go" signal (82 dB) during a simple reaction time (RT) task. Valls-Solé et al. (1999) demonstrated that premotor RT could be reduced by as much as 77 ms when participants were startled. Valls-Solé et al. (1999) suggested that the prepared response was triggered from subcortical areas because the voluntary response was produced at latencies too short to have involved the cerebral cortex. Two other lines of evidence also support the notion of a startle-elicited response. First, the observed response-related EMG activation pattern (e.g. Wadman et al. 1979) triggered by the startling stimulus was similar in both burst duration and timing to that produced when participants performed the task in the absence of a startling stimulus (Carlsen et al., in press; Valls-Solé et al. 1999). Thus it was argued that effect of a speeded response was not produced simply by an early startle response superimposing on to a later voluntary response. Second, task accuracy was maintained during the startle-elicited response. Specifically the startle had no effect on aiming accuracy, suggesting that the response produced at a short latency was indeed the one that was prepared (Carlsen et al., in press).
Due to habituation of the startle response, employing it as a tool in RT investigations can be problematic. Several difficulties arise when startling a participant multiple times in the course of an experiment. For instance, there are conflicting reports regarding the rate of habituation depending on the activity required of the participant. Brown et al. (1991) found that when participants were sitting quietly, there was no measurable EMG response present following two to six random presentations of the startling stimulus. In contrast, a recent study by Siegmund et al. (2001) reported that no habituation occurred when participants were engaged in a RT task. Specifically, participants were instructed to make a swift rotational head movement in order to look at a target. Because reaction times remained speeded in the startle condition throughout the study, and response kinematics and EMG amplitudes were unchanged after 14 presentations of the startling stimulus, the authors argued that habituation did not occur. This was in contrast to observed habituation in startle only (no movement) trials. However, the most important independent indicator that a startle occurred, the SCM muscle (Brown et al. 1991), was also the prime mover in this study. Therefore, the suggestion that a startle occurred was inferred from other factors. First, the RT during the startle (ST) trials was significantly shorter than control trials, and voluntary activation of the SCM and paraspinal muscles (PARA) in the ST trials was aligned with startle activation of these muscles in the startle-only trials. Thus, although the amplitudes and latencies revealed a startle-like pattern throughout the experiment, the results may have been contaminated, as voluntary activation was required by the same muscles. The OOc and masseter muscles, which were also measured, have been shown to be unreliable and disputed indicators of a startle (Brown et al. 1991).
The effect of repeated startle stimulation was also investigated by Valls-Solé et al. (1997) who observed no habituation under certain conditions. Specifically, participants who were startled when preparing to react to a "go" signal in a RT task showed no habituation to the startling stimulus, while participants sitting quietly or resting in a busy environment did show habituation. During the RT task, the rate of habituation was significantly decreased following five presentations of the stimulus. Specifically, peak EMG amplitude in the SCM and MAS did not decrease below 60% of initial amplitude, whereas in all other conditions, peak EMG amplitude in these muscles fell below 20% of initial values by the fifth presentation of the stimulus. Unfortunately, the study did not determine how many startling stimuli could be presented before habituation of the response occurred.
Taken together, it appears from these studies that habituation does not occur in a similar manner or with a similar time course when participants are engaged in voluntary activities compared to when they are sitting quietly. However, what remains unclear is the time course of the habituation process when a RT task is involved. The aim of the present experiment was to determine how many times a participant could be startled in a RT experiment before the stimulus became ineffective in producing a startle response and significantly speeding the prepared action in a RT task. The study was designed to determine the effects of repeated random startle stimulation on the EMG responses of the startle indicators (orbicularis oculi and sternocleidomastoid) and on premotor RT (PMT). The task employed was a targeted wrist extension involving the wrist extensors (extensor carpi radialis longus) and flexors (flexor carpi radialis). These results will be important in informing the design of future experiments involving the startle response and RT tasks.
Materials and methods
Participants
Twelve right-handed volunteers (8 M, 4F; ages 25±5 years) with no obvious upper body abnormalities or sensory or motor dysfunctions volunteered to participate in the study after giving informed consent. The participants were all naïve to the hypothesis under investigation. Testing of each participant took place in one afternoon session. This study was conducted in accordance with ethical guidelines established by the University of British Columbia.
Task
The experimental task was to perform a 20 deg extension movement with the right wrist to a fixed target as quickly and as accurately as possible following an auditory stimulus. Participants were encouraged to react as soon as possible following the stimulus, and were offered a monetary bonus for doing so. This was done because RT studies in which the level of motivation of the participant was manipulated have indicated that RT can be affected by motivational instructions (Shankweiler 1959).
Participant position
The participants sat in a height-adjustable chair outfitted with an automobile racing harness (Racer Components Inc.) in order to constrain any movement to the wrist joint. The right arm was secured, in a semi-prone position with the palm facing inward, to a custom-made aluminium wrist manipulandum that moved in the transverse plane with an axis of rotation at the wrist joint. The hand was secured in the hand support portion of the manipulandum to restrict any unwanted movement with the wrist joint directly in line with the axis of rotation and the manipulandum arm. The manipulandum was oriented at an angle of 15 degrees to the right of the body midline, as this has been found to be a more comfortable position than orienting the manipulandum parallel to the body midline. The starting position (20 degrees of flexion from neutral) was indicated by a mechanical stop. Prior to testing, the arm / manipulandum unit was obscured from view so that direct visual feedback was not available.
Recording equipment
Surface EMG data were collected from the muscle belly of the following superficial muscles: right flexor carpi radialis (FCR), right extensor carpi radialis longus (ECR), left orbicularis oculi (OOc), and left sternocleidomastoid (SCM) muscles using bipolar preamplified Ag/AgCl surface electrodes (Therapeutics Unlimited). The recording sites were prepared and cleansed in order to decrease electrical impedance. The electrodes were oriented parallel to the muscle fibers, and then attached using double sided adhesive strips. A grounding electrode was placed on the participant's left radial styloid process. EMG data were amplified onsite and the electrodes were connected via shielded cabling to an external amplifier system (Therapeutics Unlimited Inc. Model 544). Wrist angular displacement data were collected using a potentiometer attached to the pivot point of the manipulandum. All data were digitally sampled at 2 kHz (National Instruments® AT-MIO-16) using a customized program written with LabVIEW® software (National Instruments Inc.).
Stimuli
The warning tone consisted of three short beeps (100 ms, 1000 Hz, 80 dB each, separated by 500 ms) generated by the computer using a 16 bit sound card (Creative SoundBlaster 16®) and standard computer speakers (Juster® sp-691n). A fixed foreperiod of 2.5 s spanned the time between the end of the warning tone and the imperative stimulus. A computer program generated the trial imperative stimuli consisting of a narrow band noise pulse (1 kHz, 40 ms duration). The signal was amplified and presented via a loudspeaker (<1 ms rise time) placed directly behind the head of the participant with an intensity of either 80 dB (control imperative stimulus) or 124 dB (startle tone). The stimuli intensities were measured using a sound level meter (Cirrus Research model CR:252B) at a distance of 30 cm from the loudspeaker (approximately the distance to the ears of the participant).
Target and feedback
The target was a fixed point in space located at 20 degrees of angular displacement into extension with respect to the right wrist's starting position. A computer screen placed directly in front of the participant provided real time feedback during trials by representing the position of the manipulandum with a vertical marker line (1 cm tall) on the screen. The marker's movement corresponded directly to movement of the manipulandum and only moved in the horizontal plane. The starting position of the marker corresponded to it being stationary 5 cm from the left edge of the computer screen. The target was represented by a blue target line (1 cm tall), 10 cm from the right edge of the screen. After each trial, feedback information including trial outcome (accepted or rejected), displacement error at the end of the initial impulse (deg), reaction time (ms) and movement time (ms) were displayed on the same computer monitor display.
Training
Participants were allowed to practice the task prior to testing to familiarize themselves with the task and equipment. The participants were instructed that they would first hear a warning tone, followed by a foreperiod (duration unknown to the participants), and finally a "go" tone (imperative stimulus). Instructions emphasised fast reaction times and fast movement times, as well as minimising target error. Participants were also instructed that the loudness of the stimulus would be variable. Participants received blocks of ten practice trials, and were deemed to have reached an adequate level of task competence to start the testing trials when they could successfully hit the target (±5 deg) four out of the last five practice trials in a block. No participants performed more than two practice blocks.
Experimental trial types
Control trials were trials in which the participant carried out the normal protocol of the experiment. Startle trials (ST) consisted of trials in which the startle stimulus was given in place of the imperative non-startle stimulus.
Participants performed 4 blocks of 25 accepted trials in which 5 ST trials were randomly dispersed for a total of 20 startle trials per participant out of 100 total accepted trials. Control Trials in which the participant did not react, in which displacement RT was more than 500 ms or less than 50 ms, or in which there was more than ±10 degrees error, were rejected. Startle trials were never rejected. ST trials did not occur within the first three trials of any block and there were never two consecutive ST trials. Four catch trials (also excluded from analysis) in which there was no imperative stimulus occurred randomly in each block. This was done to discourage incorrect anticipation and false starts.
Data reduction
Movement onset was defined as the first point of a change of more than 0.2 deg of angular displacement from the starting position following the stimulus. Peak displacement was determined by identifying the point at which velocity returned to zero following movement onset. The final position of the movement was defined as the first point at which angular velocity remained below 8 deg/sec for at least 150 ms. Movement time was defined as the time (in ms) between movement onset and final position.
Surface EMG burst onsets were defined as the point at which the EMG first began a sustained rise above baseline levels. The location of this point was determined by first displaying the EMG pattern on a computer monitor with a superimposed line indicating the point at which activity increased to more than 2 standard deviations above baseline (mean of 50 ms of EMG activity preceding movement). Onset was then verified by visually locating and manually adjusting the onset mark to the point at which the activity first increased. This method allowed for correction of errors due to the strictness of the algorithm. Premotor RT (PMT) was defined as EMG onset in the ECR muscle. Peak EMG amplitudes were defined as the largest EMG amplitude, rectified and filtered with a 25 Hz lowpass elliptic filter, recorded within an interval of 100 ms following EMG burst onset. EMG offsets were marked in a similar fashion, with the activity between EMG onset and EMG offset being defined as a distinct burst. To normalise the EMG for comparison between participants, ST trial EMG burst amplitudes for the ECR and FCR were expressed as a percentage of the mean peak EMG amplitude for each respective muscle in the Control condition for each participant. Since there was not normally SCM or OOc activity in the Control condition, ST trial peak EMG amplitude for these muscles was expressed as a percentage of the respective EMG amplitudes in the first ST trial in which SCM and OOc activity was observed.
Statistical analyses
Dependent measures (PMT, peak displacement, movement final position, peak EMG amplitude, and EMG burst timings) were analyzed using one-way repeated measures analysis of variance (ANOVA), to determine if differences existed between Control and Test trials. Differences with a probability of less than .05 were considered to be significant. Tukey's Honestly Significant Difference (HSD) post-hoc tests were administered to determine the locus of the differences.
Results
Startle response indicators
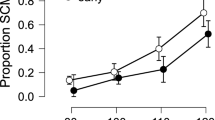
The amplitudes of rectified and filtered EMG from both startle response indicators, SCM and OOc, were compared between the successive ST trials and results are presented in Fig. 1. Amplitudes were subjected to a one-way (20 ST trials) repeated measures ANOVA. No main effect was found for trial position for either the SCM, F (19,209)=1.334, p=0.165, or the OOc, F (19,209)=1.503, p=0.124, indicating EMG amplitude was not different for any of the ST trials for either SCM or OOc. No significant trend was found for the SCM. However, there was a significant linear trend in OOc, F (1,11)=23.230, p=0.001, indicating that in the OOc, EMG amplitude tended to decrease linearly from the first ST trial to the 20th ST trial.
Mean peak EMG amplitude (SE) of startle indicator muscles, sternocleidomastoid (SCM) and orbicularis oculi (OOc), for each startle (ST) trial in order of presentation as a percentage of the EMG amplitude in the first ST trial in which activity was observed (activity was absent in the first ST trial for two participants; therefore mean % amplitude in ST1 was less than 100)
Although startle response EMG burst activity in the OOc was present in 89.4% of the ST trials, SCM burst activity was only present in 66.1% of the ST trials. However, the incidence of ST trials in which there was no SCM activity recorded was no higher in the last ten ST trials (39.2% of ST trials) compared to the first ten ST trials (27.5%), χ 2=3.15, p>0.05, across all participants (see Fig. 2). Observed mean onset latencies for SCM (62.9 ms) and OOc (43.0 ms) were similar to those reported previously (see Brown et al. 1991).
Response EMG
Analysis of PMT revealed that RT was significantly shorter in the ST condition (98.6 ms) compared to the Control condition (127.9 ms), F (1,11)=35.057, p<0.001. Furthermore, there was no difference in PMT in the ST condition between the first startle and the 20th startle, F (19,209)=0.632, p=0.767, indicating that throughout the experiment in the ST condition, PMT remained consistently shorter than in the Control condition (see Fig. 3).
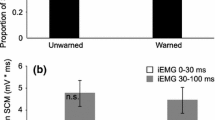
Given that in some ST trials startle EMG activity was absent, it was unclear if the participants were actually startled. Therefore, we separated ST trials in which there was no startle indicator activity from the other ST trials. Premotor RT was compared between Control trials, ST trials in which SCM activity was observed (ST+) (n=160), and ST trials in which no SCM activity was observed (ST-) (n=80). Results are summarized and illustrated in Fig. 4 and Table 1. A main effect was found for trial type, F (2,18)=27.786, p<0.001, with post-hoc analysis indicating that PMT was significantly different (p<0.05) between all three conditions. PMT was significantly shorter in ST+ (91.3 ms) than ST- trials (110.8 ms). Furthermore, RT was significantly shorter in both ST situations than in Control trials (127.9 ms).
Premotor reaction time (SE) in each condition. Control trials (striped) can be compared to startle (ST) trials (black) or the components of the ST trials (white). Startle (ST) trials comprise ST trials in which sternocleidomastoid (SCM) activity was observed (ST+) and ST trials in which no SCM activity was observed (ST-)
Similar analysis was also performed on ST trials with or without OOc activity. In contrast to differences in RT with and without the presence of SCM activity, there were no differences in RT between ST trials in which OOc activity was observed (99.9 ms) versus ST trials with no OOc activity (99.8 ms).
Analysis of the timing characteristics of the triphasic EMG pattern revealed no differences between the Startle and Control conditions in the initial agonist (ECR1) burst duration, F (2,18)=0.768, p=0.479, antagonist (FCR) burst duration, F (2,18)=0.298, p=0.746, second agonist (ECR2) burst duration, F (2,18)=1.356, p=0.283, ECR to FCR inter-onset time, F (2,18)=0.329, p=0.724, or in ECR1 to ECR2 inter-onset time, F (2,18)=0.348, p=0.711 (Table 1), suggesting that the timing of the triphasic EMG pattern was unchanged across conditions. However, EMG amplitude differences were found, between the conditions in all three bursts including ECR1, F (2,18)=4.573, p=0.025, FCR, F (2,18)=6.359, p=0.008, and ECR2, F (2,18)=3.885, p=0.040 (Table 1). Post-hoc analysis revealed that EMG burst amplitude was larger (p<0.05) in the ST+ condition than the ST- and Control conditions for all bursts except ECR1, in which ST+ amplitude was found to be larger than Control amplitude, however, not different than ST- amplitude (see Table 1).
Response kinematics
Response kinematics were analyzed to determine if differences existed in movement production variables between the conditions. No significant differences were found between the conditions in final position, F (2,18)=2.385, p=0.115, or movement time, F (2,18)=0.957, p=0.403 (see Table 1). However, significant differences in peak displacement, F (2,18)=13.703, p<0.001, and time to peak displacement, F (2,18)=15.843, p<0.001, were found. Post-hoc comparison revealed that in both ST situations (ST+ and ST-), peak displacement was significantly larger than in the Control condition, although peak displacement was not different between the ST+ and ST- trials. In addition, time to peak displacement was significantly shorter for the ST+ condition compared to the ST- and Control conditions (Table 1). Only two Control trials were removed from analysis due to errors (>10 deg target error). No errors occurred during ST trials.
Discussion
Startle response habituation
Previous reports have indicated that in humans sitting quietly, and not engaged in a motor activity, startle habituation was complete (disappearance of startle response EMG activity) after two to six random presentations of the startling stimulus (Brown et al. 1991; Valls-Solé et al. 1997). However, other evidence suggested that when participants were prepared to perform a RT task, habituation was diminished or absent (Siegmund et al. 2001; Valls-Solé et al. 1997). In the present study, peak EMG amplitude in both SCM and OOc was used to measure the startle response amplitude, with emphasis on the SCM. No significant decrease in SCM or OOc peak amplitude was found from the first startle trial (ST1) to the 20th startle (ST20) across subjects. These findings indicate that when engaged in a RT task, startle response habituation was absent, even after 20 startle trials. This finding is in agreement with previous reports (Valls-Solé et al. 1997), and extends the previous findings to include up to 20 startle trials. In the current study, the ratio of Control to ST trials was 5:1. It remains unclear, however, whether habituation might occur differently if the startling stimulus was presented on every RT trial. We therefore suggest that the role of the ratio of ST trials to Control trials on startle response habituation deserves further examination. In addition, upon examination of individual data (Fig. 2), it appears that two participants did habituate to the startling stimulus, since no SCM activity was observed in any of the last ten ST trials.
The previously observed reduction in startle habituation when participants were engaged in a RT task (Siegmund et al. 2001; Valls-Solé et al. 1997) has been attributed to two main factors. First, since the startle response has been shown to be modulated by cortical structures, cortical processes such as attention and gating might play a role in the excitability of the startle circuit. Although it has been argued that startle habituation is a process that occurs in the brainstem (Leaton et al. 1985), several studies have implicated higher brain centres in modulating the habituation. For example, in a review of lesioning studies involving the startle circuit, Davis (1984) reported that a decrease in habituation was observed in rats with lesions of either the hippocampus, the midbrain reticular formation, or with complete cerebral cortex transection. In addition, studies involving humans with cortical lesions have revealed decreased habituation and an increase in startle response (Liégeois-Chauvel et al. 1989). Similarly, Timmann et al. (1998), using positron emission tomography (PET), reported that during habituation, there is a decrease in cerebellar activity reflecting a decrease in tonic activity on reticular neurons involved in the startle circuit. These studies indicate that the excitability of the startle response may be at least partially under cortical or cerebellar control, and that the cortex as a whole may be inhibitory to the startle response.
Since cortical structures can influence the excitability of the startle response, cortical processes may also play a role in the modulation of the startle response. Behavioural evidence substantiates this possibility. For example, the use of a weak antecedent stimulus or "prepulse" results in reduced startle response amplitude (Graham 1975). This type of reflex modification has become known as prepulse inhibition (PPI) of startle (Davis 1984; Hoffman 1984; Lehmann et al. 1999) and is thought to reflect the ability of higher brain centres to filter or "gate" incoming stimuli (Abel et al. 1998; Blumenthal 1996; Fendt et al. 2001; Zhang et al. 1998). The extent of the startle modulation by a prepulse has been thought to be affected by the extent to which the prepulse can attract attention (Lipp et al. 2000). Similarly, attention directed towards a stimulus of the same modality as the startling stimulus has been shown to increase startle amplitude, whereas directing attention towards a different stimulus modality has been shown to decrease startle amplitude (Acocella and Blumenthal 1990; Richards 2000; Schicatano and Blumenthal 1998). Directed attention may modulate the startle response by enhancing the complimentary sensory systems and attenuating competing sensory systems (Richards 1998, 2000). Thus, by directing attention to a given sensory modality, the excitability of the involved neural networks may be increased.
A second argument explaining the reduction in startle habituation when participants are engaged in a RT task involves the influence of motor preparation (Siegmund et al. 2001; Valls-Solé et al. 1997). It has been demonstrated repeatedly using electroencephalography (EEG) that motor readiness is reflected by a slowly increasing bilaterally recorded negative potential (e.g., Brunia 1993). This negativity represents an increase in excitatory post-synaptic potentials (EPSPs); moreover, motor preparation results in increased excitability of all the structures involved in the execution of a motor command. For example, using transcranial magnetic stimulation (TMS) resulted in increased amplitude of evoked muscle potentials (MEPs) in the short time (up to 23 ms) preceding agonist onset, indicating an increased excitability of the motor response pathway (MacKinnon and Rothwell 2000; Rothwell et al. 2002). Thus readiness to perform a motor act may increase the excitability of both the cortical and subcortical components of the response pathway.
Dishabituation during a RT task
In the present study there were few ST trials in which OOc activity was absent (10.6%). Since the activity in the OOc was almost always present in ST trials, it is unlikely that its presence was a good indicator of a startle response. This same position was taken by Brown et al. (1991) who suggested that the blink reflex, also elicited by non-startling acoustic stimuli, may be physiologically separate from the startle response since the blink reflex continued to be produced despite no other manifestation of a startle response (Brown et al. 1991). In contrast to ST trials with no OOc activity, we observed several ST trials in which SCM activity was absent (33.9%). Only one participant exhibited SCM activity (indicating the presence of a startle response) in all 20 ST trials, and two participants out of 12 (16.7%) did not exhibit SCM activity in either of the first two trials. This number of low responders agrees with previous reports (Abel et al. 1998; Geyer and Braff 1982). However, data from these two participants were still taken into account in the present analysis. Interestingly, these same two participants appeared to habituate to the startle (no SCM activity observed in the last ten trials). This indicated that certain participants may be more prone to startle habituation. Across participants, however, the incidence of observing a ST trial in which there was no SCM activity was no higher in the last 10 ST trials as compared to the first ten ST trials (χ 2=3.15).
If habituation was reduced by a RT task requirement, it is unclear why there was an observed incidence of ST trials in which the participant was not startled (ST-) at random in nearly 34% of ST trials. Our contention is that habituation of the startle circuit itself may have still occurred normally as described previously (see Brown et al. 1991; Valls-Solé et al. 1997); but it may have been transiently overridden by other processes. Under normal circumstances (participants sitting quietly), startle habituation is complete after two to six presentations of the stimulus (Brown et al. 1991). Similarly, Siegmund et al. (2001) showed that no measurable startle response was elicited in control ST trials (participants sitting quietly) following either 7 or 14 startled RT trials. This was observed although no habituation was evident when participants were actively performing the RT task. This evidence seems to indicate that while the normal process of habituation was still occurring, other factors were overriding the habituation, allowing the startle response to continue to be elicited. Thus the reduced habituation reported by both Valls-Solé et al. (1997) and Siegmund et al. (2001) as a result of a RT task requirement might have instead been an "overriding of habituation" or dishabituation as opposed to a reduction of the neural processes leading to habituation. Dishabituation has been previously described as a sensitizing stimulus overriding the effects of habituation (Kupfermann 1991). In a similar way, in the present experiment, directed attention to the same stimulus modality as the startling stimulus, as well as motor readiness, may have both led to increased excitability of both the efferent and afferent pathways, transiently enabling the startle response. In this way, if participants were not sufficiently attending to the task, or if the participants were not sufficiently "ready" on a particular startle trial, the startle would not be enabled, leading to a non-startled ST trial (ST-), with no evidence of SCM activity.
Reaction time data
It has been shown that a startling acoustic stimulus can elicit a prepared ballistic response at very short onset latencies (Carlsen et al. 2003; Siegmund et al. 2001; Valls-Solé et al. 1995, 1999). In the present study, PMT was consistently and significantly lower across all ST trials (98.59 ms) compared to Control trials (127.92 ms). This result agrees well with previous findings (Carlsen et al., in press; Siegmund et al. 2001; Valls-Solé et al. 1995, 1999) indicating that the prepared response was speeded by the startle. Additionally, there was no significant difference in PMT in ST trials from ST1 to ST20, indicating that PMT remained significantly shortened throughout all ST trials. However, in comparing ST trials in which SCM activity was observed (ST+) to ST trials in which no SCM activity was observed (ST-), it was found that PMT was significantly lower when SCM activity was present (91.4 ms) than when SCM activity was not present (110.8 ms). This seems to indicate that in an auditory RT task, that both the observed startle response and the early triggering of the voluntary response have the same initial physiological basis. Therefore, the prepared movement may have been initiated by separate processes depending on whether a startle response occurred or not. We suggest that in certain ST trials, the participant was not startled. If the participant had habituated to the startle, but was not sufficiently ready, or was insufficiently attending to the task, the habituation may not have been overridden and the participant may not have been startled by the loud stimulus. Therefore if the reticular formation was not sufficiently activated to produce a startle response (as evidenced by activity in the SCM), it is unlikely that there would have been sufficient activity to trigger or release the prepared response (as evidenced by significantly slower PMTs). However, PMTs in ST trials (110.8 ms) were still significantly shorter than in Control trials (127.9 ms). Since it has been shown that louder stimulus intensities result in shortened reaction time (first recognized by Piéron 1919, cited in Woodworth 1938 p. 318; see also Kohfeld 1969) and the ST- trial responses occurred with a sufficient latency to have involved cortical areas, we propose that in the ST- trials the shorter PMT observed compared to the Control PMT was a result of sensory facilitation due to increased stimulus intensity.
Although the incidence of ST trials in which there was no OOc activity was much lower (10.6%) than ST trials with no SCM, these were also analysed for differences in RT. In contrast to RT differences due to the presence of SCM activity, there was no difference in PMT between ST trials with OOc activity (99.9 ms), or ST trials without OOc activity (99.8 ms). Because activity in the OOc did not allow for the discrimination between ST trials in which PMT was significantly shortened or not, we presume that OOc was not a good indicator of the presence of a startle response.
Kinematic analysis
Kinematic analysis revealed that there were no differences in movement time (MT) or final position (accuracy) between Control and ST trials, indicating that the required response was produced with similar timing and accuracy across conditions (Table 1). This finding is in agreement with previous reports (Carlsen et al., in press). Furthermore, no differences were found in either EMG durations or EMG interburst intervals between Control and ST trials, further indicating that the response that was produced was unchanged (in terms of EMG timing characteristics) between the conditions (Table 1). However, results showed that peak displacement and the time to peak displacement were different between the ST+ and Control conditions (see Table 1). Specifically, compared to the Control condition, participants produced a movement in the ST+ trials with a larger peak displacement (31.2 deg vs. 24.9 deg), while reaching peak displacement in a shorter time (105.8 ms vs. 119.2 ms). Interestingly, however, final position was unchanged, as was MT. The observed larger peak displacement might be explained by the differences observed in the EMG amplitudes which were found to be significantly larger in the ST+ trials compared to the Control condition. Increased amplitude of the initial agonist burst has been attributed to the startle volley summing with the voluntary response (Siegmund et al. 2001). In this way, an increased EMG burst amplitude due to startle and voluntary response summation may have resulted in a larger impulse and a larger peak displacement. Interestingly, in all conditions the movement was competed accurately, and with the same total MT. However, it is unclear how accurate completion of the task could have been accomplished if a motor program was released by the startle. It is unlikely that detection of an error and subsequent voluntary corrections to the movement could have been completed in the short amount of time (~150 ms) between peak displacement and final position, since this amount of time is similar to voluntary reaction time estimates. Furthermore, in the ST+ trials, EMG amplitude was elevated for all three phases of the triphasic pattern (Table 1). Summation with the startle volley cannot explain elevated amplitudes for the antagonist and second agonist bursts since they occur much later than the startle volley. This pattern of results seems to indicate that what is triggered by the startle is not a prepared set of muscle actions (i.e. motor program, see Keele 1968), but a single control variable that defines the movement endpoint or equilibrium point. This control variable has been proposed to be the threshold (λ) of a length sensitive reflex (Feldman 1986; Latash and Gottlieb 1991). Based on this model, the EMG would arise as a consequence of the movement. Thus, if the control variable was released, but EMG "leakage" from the startle volley (Siegmund et al. 2001) summed with the agonist EMG burst this would result in a larger peak displacement. Furthermore, since the control variable (λ) was unchanged, compensatory EMG would result automatically in the antagonist to bring the wrist back to the correct endpoint. In this way, if the startle actually releases a control variable that defines the goal of the movement, the startled movement might be thought of as evidence for endpoint control. However, more research is required in order to support this hypothesis.
Conclusions
In summary, we suggest that although physiological habituation of the startle response occurs even when the participant is engaged in a RT task, that the increased excitability of the response pathway due to motor readiness and attentional processes may be sufficient to allow the startle response to be elicited indefinitely. Furthermore, it appears that activity in the SCM is the minimum adequate EMG indicator of whether a physiological startle response has been elicited. Results indicate that when there is activity in the SCM, there is sufficient activity to trigger a prepared response, whereas if there is no SCM activity, the response is triggered normally via cortical control. We suggest that the startle is a useful tool for probing RTs and investigating neural processes involved in response preparation; however, startle trials in which there is no SCM activity present should be treated separately from ones in which there is SCM activity observed. This would allow for the treatment of truly startled trials as a fully separate group of trials from stimulus intensity facilitated trials.
References
Abel K, Waikar M, Pedro B, Hemsley D, Geyer M (1998) Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls. J Psychopharmacol 12:330–337
Acocella CM, Blumenthal TD (1990) Directed attention influences the modification of startle reflex probability. Psychol Rep 66:275–285
Blumenthal TD (1996) Inhibition of the human startle response is affected by both prepulse intensity and eliciting stimulus intensity. Biol Psychol 44:85–104
Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD (1991) New observations on the normal auditory startle reflex in man. Brain 114:1891–1902
Brunia CH (1993) Waiting in readiness: gaiting in attention and motor preparation. Psychophysiology 30:327–339
Carlsen AN, Nagelkerke P, Garry M, Hodges N, Franks IM (2000) Using the startle paradigm to investigate the nature of a prepared response. J Sport Exerc Psychol 22S:S24
Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R (2003) Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol 89:1857–1863
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (in press) Prepared movements are elicited early by startle. J Motor Behav
Davis M (1984) The mammalian startle response. In: RC Eaton (ed) Neural mechanisms of startle behavior. Plenum, New York, pp 287–351
Davis M, Henninger GR (1972) Comparison of response plasticity between the eyeblink and vertex potential in humans. Electroencephalogr Clin Neurophysiol 33:283–293
Feldman AG (1986) Once more on the equilibrium-point hypothesis (λ model) for motor control. J Mot Behav 18:17–54
Fendt M, Li L, Yeomans JS (2001) Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology 156:216–224
Fox JE (1978) Excitatory and inhibitory components of the eye-blink response to startle evoking stimuli, studied in the human subject. Electroencephalogr Clin Neurophysiol 44:490–501
Geyer MA, Braff DL (1982) Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology 19:1–6
Graham FK (1975) The more or less startling effects of weak prestimulation. Psychophysiology 12:238–248
Hoffman HS (1984) Methodological factors in the behavioral analysis of startle: the use of reflex modification procedures in the assessment of threshold. In: Eaton RC (ed) Neural mechanisms of startle behavior. Plenum, New York, pp 267–286
Jordan WP, Strasser HC, McHale L (2000) Contextual control of long-term habituation in rats. J Exp Psychol Anim Behav Proc 26:323–339
Kandel ER (1991) Cellular mechanisms of learning and the biological basis of individuality. In: Kandel ER, Schwartz JH, Jessel TM (eds) Principles of neural science, 3rd edn. Elsevier, New York, pp 816–834
Keele SW (1968) Movement control in skilled motor performance. Psychol Bull 70:387–403
Kohfeld DL (1969) Effects of the intensity of auditory and visual ready signals on simple reaction time. J Exp Psychol 82:88–95
Kupfermann I (1991) Learning. In: Kandel ER, Schwartz JH, Jessel TM (eds) Principles of neural science, 3rd edn. Elsevier, New York, pp 805–815
Latash ML, Gottlieb GL (1991) An equilibrium-point model for fast single-joint movement: I. emergence of strategy-dependent EMG patterns. J Mot Behav 23:163–177
Leaton RN, Cassella JV, Borszcz GS (1985) Short-term and long-term habituation of the acoustic startles response in chronic decerebrate rats. Behav Neurosci 99:901–912
Lehmann J, Pryce CR, Feldon J (1999) Sex differences in the acoustic startle response and prepulse inhibition in Wistar rats. Behav Brain Res 104:113–117
Liégeois-Chauvel C, Morin C, Musolino A, Bancaud J, Chauvel P (1989) Evidence for a contribution of the auditory cortex to audiospinal facilitation in man. Brain 112:375–391
Lipp OV, Siddle DAT, Dall PJ (2000) The effect of warning stimulus modality on blink startle modification in reaction time tasks. Psychophysiology 37:55–64
MacKinnon CD, Rothwell JC (2000) Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol 528.3:633–645
Ornitz EM, Russell AT, Yuan H, Liu M (1996) Autonomic, electroencephalographic and myogenic activity accompanying startle and its habituation during mid-childhood. Psychophysiology 33:507–513
Richards JE (1998) Development of selective attention in young infants: enhancement and attenuation of the startle reflex by attention. Dev Sci 1:45–51
Richards JE (2000) Development of multimodal attention in young infants: modification of the startle reflex by attention. Psychophysiology 37:65–75
Rimpel J, Geyer D, Hopf HC (1982) Changes in the blink responses to combined trigeminal, acoustic, and visual repetitive stimulation, studied in the human subject. Electroencephalogr Clin Neurophysiol 54:552–560
Rothwell JC, MacKinnon CD, Valls-Solé J (2002) Role of brainstem-spinal projections in voluntary movement. Mov Disord 17:S27–S29
Schicatano EJ, Blumenthal TD (1998) The effects of caffeine and directed attention on acoustic startle habituation. Pharmacol Biochem Behav 59:145–150
Scott BW, Frankland PW, Li L, Yeomans JS (1999) Cochlear and trigeminal systems contributing to the startle reflex in rats. Neuroscience 91:1565–1574
Shankweiler DP (1959) Effects of success and failure instructions on reaction time in patients with brain damage. J Comp Physiol Psychol 52:546–549
Siegmund GP, Inglis JT, Sanderson DJ (2001) Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535:289–300
Timmann D, Musso C, Kolb FP, Rijntjes M, Jüptner M, Müller SP, Diener HC, Weiller C (1998) Involvement of the human cerebellum during habituation of the acoustic startle response: a PET study. J Neurol Neurosurg Psychiatry 65:771–773
Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES (1995) Reaction time and acoustic startle in normal human subjects. Neurosci Lett 195:97–100
Valls-Solé J, Valldeoriola F, Tolosa E, Nobbe F (1997) Habituation of the auditory startle reaction is reduced during preparation for execution of a motor task in normal human subjects. Brain Res 751:155–159
Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E (1999) Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516.3:931–938
Wadman WJ, Denier van der Gon JJ, Geuze RH, Mol CR (1979) Control of fast goal-directed arm movements. J Hum Mov Stud 5:3–17
Wickelgren BG (1967) Habituation of spinal interneurons. J Neurophysiol 30:1424–1438
Woodworth RS (1938) Experimental psychology. Henry Holt, New York
Yeomans JS, Frankland PW (1996) The acoustic startle reflex: neurons and connections. Brain Res Rev 21:301–314
Zhang J, Engel JA, Ericson M, Svensson L (1999) Involvement of the medial genticulate body in prepulse inhibition of acoustic startle. Psychopharmacology 141:189–196
Author information
Authors and Affiliations
Corresponding author
Additional information
Anthony N. Carlsen, Romeo Chua, J. Timothy Inglis, David J. Sanderson, and Ian M. Franks, School of Human Kinetics, University of British Columbia, Vancouver, Canada, and the International Collaboration on Repair Discoveries (ICORD). This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada awarded to Ian M Franks.
Rights and permissions
About this article
Cite this article
Carlsen, A.N., Chua, R., Inglis, J.T. et al. Startle response is dishabituated during a reaction time task. Exp Brain Res 152, 510–518 (2003). https://doi.org/10.1007/s00221-003-1575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1575-5