Abstract
A number of studies have shown the importance of halophyte species as sources of natural antioxidants. Crithmum maritimum is considered an edible and medicinal plant, though in vivo studies of this plant still are necessary to elucidate its potential benefits in alleviating toxicity. The present work was undertaken to investigate the major components and the antioxidant profile of C. maritimum leaf hydro-methanolic extract (CME) and the protective effects of C. maritimum (CM) against CCl4-induced toxicity in rats. Using LC–ESI–MS analysis, 17 phenolic compounds were identified in plant extract, with chlorogenic acid being the major component. The levels of total phenolic acids, total flavonoids and flavonols were ca. 26 mg GAE/g DW, 15.6 mg CE/g DW and 12 mg QE/g DW, respectively. Accordingly, CME showed important in vitro antioxidant activities (DPPH and ORAC). Moreover, CM supplementation to CCl4-intoxicated rats partially restored the impaired hepatic markers (ALT and AST activities and creatinine level), and reduced the CCl4-induced oxidative stress as shown by lipid peroxidation, GSH and protein carbonyl levels. The drug metabolizing system was also evaluated in liver by the measurement of some cytochrome P450-dependent activities such as ECOD, EROD and pNPH and the antioxidant DT-diaphorase, CAT and heme oxygenase activities. Thus, CM treatment reduced the enzymatic perturbations induced by CCl4 and hepatic damages observed from histopathological examination. The obtained results highlight the potential interest of C. maritimum as a source of bioactive compounds with relevant hepatoprotective effects.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Considering increasing global climate change and severe conditions that prevail throughout the world, cultivation of conventional crops is facing various limitations related with scarcity of good quality water, temperature increase, salinization, heavy metals contamination and degradation of soil properties, especially in arid and semi-arid regions of the Mediterranean basin [1, 2]. The common feature of the plant responses to different stresses is the production of reactive oxygen species (ROS) [3]. The overproduction of ROS in plants causes impairment of redox homeostasis, resulting in oxidative stress [4, 5]. The oxidative stress generated by ROS in plants has detrimental effects on crop production [6]. Therefore, it is of utmost importance to valorize other edible and medicinal species that can adapt under harsh conditions within the framework of saline agriculture, thus being good candidates as potential cash food and medicinal crops [7].
More than 100 halophyte species are promising candidates with important nutritional, medicinal and economic interests [2]. These salt-tolerant plants have developed adaptive responses, including the accumulation of highly bioactive molecules with powerful antioxidant capacities (e.g. phenolic compounds, antioxidant enzymes and vitamins), to cope with the production of ROS and to protect the cellular structures from these highly toxic entities [3, 8]. These natural antioxidants generally exhibit strong biological activities, such as radical scavenging, metal chelation and enzyme induction capabilities, leading to beneficial therapeutic properties. Thus, some halophytic species which accumulate them are used as ingredients in many traditional dishes, recipes and beverages [9,10,11,12]. Along, the recent advances in food science and technology dictate the evaluation of plant safety profile via metabolomic and toxicological studies to produce a safety and healthy food [8, 12].
The perennial halophyte sea fennel (Crithmum maritimum, Apiaceae) is a typical plant of the Mediterranean, Pacific and Atlantic coasts [4, 5, 13]. Interestingly, its growth is stimulated by low salinity, and it is tolerant to salt stress and climate variations [14], making it a promising crop in the context of biosaline agriculture. C. maritimum was recognized as a promising crop for human nutrition [15] since its leaves are a source of minerals and vitamins and have a convenient nutritional profile for human consumption and animal farming. Already, they are currently used as salads, pickles and to prepare soups [13, 14]. The areal parts of this perennial halophyte represent various economical interests, due to its high contents in flavonoids, carotenoids, vitamin C and substances with nutraceutical and antimicrobial properties [14]. In the Mediterranean regions, sea fennel has a good reputation as a traditional remedy. As an example, in Italy, the decoction of aerial parts has been used to treat whooping cough and pain, inflammations of urinary tracts and prostate, colics, as well as a liver-detoxifying remedy [16, 17].
Many plant extracts and plant-derived compounds have been tested using animal models for their potential protective activities against hepatotoxic agents such as carbon tetrachloride (CCl4) [18, 19]. Although the aerial parts of C. maritimum are widely studied in vitro [8, 12, 14, 20, 21], no in vivo studies using this plant have been reported hitherto. Therefore, the present study was undertaken to explore along with the antioxidant properties of C. maritimum leaf hydro-methanolic extract (CME), and the possible protective effects of C. maritimum (CM) leaves against CCl4-induced hepatotoxicity in rats.
Materials and methods
Plant material
Leaves of Crithmum maritimum L. were identified and sampled on rocks along the shoreline at “Sainte Anne du Portzic” by Professor Christian Magné (Brittany, France) in August 2016. The leaves were cleaned with deionized water, rapidly soaked, stored at − 20 °C and then freeze dried. The dry material was ground to a fine powder, and stored until analysis.
Methanolic extraction
One gram of sea fennel leaf powder was macerated in 10 mL of 80% methanol overnight under stirring at room temperature. The mixture was centrifuged for 10 min at 3500×g at 4 °C (Jouan CR3i centrifuge, Newport Pagnell, UK) and supernatant was collected, filtered (0.2 mm VWR International PBI, Milan, IT), and kept at 4 °C in the dark until use. The extraction was repeated twice on the pellet and the three filtered supernatants were gathered. This procedure resulted in a C. maritimum extract (CME) with a yield of 22%.
Phytochemical characterization and phenolic compound profiling by LC–ESI–MS analysis
The total phenolic content was determined by the Folin Ciocalteu colorimetric method [22] and expressed as mg of gallic acid equivalents/g dry weight (mg GAE/g DW). The total flavonoid contents were quantified using the aluminum chloride colorimetric method [23] and expressed as mg catechin equivalent (CE)/g DW. The total flavonols were measured according to the method described by [24] and expressed as mg quercetin equivalent (QE)/g DW. The phenolic profile of C. maritimum extract was characterized by LC–ESI–MS analysis.
Determination of antioxidant activities (DPPH test and ORAC assay)
The scavenging activity on DPPH radical of methanolic extracts of C. maritimum leaves extracts was determined following the method reported by Sokmen et al. [25]. The Oxygen Radical Absorbance Capacity (ORAC) of C. maritimum leaf extract was evaluated according to the method reported by Bacchiocca et al. [26].
In vivo hepatoprotective assay
Animal treatments
This study was performed using male Wistar rats with body weight of 180–200 g. The animals received food and drinking water ad libitum and were maintained in cages under a 12 h light/dark cycle at room temperature with 55% relative humidity. The rats were separated into four groups of five animals: control rats (CNT), rats supplemented daily during 5 days with a water suspension of C. maritimum leaves by gavage at 300 mg/kg bw (CM), rats injected intra-peritoneally (i.p.) with a single dose of 1.5 mL CCl4/kg bw dissolved in corn oil (CCl4) [18] and sacrificed after 24 h, and rats given daily a water suspension of C. maritimum leaves at 300 mg/kg bw during 5 days followed on day fifth by a single i.p. dose of 1.5 mL CCl4/kg bw (50% in corn oil) (CCl4 + CM). Rats from each group were sacrificed at the end of day 6. The dose of the suspension of C. maritimum leaves was selected on the basis of previous studies on other halophyte plants [27]. Rats from each group were sacrificed at day sixth 24 h after the last injection. At the sacrifice, the animals did not show any visual symptoms.
All animal procedures were performed with the approval of the Local Ethical Committee and in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Biological sample collection
The blood samples were drawn by cardiac puncture and centrifuged at 3000×g for 10 min. The serum was stored at − 20 °C until analyses. Liver was carefully removed, washed with ice-cold saline solution and then stored at − 80 °C for future analysis. A portion of liver was collected and fixed in 10% formalin solution and immediately processed for histological study.
Serum biochemical analysis
The levels of creatinine and the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using a semiautomatic clinical chemistry analyser (model ARCO Biotecnica Instruments, SPA, Italy).
Hepatic oxidative stress biomarkers
The malondialdehyde (MDA) concentration of liver homogenates was analyzed as marker of lipid peroxidation according to [28], with some modifications. A standard curve was made from a solution of the hydrolyzed 1,1,3,3-tetramethoxypropane (TEP) in water. MDA concentrations were calculated as µmol/g tissue. The protein oxidation level was determined by the carbonyl protein assay according to [29]. Concentration of carbonylated proteins was calculated as nmol/g tissue. The glutathione (GSH) content was determined following the method described by [30].
Liver enzyme assays
The microsomal and cytosolic fractions from liver were prepared according to Longo et al. [50]. Protein content was determined according to the method of [31], using bovine serum albumin (BSA) as the standard.
The heme oxygenase activity was spectrophotometrically determined by quantifying the bilirubin produced from the reduction of biliverdin [32]. To determine catalase (CAT) activity, hydrogen peroxide (H2O2) was used as substrate [33]. The degradation of H2O2 was detected by a decrease in absorbance at 240 nm for 1 min and the enzyme activity was expressed as µmol H2O2 consumed per minute per mg of protein.
Ethoxycoumarin-O-deethylase (ECOD) and ethoxyresorufin O-deethylase (EROD) were determined fluorometrically by measuring the formation of 7-hydroxycoumarin and resorufin, respectively [34]. The pNPH activity was determined by measuring the formation of p-nitrocatechol [35]. The DT-diaphorase activity was assayed by following the reduction in dichlorophenolindophenol at 630 nm [36].
Histopathological analysis
Liver samples (n = 5) were collected from the rats of each experimental group for histological analysis and fixed in 10% neutral buffered formalin. After overnight fixation, livers were routinely dehydrated (throughout alcohol series 40%, 70%, 95%, 100% and xylene) and embedded in paraffin wax blocks, sectioned at a thickness of 5 μm and stained with haematoxylin and eosin [37]. All the slide sections were examined under a light microscope.
Statistical analyses
Statistical analyses were performed using XLStat 2016®. Results were expressed as mean ± standard deviation (SD) and experiments were conducted at least in triplicate. Significant differences (p < 0.05) were assessed by one-way analysis of variance (ANOVA). If significant, the pairwise multiple comparison tests Tukey or Dunn's were applied.
Results and discussion
Antioxidant properties
The content of total polyphenols, flavonoids, flavonols and the antioxidant capacity were measured in C. maritimum leaf extract and depicted in Table 1. The extract contained 26.25 mg GAE/g DW of polyphenols, 15.6 mg CE/g DW of flavonoids, and 12.2 mg QE/g DW of flavonols. Similar results were found in sea fennel plants harvested during summer from the Breton cliffs [14] or in Tighzert region (Bejaïa, Algeria) [21]. Overall, sea fennel was confirmed to be rich in phenolic compounds, compared to other Apiaceae such as Foeniculum vulgare [38] or Eryngium maritimum [14].
The in vitro antioxidant capacity and the radical scavenging activity of CME, as measured using the ORAC and the DPPH assays, are shown in Table 1. CME had 11,253 ORAC units per gram of DW, and a DPPH IC50 of 0.25 mg/mL. Other studies reported high antioxidant activities of C. maritimum extract [8, 14, 39]. The concomitant high phenolic contents and antioxidant activities confirm that phenolic compounds are likely major contributors to the antioxidant activities of C. maritimum extracts [12, 40], as commonly considered for halophytic species [41].
Phytochemical profile of CME extract
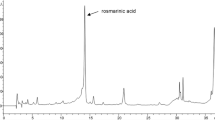
The phenolic profile of C. maritimum extract was characterized by LC–ESI–MS analysis and depicted in Fig. 1. Seventeen different compounds were detected and quantified, totalizing 18.19 mg/g DW (Fig. 1, Table 2). Other authors have reported a similar yield of these phytochemical compounds extracted from C. maritimum although with different extraction methods [12, 21]. Besides, [14] observed by NMR analysis that the most part of methanolic extract of this plant is represented by soluble sugars and organic acids (malate and quinate).
LC–ESI–MS analysis (280 nm) of soluble phenolic compounds in hydro-methanolic extract of C. maritimum. Peak numbers refer to the compounds shown in Table 2
Our analyses showed that the most represented phenolic classes in CME are phenolic acids, flavonoids and flavonols. Indeed, the high content in phenolic acids (and allied compounds) in the leaves of C. maritimum extract was mainly composed of chlorogenic acid (28.36%), quinic acid (organic acid) (20.12%), neochlorogenic acid (9.40%) and trans ferulic acid (6.32%). Among the flavonoids, rutin was the major compound detected. Besides, kaempferol (7.75%) and quercetrin (6.54%) were the most abundant flavonols [14] reported that C. maritimum extract exhibited a significant concentration of chlorogenic acid and quinic acid. Our analyses showed the presence of six hydroxycinnamic acids (n° 2, 3, 4, 7, 8, 9 in Table 2), in accordance with the results described by [12] in the water infusion or decoction of sea fennel leaves. However, these authors did not detect free quinic and caffeic acids [21] reported the HPLC/DAD–ESI–MS analysis of the hydro-methanolic extract of aerial parts of C. maritimum. They identified the six hydroxycinnamic acids we also measured and, at a lower level, 3-coumaroylquininic acid, cis-5-coumaroylquininic acid, 3,4-dicaffeoylquinic acid and 3,5-dicaffeoylquinic acid. The presence in the boiling water extract of sea fennel of these six major phenolic acids was also reported by [20]. On the other hand, among the flavonoids and flavonols in C. maritimum extract, only rutin and hyperoside have been previously reported [42], though kaempferol was found here as the major flavonol. All these secondary metabolites have been associated with the strong antioxidant activity of several vegetables and their health-promoting effects, as for example the modulation of glucose and lipid metabolism and the protective effects against ROS damages [43].
In vivo effects of CCl4 treatment and CM administration
CCl4 induces toxicity by generating the reactive intermediates trichloromethyl (CCl3*) and trichloromethyl peroxyl radical [44]. The activation of CCl4 is performed by the hepatic cytochrome P450 system and in particular by the P450 2E1 isoform [45]. These radicals alkylate cellular proteins and other macromolecules including polyunsaturated fatty acids, giving rise to lipid peroxidation (MDA) and protein carbonylation. Moreover, they cause damages to hepatic cells and kidney, leading to a leakage of aminotransferases (ALT and AST) and creatinine in plasma [45,46,47]. The GSH level, which is the prominent antioxidant in liver, is reduced by the reaction with hydrogen peroxide and hydroperoxides formed by the CCl4 treatment [48].
To investigate a possible protective role of CM against hepatotoxicity, we treated rats by CCl4. We used a suspension of C. maritimum leaves and not the extract at the light of recent studies that show how the administration of an extract can lead to a significant different modulation pathways of xenobiotic metabolizing enzymes, compared to the whole vegetable [49]. As a consequence, AST and ALT levels in plasma increased markedly (p < 0.01), indicating CCl4-induced liver injury (Table 3). Moreover, CCl4 treatment caused a strong oxidative stress, as seen by the significant increase of MDA level and protein carbonylation, as well as the decrease of the hepatic glutathione content and plasmatic creatinine (Table 3). These metabolic markers were not affected by the CM treatment alone highlighting that the dose of CM administered for 5 days was not toxic for animals. Interestingly, the pre-treatment of rats with CM prior to administration of CCl4 partially maintained control levels of GSH, protein carbonylation and creatinine. Moreover, CM pre-treatment significantly reduced the activities of ALT and AST (p < 0.05), when compared to CCl4-treated group. All these observations strongly suggest a protective role of CM against CCl4 toxicity, though it failed to impede lipid peroxidation (TBARS levels being similar in the CCl4 and CM + CCl4 groups).
To see the effect of CCl4 on drug metabolizing system, we have measured some P450-dependent activities such as EROD, ECOD, pNPH and other activities of phase II enzymes. As shown in Fig. 2, the administration of CM at the dose of 300 mg/kg bw did not significantly affect the activities of hepatic ECOD, EROD, and pNPH, which are biomarkers of multiple P450 isoforms, P450 1A1/2, and of P450 2E1, respectively [50]. The CCl4 treatment markedly induced the pNPH activity compared to control group, whereas EROD and ECOD activities were strongly decreased. Some studies have reported the reduction of many P450 activities after application of CCl4, and conversely the induction of P450 2E1 to perform the CCl4 metabolism, since this compound is a selective substrate of this enzyme [43]. Noteworthy, EROD and particularly pNPH did not change much in the CCl4-intoxicated rats when pre-treated with CM.
As observed for the P450-dependent oxidative metabolism, the activity of DT-diaphorase, CAT and heme oxygenase enzymes was not significantly affected by the CM treatment alone, compared to control (Table 3). Conversely, CCl4 treatment significantly increased the DT-diaphorase and heme oxygenase activities and reduced that of CAT (Table 4). These results are in agreement with the literature showing that CCl4 significantly decreases CAT activity [51], whereas it induces the activity of DT-diaphorase and heme oxygenase as an adaptive response to oxidation injury [52, 53]. Interestingly, pre-treatment of rats with CM partially mitigated the above-mentioned changes. Overall, these findings indicate the partial preventive protection against hepatic damages by CM administration at 300 mg/kg bw for 5 days. The disease prevention by natural products is at the heart of the scientific debate and the dose of the substance taken can be crucial. In the last decade, the scientific community has experienced how several compounds, specifically hypothesized to protect or reduce risk, have actually increased the incidence of the disease they hoped to prevent [54, 55].
Histopathological analysis
Histopathological examination of the rat liver tissues from the different groups is shown in Fig. 3. Control group (Fig. 3a, b) showed normal liver morphology with well-preserved cytoplasm and prominent nucleus in hepatocytes; same tissue architecture was observed in the liver from CM group (Fig. 3c, d), indicating that the CM alone had no negative effects on the liver anatomy and functionality. Conversely, treatment with CCl4 induced severe injuries in liver tissues such as cytoplasmic vacuolization of hepatocytes, cellular swelling, fatty degeneration, obvious tissue necrosis, congested central vein, and infiltration by inflammatory cells (Fig. 3e, f, arrows). Such observations confirm those previously reported by [53] and [56].
Photomicrographs of hepatic tissue in control and experimental treated rats. liver sections stained with hematoxylin and eosin (magnification ×50 and ×100). a, b Control group; c, d CM-treated rats; e, f CCl4-treated rats; and g, h CCl4 + CM-treated rats, arrows indicates lipids and erythrocyte infiltration in central vein
Noteworthy, CM pre-treatment mitigated histological features of CCl4-induced liver injuries and clearly ameliorated the pathological changes in liver tissues: steatosis and hepatocyte vacuolization were alleviated and the liver anatomy looked similar to those of the control or CM groups, indicating an hepatoprotective effect of the plant (Fig. 3g, h). The remarkable anti-steatosis effect of CM pre-treatment is likely due to the antioxidant compounds present in the plant leaves, since recent studies have shown that the use of natural antioxidants can positively act against steatosis and fibrosis [19, 56].
Conclusion
The present study confirmed that the hydro-alcoholic extract of C. maritimum leaves contains abundant soluble polyphenols with important antioxidant properties as demonstrated by their high ORAC and DPPH-scavenging activities. The phytochemical analysis using LC–ESI–MS allowed us to identify 17 compounds among hydroxycinnamic acids, flavonoids and flavonols. Moreover, our study used the highly toxic CCl4 as a model to induce acute liver damage. The administration of a suspension of C. maritimum leaves significantly prevented rats from the toxic effects of CCl4, as evidenced by its ability to partially restore the hepatic activities of some P450 enzymes, antioxidant enzymes such as catalase, DT-diaphorase and heme oxygenase, and the contents of GSH and protein carbonyls. Moreover, the histopathological results also suggested that C. maritimum has no adverse effects. Thus, the medicinal properties of C. maritimum are broaden by this work, and its richness in bioactive compounds with a great antioxidant potential makes CM a good candidate to be used as natural food and/or as a source of natural bio-antioxidants.
References
Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:1–15
Petropoulos SA, Karkanis A, Martins N, Ferreira ICFR (2018) Edible halophytes of the Mediterranean basin: potential candidates for novel food products. Trends Food Sci Technol 74:69–84
Souid A, Bellani L, Magné C, Zorrig W, Smaoui A, Abdelly C, Longo V, Ben Hamed K (2018) Physiological and antioxidant responses of the sabkha biotope halophyte Limonium delicatulum to seasonal changes in environmental conditions. Plant Physiol Biochem 123:180–191
Ben Hamed K, Castagna A, Elkahoui S, Ranieri A, Abdelly C (2007) Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regul 53:185–194
Atia A, Barhoumi Z, Mokded R, Abdelly C, Smaoui A (2011) Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J Med Plant Res 5:3564–3571
Ben Hamed K, Ellouzi H, Talbi OZ, Hessini K, Slama I, Ghnaya T, Munné Bosch S, Savouré A, Abdelly C (2013) Physiological response of halophytes to multiple stresses. Funct Plant Biol 40:883–896
Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S (2014) Halophyte agriculture: success stories. Environ Exp Bot 107:71–83
Jallali I, Zaouali Y, Missaoui I, Smaoui A, Abdelly C, Ksouri R (2014) Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem 145:1031–1038
Kang LP, Wu KL, Yu HS, Pang X, Liu J, Han LF, Zhang J, Zhao Y, Xiong CQ, Song XB, Liu C, Cong YW, Ma BP (2014) Steroidal saponins from Tribulus terrestris. Phytochemistry 107:182–189
Petropoulos S, Karkanis A, Martins N, Ferreira ICFR (2016) Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci Technol 55:1–10
Chedraoui S, Rajjou L (2017) Capparis spinosa L. in a systematic review: a xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front Plant Sci 8:1–18
Pereira CG, Barreira L, da Rosa NN, Nogueira JMF, Marques C, Santos TF, Varela J, Custódio L (2017) Searching for new sources of innovative products for the food industry within halophyte aromatic plants: in vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem Toxicol 107:581–589
Renna M, Gonnella M, Caretto S, Mita G, Serio F (2017) Sea fennel (Crithmum maritimum L.): from underutilized crop to new dried product for food use. Genet Resour Crop Evol 64:205–216
Méot-Duros L, Magné C (2009) Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol Biochem 47:37–41
Renna M, Gonnella M (2012) The use of the sea fennel as a new spice-colorant in culinary preparations. Int J Gastron Food Sci 1:111–115
Cornara L, La Rocca A, Marsili S, Mariotti MG (2009) Traditional uses of plants in the eastern riviera (Liguria, Italy). J Ethnopharmacol 125:16–30
Savo V, Giulia C, Maria GP, David R (2011) Folk phytotherapy of the Amalfi Coast (Campania, Southern Italy). J Ethnopharmacol 135:376–392
Longo V, Chirulli V, Gervasi PG, Nencioni S, Pellegrini M (2007) The Lisosan G, a powder of grain, does not interfer with the drug metabolizing enzymes and has a protective role on carbon tetrachloride-induced hepatotoxicity. Biotechnol Lett 29:1155–1159
Mejri H, Tir M, Feriani A, Ghazouani L, Allagui SM, Saidani-Tounsi M (2017) Does Eryngium maritimum seeds extract protect against CCl4 and cisplatin induced toxicity in rats: preliminary phytochemical screening and assessment of its in vitro and in vivo antioxidant activity and antifibrotic effect. J Funct Foods 37:363–372
Siracusa L, Kulisic-Bilusic T, Politeo O, Krause I, Dejanovic B, Ruberto G (2011) Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J Agric Food Chem 59:12453–12459
Nabet N, Boudries H, Chougui N, Loupassaki S, Souagui S, Burlo F, Hernandez F, Carbonell-Barrachina AA, Madani K, Larbat R (2017) Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int J Food Prop 20:1843–1855
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299:152–178
Kim DO, Chun OK, Kim YJ, Moon HY, Lee CY (2003) Quantification of polyphenolics and their antioxidant capacity in fresh plums. J Agric Food Chem 51:6509–6515
Romani A, Mancini P, Tatti S, Vincieri F (1996) Polyphenols and polysaccharides in Tuscan grapes and wines. Ital J Food Sci 8:13–24
Sokmen A, Gulluce M, Akpultat HA, Daferera D, Tepe B, Polissiou M (2004) The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control 15:627–634
Bacchiocca M, Biagiotti E, Ninfali P (2006) Nutritional and technological reasons for evaluating the antioxidant capacity of vegetable products. Ital J Food Sci 18:1–9
Gnanadesigan M, Ravikumar S, Inbaneson SJ (2011) Hepatoprotective and antioxidant properties of marine halophyte Luminetzera racemosa bark extract in CCL4 induced hepatotoxicity. Asian Pac J Trop Med 4:462–465
Seljeskog E, Hervig T, Mansoor MA (2006) A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clin Biochem 39:947–954
Terevinto A, Ramos A, Castroman G, Cabrera MC, Saadoun A (2010) Oxidative status, in vitro iron-induced lipid oxidation and superoxide dismutase, catalase and glutathione peroxidase activities in rhea meat. Meat Sci 84:706–710
Ellmann GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82:70–77
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Naughton P, Foresti R, Bains SK, Hoque M, Green CJ, Motterlini R (2002) Induction of heme oxygenase 1 by nitrosative stress—a role for nitroxyl anion. J Biol Chem 277:40666–40674
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Turini A, Amato G, Longo V, Mazzaccaro A, Gervasi PG (1998) Oxidation of methyl- and ethyl-tertiary-butyl ethers in rat liver microsomes: role of the cytochrome P450 isoforms. Arch Toxicol 72:207–214
Vornoli A, Pozzo L, Della Croce CM, Gervasi PG, Longo V (2014) Drug metabolism enzymes in a steatotic model of rat treated with a high fat diet and a low dose of streptozotocin. Food Chem Toxicol 70:54–60
La Marca M, Beffy P, Pugliese A, Longo V (2013) Fermented wheat powder induces the antioxidant and detoxifying system in primary rat hepatocytes. PLoS ONE 8(12):e83538
Mark M, Teletin M, Antal C, Wendling O, Auwerx J, Heikkinen S, Khetchoumian K, Argmann CA, Dgheem M (2007) Histopathology in mouse metabolic investigations. Curr Protoc Mol Biol 78:4.1–4.32
Roby MHH, Sarhan MA, Selim KAH, Khalel KI (2013) Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind Crop Prod 44:437–445
Mekinić IG, Šimat V, Ljubenkov I, Burčul F, Grga M, Mihajlovski M, Lončar R, Katalinić V, Skroza D (2018) Influence of the vegetation period on sea fennel, Crithmum maritimum L. (Apiaceae), phenolic composition, antioxidant and anticholinesterase activities. Ind Crop Prod 124:947–953
Houta O, Akrout A, Neffati M, Amri H (2011) Phenolic contents, antioxidant and antimicrobial potentials of Crithmum maritimum L. cultivated in Tunisia arid zones. J Biol Act Prod Nat 1:138–143
Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, Abdelly C (2008) Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol 331:865–873
Jallali I, Megdiche W, M’Hamdi B, Oueslati S, Smaoui A, Abdelly C, Ksouri R (2012) Changes in phenolic composition and antioxidant activities of the edible halophyte Crithmum maritimum L. with physiological stage and extraction method. Acta Physiol Plant 34:1451–1459
Shahidi F, Ambigaipalan P (2015) Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects: a review. J Funct Food 18:820–897
Weber LW, Boll M, Stampfl A (2003) Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33:105–136
Wang FW, Chan WY, Lee SS (1998) Resistance of carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharm 153:109–118
Drotman RB, Lawhorn GT (1978) Serum enzymes are indicators of chemical induced liver damage. Drug Chem Toxicol 1:163–171
Recknagel RO, Glende-Jr EA, Dolak JA, Waller RL (1989) Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther 43:139–154
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implication for health. J Nutr 134:489–492
Canistro D, Vivarelli F, Cirillo S, Costa G, Andreotti C, Paolini M (2016) Comparison between in toto peach (Prunus persica L. Batsch) supplementation and its polyphenolic extract on rat liver xenobiotic metabolizing enzymes. Food Chem Toxicol 97:385–394
Longo V, Mazzaccaro A, Naldi F, Gervasi PG (1991) Drug metabolizing enzymes in liver, olfactory and respiratory epithelium of cattle. J Biochem Toxicol 6:123–128
Szymonok-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Słomka M, Madro A, Celiński K, Wielosz M (2003) Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepato-Bil-Pan Surg 10:309–315
Nakahira K, Takahashi T, Shimizu H, Maeshima K, Uehara K, Fujii H, Nakatsuka H, Yokayanna M, Akagi R, Morita K (2003) Protective role of heme oxygenase-1 induction in carbon tetrachloride-induced hepatotoxicity. Biochem Pharmacol 66:1091–1105
Abdelhafez OH, Fawzy MA, Fahim JR, Desoukey SY, Krischke M, Mueller MJ, Usama RA (2018) Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE 13:e0202362
Potter JD (2014) The failure of cancer chemoprevention. Carcinogenesis 35:974–982
Bonamassa B, Canistro D, Sapone A, Vivarelli F, Vornoli A, Longo V, Paolini M (2016) Harmful effects behind the daily supplementation of a fixed vegetarian blend in the rat model. Food Chem Toxicol 97:367–374
Yang CC, Fang JY, Hong TL, Wang TC, Zhou YE, Lin TC (2013) Potential antioxidant properties and hepatoprotective effects of an aqueous extract formula derived from three Chinese medicinal herbs against CCl4-induced liver injury in rats. Int Immunopharmacol 15:106–113
Acknowledgements
This work was supported by the Institute of Biology and Agricultural Biotechnology (IBBA), CNR Pisa. Aymen Souid acknowledge the Italian minister of foreign affair and international cooperation (MAECI) for the Post-Doc grant. Also, we acknowledge Dr. Maristella Maltinti (Fondazione Toscana Gabriele Monasterio, Pisa) for her scientific support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with Ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Souid, A., Croce, C.M.D., Pozzo, L. et al. Antioxidant properties and hepatoprotective effect of the edible halophyte Crithmum maritimum L. against carbon tetrachloride-induced liver injury in rats. Eur Food Res Technol 246, 1393–1403 (2020). https://doi.org/10.1007/s00217-020-03498-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03498-9