Abstract
Lisosan G is a powder of grain registered as an alimentary integrator. The treatment of rats for 4 days with 0.5 g Lisosan G/kg had no effect on various drug metabolizing enzymes. Experiments in vitro showed that Lisosan G had radical scavenger activity. A confirmation of the antioxidative property of Lisosan G was also confirmed when it was administered in vivo to carbon tetrachloride (CCl4)-intoxicated rats. The toxicity caused by CCl4-treatment of rats was restored to the control levels when the rats were given Lisosan G for 4 days before CCl4. Lisosan G thus does not interfer with drug metabolizing system but has antioxidant properties and protects against CCl4-induced hepatotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years herbs have become an important form of healthcare (Ernst 2005; Bianchini and Vainio 2001; Zhou et al. 2004). However, problems may arise from their use due to intrinsic toxicity, adulteration, contamination and lack of standardization; in some cases their use has been associated with toxicity of heart, liver, blood, central nervous and skin (Zhou et al. 2004). The cytochrome P450 (CYP) system and enzymes such as glutathione-S-transferase (GST), NAD(P)H:quinoneoxidoreductase and UDP-glucuronosyl transferase (UDP-GT) contribute to the metabolism of a variety of xenobiotics, including therapeutic drugs, carcinogens, steroids and eicosanoides (Nelson et al. 1996). This metabolism increases the polarity of the xenobiotics and facilitate their excretion. It can, therefore, be considered a detoxication process but, in some instances, the compounds are converted by metabolism to reactive toxic metabolites (Nelson et al. 1996). It is likely that constituents in herbal preparations may be substrates, inhibitors or inducers of CYPs (Zhou et al. 2004). Several plants, including grain extracts, exhibit antioxidant properties that could be useful in the prevention of oxidative stress reactions, such as those mediated by the formation of free radical species in different pathological situations (Janbaz and Gilani 2000; Jin Lee et al. 2004; Truswell 2002; Jeon et al. 2003). Up to now there have been no studies of the toxicity and antioxidant properties of the powder of grain (Lisosan G) used as alimentary integrator. For this reason we have now studied whether Lisosan G affects the drug-metabolizing system and exhibits antioxidant properties. The hepatoprotective effects of Lisosan G in rats toxicated by CCl4 was also investigated.
Materials and methods
Chemicals
Lisosan G is registered as alimentary integrator by the Italian Minister of Health and was supplied by Agrisan Company, Larciano (PT), Italy. All other chemicals and solvents were of the highest grade available.
Animals
Nine male Sprague Dawley rats, 200–230 g, were used for each group. In the first group (group Lis) animals were given by gavage a water suspension of Lisosan G, at a daily dose of 0.5 g/kg, for 4 days. Rats of a second group (group CCl4) were injected intraperitoneally (i.p.) with a single dose of 100 mg CCl4/kg dissolved in corn oil at 50 mg/ml. A third group (group Lis + CCl4) was daily injected with Lisosan G for 4 days at 0.5 g/kg and on the 3rd day after administration of Lisosan G received also a dose of 100 mg CCl4/kg. The animals were killed 24 h after the last injection. The fourth group of rats (controls) was treated with corn oil only. Microsomal and 100,000g supernatant fractions were prepared from the liver as previously described (Longo et al. 1991).
Enzymatic activities
Microsomal p-nitrophenol hydroxylase (PNH) activity was determined by measuring the formation of p-nitrocatechol as described by Reinke and Moyer (1985). Erythromycin demethylase (ErD) activity was assayed by measuring the formation of formaldehyde (Tu and Yang 1983). Ethoxyresorufin-O-deethylase (EROD) and pentoxyresorufin-O-depentylase (PROD) activities were determined by measuring the formation of resorufin spectrofluorimetrically (Lubet et al. 1985). Ethoxycoumarin-O-deethylase (ECOD) and coumarin 7-hydroxylase (COH) activities were assayed by the fluorimetric determination of 7-hydroxycoumarin (Aitio 1978). NAD(P)H:Quinone oxidoreductase activity was assayed by following the reduction of dichlorophenolindophenol at 600 nm and calculating the rate which could be inhibited by 1 μM dicoumarol (Wermuth et al. 1986). Glutathione-S-transferase (GST) activity was quantified as previously described (Habig et al. 1974) using as substrate 1-chloro-2,4-dinitrobenzene. UDP-glucuronosyl transferase (UDP-GT) activity was determined using 1-naphthol as substrate (Mackenzie and Hanninen 1980). Thiobarbituric acid-reactive substances were measured in the liver homogenate, as described by Johansson and Ingelman-Sundberg (1985). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured in plasma according to the instructions of a commercial kit (Beckman, USA). In order to estimate the total antioxidant activity of Lisosan G we measured its ability to suppress the generation of 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) radical, as monitored at 730 nm (Arnao et al. 1999). The GSH level was estimated by a colorimetric method using Ellman’s reagent (Sedlak and Lindsay 1968).
Statistical analysis
Results are reported as means ± SD. Significant differences between means of various rat treatment groups were determined by analysis of variance (ANOVA) and means were compared using Dunnett’s t-test. In some cases comparisons were performed by Student’s t-test. All statistical analyses were carried out using Prism, GraphPad Software (San Diego, CA, USA).
Results and discussion
Effects of Lisosan G on drug metabolizing system
The major components of Lisosan G are reported in Table 1. Some of these components such as lipid, vitamins and oligoelements can modulate the drug metabolizing enzymes (Yang et al. 1992; Ioannides 1999).
As shown in Fig. 1 the treatment of rats with Lisosan G did not significantly affect the hepatic ECOD, EROD, ErD, CoH, PNH, PROD activities, which can be taken as enzymatic markers of the CYP1A, CYP2A, CYP2B, CYP2E1 and CYP3A isoforms (Longo et al. 1991). Also the ECOD activity which account for several CYPs including the CYP2C11, the major constitutive CYP isoform in the rat, was not influenced by the treatment. As observed for the CYP dependent oxidative metabolism, also the GST, DT-diaphorase and UDP-GT enzymes were not significantly altered by the Lisosan G treatment (Table 2). These results suggest that the amounts of either the lipid or the oligoelements present in Lisosan G are under the levels which are needed to alter the drug metabolizing system of the liver of rats.
Cytochrome P450-dependent enzymatic activities in liver microsomes from control- (▥)and Lisosan- (▤) treated rats. Each bar represents the means ± SD of three experiments performed with hepatic microsomes. Each experiment was carried out with microsomes pooled from three rats. Abbreviations: COH: coumarin 7-hydroxylase; ECOD: Ethoxycoumarin-O-deethylase; ErD: Erythromycin demethylase; EROD: Ethoxyresorufin-O-deethylase; pNPH: p-nitrophenol hydroxylase; PROD: Pentoxyresorufin-O-depentylase
Antioxidant activity of Lisosan G
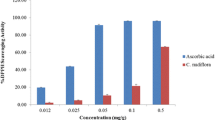
The antioxidant activity of Lisosan G was evaluated by in vitro and in vivo experiments. The in vitro method involved the formation of ABTS radical by the peroxidase/H2O2 system, a radical that absorbs at 730 nm and can be inhibited or suppressed by antioxidant compounds. Figure 2 shows the progressive loss in absorbance at 730 nm when increasing amounts of Lisosan G were added, indicating an antioxidant activity, which was compared to that of a standard antioxidant (ascorbate—ASC) and expressed as ASC equivalents. The total antioxidant activity of Lisosan G was 80 ± 20 μmol per g, showing that it is a good scavenger of radical species.
To investigate a possible chemoprotective role of Lisosan G we induced hepatotoxicity in rats by CCl4. This compound is known to cause extensive liver damage, by forming reactive intermediates, such as the thrichloromethyl radical (Jin Lee et al. 2004). Activities of plasmatic ALT and AST (two marker enzymes indicative of liver damage) increased significantly in the group of rats treated with CCl4 as compared to controls (Table 3). CCl4 treatment also increased the hepatic LP and decreased significantly the GSH content in the liver, without modifying its GST activity (Table 3).
Interestingly, pretreatment of rats with repeated doses of Lisosan G prior to the administration of CCl4 restored ALT, AST activities along the LP, GSH levels to the control values. This is similar to the protective effect against CCl4 liver damage reported by administering other antioxidants (Jin Lee et al. 2004; Jeon et al. 2003; Sheweita et al. 2001). When the pretreatment of Lisosan G was performed at 0.1 g/kg no protection on the toxicity CCl4-dependent was observed (data not shown).
In conclusion, the constituents of Lisosan G and their metabolites did not interfer with the phase 1 and 2 drug metabolizing enzymes and for this reason Lisosan G can be coadministrated during drug therapy. In fact, a number of clinically important herb-drug interactions have been reported and some of them have led to altered efficacy and/or adverse events (Zhou et al. 2004). Moreover, the results demonstrated a hepatoprotective antioxidant activity suggesting that the protection activity of Lisosan G against hepatotoxins observed in rat might occur also in human using a diet supplemented with Lisosan G.
References
Arnao MB, Cano A, Acosta M (1999) Methods to measure the antioxidant activity in plant material. A comparative discussion. Free Radic Res 31:89–96
Aitio A (1978) A single sensitive assay of ethoxycoumarin deethylation. Anal Biochem 85:488–491
Bianchini F, Vainio H (2001) Allium vegetables and organosulfur compounds: do they help prevent cancer? Environ Health Perspect 109:893–902
Ernst E (2005) The efficacy of herbal medicine-an overview. Fundam Clin Pharmacol 19:405–409
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione transferase, the first step in mercapturic acid formation. J Biol Chem 249:7140–7149
Ioannides C (1999) Effect of diet and nutrition on the expression of cytochromes P450. Xenobiotica 29:109–154
Janbaz KH, Gilani AH (2000) Studies on preventive and curative effects of barberine on chemical-induced hepatotoxicity in rodents. Fitoterapia 71:25–33
Jeon TI, Hwang SG, Lim BO et al (2003) Extract of Phellinus linteus grown on germinated brown rice suppress liver damage induced by carbon tetrachloride in rats. Biotech Lett 25:2093–2096
Jin Lee K, Woo ER, Choi CY et al (2004) Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci 74:1051–1064
Johansson I, Ingelman-Sundberg M (1985) Carbon tetrachloride-induced lipid peroxidation dependent on an ethanol-inducible form of rabbit liver microsomal cytochrome P-450. FEBS Lett 183:265–269
Longo V, Mazzaccaro A, Naldi F, Gervasi PG et al (1991) Drug metabolizing enzymes in liver olfactory and respiratory epithelium of cattle. J Biochem Toxicol 6:123–128
Lubet RA, Mayer RT, Cameron JW et al (1985) Dealkylation of pentoxyresorufin: a rapid and sensitive assay for measuring induction of cytochrome(s) P-450 by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys 238:43–48
Mackenzie PI, Hanninen O (1980) A sensitive kinetic assay for UDP glucuronosyl transferase using 1-naphthol as substrate. Anal Biochem 109:362–368
Nelson DR, Koymans L, Kamataky T, Stageman JJ, Feyereisen R, Waxman DJ, Waterman MR, Gotoh O, Coon MJ, Estabrook RW, Gunsalus IC, Nebert DW et al (1996). P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6:1–42
Reinke LA, Moyer MJ (1985) P-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos 13:548–552
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sheweita SA, El-Gabar MA, Bastawy M (2001) Carbon tetrachloride changes the activity of cytochrome P450 system in the liver of male rats: role of antioxidants. Toxicology 165:217–224
Truswell AS (2002) Cereal grains and coronary heart disease. Eur J Clin Nutr 56:1–14
Tu YY, Yang CS (1983) High-affinity nitrosamine dealkylase system in rat liver microsomes and its induction by fasting. Cancer Res 43:623–629
Zhou S, Koh HL, Gao Y et al (2004) Herbal bioactivation: the good, the bad and the ugly. Life Sci 74:935–968
Wermuth B, Platt KL, Scidel A et al (1986) Carbonyl reductase provides the enzymatic basis of quinone detoxication in man. Biochem Pharmacol 35:1277–1282
Yang CS, Brady JF, Hong JY (1992) Dietary effects on cytochrome P450, xenobiotic metabolism, and toxicity. Faseb J 6:737–744
Acknowledgements
We acknowledge Dr. F. De Matteis from MRC Bioanalytical Science Group, Birkbeck College, University of London, for critical rewiew of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Longo, V., Chirulli, V., Gervasi, P.G. et al. Lisosan G, a powder of grain, does not interfer with the drug metabolizing enzymes and has a protective role on carbon tetrachloride-induced hepatotoxicity. Biotechnol Lett 29, 1155–1159 (2007). https://doi.org/10.1007/s10529-007-9378-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9378-6