Abstract
The effect of different yeast strains on the quality of kiwi wine was investigated by polyphase determine approaches in the present research. The influence of co-culture and inoculation sequence on the quality was also explored simultaneously. Results suggested that the characteristics of the kiwi wine were affected by the metabolic characteristic of strains. The flavor content and their flavor profile of samples fermented by co-culturing of strain among species, genus, and families. When Saccharomyces bayanus (Y5 or Y6) co-cultured with Torulaspora delbrueckii Y7, the ratio of phenethyl alcohol increased, but that of octanoic acid and ethyl octanoate decreased significantly. The odor activity value (OAV) of ethyl octanoate and ethyl hexanoate was increased by co-culturing Saccharomyces with T. delbrueckii, and that of decanal and terpinen-4-ol was enhanced by co-culturing of different strains of Saccharomyces. It was an excepting process to obtain high quality of kiwi wine by co-culturing technology of yeasts, and was very effective to optimize the process by polyphase analysis approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, Saccharomyces cerevisiae is considered as an important starter in the wine fermentation process, whether it is applied as a commercial starter or indigenous yeasts, to regulate the microbial community diversity and their metabolism. But it is homogenizing for the quality feature of the final wine. In fact, the contribution to grape wine characteristics was not only depended on Saccharomyces, but also was closely related to non-Saccharomyces whether the wine belonged to Old World or the New World. Therefore, it is one of the focuses on co-culturing S. cerevisiae with non-Saccharomyces cerevisiae or non-Saccharomyces to enhance the quality of wine. In this way, the role of dominant flora originated from raw material and winery environment can be simulated effectively, and the wine fermentation process was regulation whether quality enhancing or avoiding safety risk. Different species of Saccharomyces involved in S. cerevisiae, S. bayanus, and S. uvarum, etc. have their own unique feature. S. cerevisiae is mainly used to produce ethanol and S. bayanus and S. uvarum are characterized by low acetic acid production and high yields of glycerin and lactic acid. The latter two are used to produce high-quality wines [1, 2]. For example, the characteristics of Malvasia delle Lipari wine fermented by S. uvarum differ compared with the wine fermented by S. cerevisiae, in which volatile acidity and ethanol were lower [3]. Similarly, it was observed that significant difference of flavor profile among two kinds of Chardonnay wines, which one was fermented by S. bayanus, and another was fermented by S. cerevisiae [4]. Non-Saccharomyces were originated from raw material and wine brewing environment. Of these non-Saccharomyces, grape, longan, lychee, and cherry wine were produced by coculturing S. cerevisiae with T. delbrueckii, and the content of acetate and ethyl ester were higher than that of ones only brewed by S. cerevisiae [5,6,7,8]. The contents of ethanol, glycerin, volatile acids, and organic acids were also different when cocoa beans, bilberry wine, and mango wine were fermented by co-cultured S. cerevisiae with T. delbrueckii, compared with the result obtained by pure fermentation pattern, especially ethanol and acetic acid content decreased [9,10,11,12]. Besides, Lu et al. reported that coculture of T. delbrueckii and Pichia kluyveri could complete alcoholic fermentation of durian wine [13].
Kiwifruit (Actinidia chinensis) is an edible body of kiwi woody vine and rich in nutrients. It is widely distributed in China, New Zealand, Italy, and Chile [14]. However, the shelf life is short and easy to over-ripe, threatening the further development of the kiwifruit chain [15]. Most nutrients and bioactive substances can be transferred to wine if it brewed wine, so that kiwifruit can be used to brew fruit wine to increase the added value [16]. S. cerevisiae has been used to brew kiwi wine. It suggested that the characteristics of the strain also affect the concentration of phenolics and volatiles [17]. However, the intensity of flavor was generally weaker than that of wine [18]. It may be caused by the difference of two raw material components. These shortcomings may be overcome by co-culturing S cerevisiae with non-Saccharomyces cerevisiae or non- Saccharomyces.
In the present, we investigated the impact of co-culturing S. cerevisiae with non-Saccharomyces cerevisiae (S. bayanus and S. uvarum), as well as inoculation mode on the kiwi wines quality compared with that of ones fermented by single strain. Additionally, the difference of volatiles profiles in kiwi wine brewed by co-culturing Saccharomyces strains and T. delbrueckii was studied. To the best of our knowledge, it was the first report on the influence of coculturing Saccharomyces and T. delbrueckii on the quality of kiwi wine.
Materials and methods
Materials and strains
Chemicals
The standards, including oxalic acid, citric acid, tartaric acid, L-malic acid, succinic acid, lactic acid, acetic acid, propionic acid, methyl octanoate, and 2-octanol were purchased from Sigma-Aldrich. Ltd. Co (Shanghai, China). Other chemicals were purchased from Chengdu Jinshan (Chengdu, China).
Microorganism
Saccharomyces cerevisiae: S. cerevisiae Y1 and S. cerevisiae Y2, S. bayanus: S. bayanus Y3, S. bayanus Y4, and S. bayanus Y5, S. uvarum Y6, T. delbrueckii Y7 were isolated from the soil located at kiwifruit wine factory and identified according to the results of physiological and biochemical experiments, cell and colony morphology, as well as ITS sequence. These strains mentioned above were all preserved in our Lab, and S. cerevisiae Y1 (CCTCCM2019521), S. bayanus Y4 (CCTCCM2019522), and T. delbrueckii Y7 (CCTCC M2019523) were also preserved in the China Center for Type Culture Collection.
Kiwi wine fermentation
Kiwifruit juice preparation
Kiwifruit (var. Hayward) were harvested in 2019 with a total sugar of 8.68% and a total acid of 12.61 g/L (tartaric acid). It was purchased from the local farm product market beaten into pulp after selection and removed of impurities. 100 mg/L of sodium hydrogen sulfite was immediately added into the pulp to control harmful bacteria and yeasts. 20 mg/L of pectinase (Lallzyme EX-V, Lallemand, France) was added, and maintained at 37 ± 2 °C for 60 min. The total soluble solids were adjusted to 19 oBrix by adding sucrose. 2 L of the pulp was spilt into a 2.5 L of the wide-mouth reagent bottle.
Starter suspension preparation
Pre-cultures were carried out initially by inoculating from the agar slant test tube of each strain into 5 mL of YPD broth (1% yeast extract, 2% peptone, 2% glucose) growing the cells at 30 ± 1 °C on a test tube rotator for approximately 24 h. Thereafter, 50 μL of this pre-culture was then re-inoculated into 5 mL fresh YPD broth and grown for approximately 24 h at 30 ± 1 °C on the test tube rotator. Cells were then inoculated from this pre-culture into 200 mL YPD broth at OD600 nm 0.1 and grown at 30 ± 1 °C with shaking at 120 rpm until the cells reach mid-exponential phase (ca. 9 h). To obtain yeast cells, the broth was centrifuged at 4500×g for 10 min to remove supernatant. Afterwards, the biomass was washed by resuspension in 0.9% sterile sodium chloride solution, followed by centrifugation at 4500 × g for 10 min. The washing was repeated three times, after which the pellet was collected and resuspended in the treated kiwifruit juice.
Fermentation procedure and condition
Prior to inoculation, the yeast cell population was determined by a hematocytometer. Four types of fermentation were conducted: (1) pure fermentations by inoculation with a single S. cerevisiae or non-Saccharomyces cerevisiae yeast strain for selecting the strains to fit kiwifruit wine-making, and the number of samples were Sample No 1, Sample No 2, and Sample No 3, respectively; (2) kiwifruit wine fermenting by co-culturing S. cerevisiae Y2 and with non-Saccharomyces cerevisiae, including S. bayanus Y5 and S. uvarum Y6 (sample No 4 and sample No 5); (3) kiwifruit wine fermenting by co-culturing S. cerevisiae Y2, S. bayanus Y5 and S. uvarum Y6 with T. delbrueckii Y7, respectively, via simultaneous inoculation, and the number of samples were Sample No 6, Sample No 7 and Sample No 8; (4) the co-culturing mode was the same as that of mode described above, but T. delbrueckii was first inoculated into kiwifruit juice, and then two days later, Saccharomyces was inoculated, and the number of samples were Sample No 9, Sample No 10 and Sample No 11, respectively.
Pre-treated kiwifruit juice was inoculated with reconstituted starter resuspension by the kiwifruit juice, and the initial concentration of starter reached the level of 2.4 × 106 CFU/mL. The co-culture of fermentation was inoculated at a rate of 1:1 for two different stains starter. The fermentation was carried out under the static condition at 15° C. The residual sugar content, ethanol content, and pH were evaluated to monitor the fermentation. The fresh kiwi wine was filtered when the fermentation finished, and then stored at 4 °C for further analyze.
Determining of physicochemical properties and antioxidant activity
The content of residual sugar, titration acidity, and ethanol was analyzed according to GB/T15038-2006 [19]. The color was determined with the method described by Bimpilas et al. [20], and absorbance was measured by UV–visible spectrophotometer (TU-1901, Beijing Purkinje General Instrument, Beijing, China), where color intensity = A420 + A520 + A620, lustre = A420/A520. The polyphenol content, DPPH and ABTS free radical scavenging activity were determined by the method reported by Wang et al. [21]. The polyphenol content was expressed as gallic acid equivalent (mg GAE/L), and DPPH and ABTS free radical scavenging activity were expressed as trolox equivalent antioxidant capacity (TEAC).
Organic acids analysis
10.0 mL of sample was centrifuged at 12,000 r/min for 10 min at 4 ℃, and the supernatant was purified by SPE column (Swell scientific instruments Co., Ltd. Chengdu, China), subsequently filtered through a 0.22 µm filter (Micron Separation Inc., Westborough, MA). The filtered samples were injected into the Agilent 1260 HPLC (Agilent Technologies, Santa Clara, USA) system equipped with an Alltech OA-1000 organic acid column (300 mm × 6.5 mm, Grace, Columbia, USA) maintained at 75 ℃ according to the method in Liang et al. [22]. Degassed H2SO4 (9 mM) was used as mobile phase and the organic acids, including oxalic, citric, tartaric, L-malic, succinic, lactic and acetic were detected using UV detector (215 nm). A 10 μL injection volume was used for samples and standards. Organic acids were quantified by external standards. The result was expressed as g/L.
Volatile compounds analysis
The analysis of volatile compounds was performed on Trace GC Ultra-DSQ II GC–MS (Thermo Electron Corporation, Waltham, USA), in combination with HS-SPME and DVB/CAR/PDMS fiber (Supelco, Bellafonte, USA), according to Niu et al. [23]. The chromatographic column for GC–MS analysis was HP-Innowax (30 m × 0.25 mm × 0.25 μm, Agilent J&W, Santa Clara, USA). Volatile compounds were identified by comparing MS with the standard library in NIST05 and verified by Kováts retention indices (RI) with that reported in literatures, which was calculated by using C8–C20 n-alkanes. At the same time, semi-quantitation of volatile compounds can be obtained by comparing the area of internal standards (methyl octanoate and 2-octanol) with the total ion chromatogram.
Statistical analysis
All analysis were performed in triplicate and the data were described as means ± standard deviation. Difference between samples was evaluated by analysis of variance (ANOVA) and Duncan’s tests using IBM SPSS Statistics 25 (SPSS Inc., Chicago, Illinois, USA), and P < 0.05 indicated statistical significance. Partial least square-discrimination analysis (PLS-DA) was employed to correlate aroma compounds with different inoculation modes using Simca 14.1 (Umetrics, Umeå, Sweden). The cluster analysis and heatmap were carried out by the R language.
Results and discussion
Characterizing major properties of kiwi wine brewed by different strains
The major properties of kiwi wine brewed by six different strains, which fall into Saccharomyces, were investigated. Significant difference of residual sugar (RS express as glucose) and the color was observed, ranged from 25.08 to 40.82 g/L. It lied on the brewing characteristics of strain used. For example, the RS content for S. cerevisiae Y1 was not only higher than that of S. cerevisiae Y2, but also higher than other species, such as S. bayanus, S. uvarum. Contrast to S. cerevisiae, S. bayanus, and S. uvarum were mainly in lower acetic acid production and lower ethanol yield. However, no significant difference in ethanol content was founded among these kiwi wines, it may be caused by raw material feature as well as operation parameter, such as aeration conditions [24]. It was reported previously that S. bayanus and S. uvarum were not differed significantly from S. cerevisiae for these indexes as mentioned above. For example, Hayasaka et al. reported that there was no difference in ethanol content in Cabernet Sauvignon red wine fermented by S. bayanus and S. cerevisiae, respectively [25]. Similarly, Furmint wine fermented by S. uvarum and S. cerevisiae, respectively, had similar volatile acid content [26]. The color of the wine is an important sensory characteristic, and closely related to consumers' acceptance. Compared to other strains, the color intensity of kiwi wine fermented by S. bayanus Y5 was lower; it may be due to anthocyanin absorbed and reaction with some chromogenic substances [27], resulting in the color loss of the wine which can be avoided by selecting the appropriate yeast. Lustre of kiwi wine brewed by S. uvarum was significantly lower contrasted to other samples, which means it's reddish.
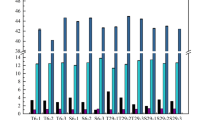
As shown in Fig. 1, no difference of total organic acid content, ranged from 27.02 to 28.34 g/L, was observed, while the profile was a little divergent among the six samples. Citric acid content from 12.63 g/L in raw material raised to 21.65–22.28 g/L constituting ca 80% of total organic acid content by fermented, while quinine acid content was decreased significantly, from 7.52 g/L to undetectable. Total organic acid contents were higher than the grape wine content [28], even though that in kiwi wine [29]. It was related to variety, origin, as well as maturity in addition to the fermentation process [30], which resulted in the increase in content of L-malic acid and succinic acid.. Compared with S. cerevisiae Y1, the content of L-malic acid and succinic acid in kiwi wine fermented by S bayanus except for Y5 was higher. The acetic acid content in the sample fermented by Y1 and Y5, respectively, was lower. However, the contents of succinic and acetic acid in the samples fermented by S. uvarum was inconsistent with the results reported previously [2], which may be also resulted by similar causes as described above.
Difference of volatiles in the kiwi wine fermented by various yeast strains
As shown in Table S2 (Online Resource), 46 volatile compounds were identified, in which 24 components were detected in all samples. These volatiles were divided into six classes according to their chemical structure (25 esters, 9 alcohols, 5 acids, 3 terpenes, 2 aldehydes, 2 phenols), and were reported in the previous document [17, 18]. Six components were dominant which were ethyl octanoate, ethyl decanoate, ethyl 9-decenoate, phenylethyl alcohol, octanoic acid, and decanoic acid, respectively. The total volatile content ranged from 5.43 to 9.40 mg/L in the six kiwi wines, and that in kiwi wine by Y5 was highest and the content of esters, acids, aldehydes, phenols were also highest, while that in kiwi wine by S. uvarum was lowest due to the lower content of esters, acids, phenols, and terpenes than that of others samples. The difference of volatile species among kiwi wine samples fermented by different yeast strains was observed, which 32, 32, 34, 37, 38 and 34 of volatile species in kiwi wine fermented by Y1, Y2, Y3, Y4, Y5, and Y6, respectively. Nine volatiles were not produced by S. cerevisiae, involved in decanal, isooctyl alcohol, 1-octanol, cis-hex-3-en-1-ol, isobutyl decanoate, pentyl decanoate, methyl decanoate, ethyl linoleate, and ethyl tetradecanoate and terpenes were not produced by Y1.
The volatiles profile among these samples were different, as shown in Fig. 2. Ethyl octanoate, ethyl decanoate, and ethyl 9-decenoate were the dominant in all samples endowed a fruity and floral scent to kiwi wine. However, their sum proportion was different, which were 72.23% (Y1), 73.05% (Y2), 81.97% (Y3), 76.67% (Y4) and 72.30% (Y5) and 71.50% (Y6), respectively. Compared to S. cerevisiae and S. uvarum, the ability of ethyl esters-producing for S. bayanus was stronger, which was also affected by fermentation temperature, aeration, and sugar contents [31]. Of alcohols, phenylethyl alcohol and isoamyl alcohol were dominant, ranged from 85.24 to 90.69%. The proportion of phenylethyl alcohol and isoamyl alcohol in other wines were also high [32]. Acids were one of the dominant volatiles ranging from 23.68 to 29.74%. The content in the samples fermented by Y4 and Y5, contributed by octanoic acid, decanoic acid, and dodecanoic acid, were higher than others which was no significant difference. Terpenes play an important role in the characteristic flavor of wine which endowed floral, rose, and bell orchid odor to wines, originated from the cell wall of grape skin. The content in wine relied on strains characteristics. In our present experiment, similar results were obtained using kiwifruit juice as raw material. Decanal was detected in the samples fermented by Y4 and Y6, which contributed an orange peel odor even though the content was very low. The content of 4-vinyl guaiacol (4-VG) in the samples fermented by Y5 was higher.
Of these volatiles, changes of eight volatiles content (OAV > 1) affected significantly the flavor characteristics of kiwi wine. In fact, the numbers of volatile with OAV > 1 were closely related with the characteristic of species and strains. For example, seven and five components were detected out in the samples fermented by Y1, Y6, and Y2, respectively, in which isoamyl acetate was only detected in Y1 sample. Although, all Y3, Y4, and Y5 belonged to S. bayanus, 6 volatiles were detected in Y3 and Y5′s, while 7 volatiles were only examined in Y4′s. Among them, S. bayanus fermented kiwi wine has a higher OAV of ethyl octanoate (487.91–588.82), ethyl decanoate (5.09–6.85), and octanoic acid (1.53–2.31), which contribute to the fruity, floral, and cheese aroma of the kiwi wine, S. uvarum has a higher OAV of decanal (2.22) with aroma of orange peel, whereas Y1 has a higher OAV of isoamyl acetate (1.38) with aroma of banana. As shown in Fig. 3, decanal was not detected in S. cerevisiae, and Y2 has a lower OAV of 4-VG (0.20) resulted in an absence of spices scent.
Physiochemical indexes and volatile compounds (OAV > 0.1) of six yeasts were used for PLS-DA analysis, which 54.40% of the total variance was explained. As shown in Fig. 4, the results showed that all samples were divided into three groups, namely the S. cerevisiae group (Y1 and Y2), the S. bayanus group (Y3, Y4 and Y5), and the S. uvarum group (Y6). Among them, S. cerevisiae Y2 was related to L-malic acid, succinic acid, ethyl hexanoate, phenylethyl alcohol, and citronellol. In addition, Y5 has a higher yield of ethyl esters when compared with Y3 and Y4, such as ethyl decanoate, ethyl 9 − decenoate, ethyl octanoate, ethyl dodecanoate, ethyl hexadecanoate. And the OAV of the aroma compounds of Y5 (OAV > 1) was larger than that of Y3 and Y4. Therefore, S. cerevisiae Y2, S. bayanus Y5, and S. uvarum Y6 were selected for the subsequent experiment.
Effect of co-cultured on major properties and flavor profile in the kiwi wine
As shown in Table S3 (Online Resource), difference of major physicochemical properties, such as RS, ethanol, acidity (expressed as tartaric acid g/L), organic acid, and free radical removal capacity was no significant among these samples, which involved in different strains belonging to Saccharomyces, i.e., co-culturing S. cerevisiae with S. bayanus and S. uvarum, respectively, as well as Saccharomyces (S. cerevisiae Y2 with S. bayanus Y5 and S. uvarum Y6) co-cultured with non-Saccharomyces (T. delbrueckii) by simultaneous or sequential inoculating.
As shown in Table 1, sixty-one volatile compounds were identified among these samples. These components were divided into seven classes which involved in esters (24), alcohols (12), acids (9), aldehydes (3), ketones (3), phenols (4), and terpenes (6) according to their chemical structure. There are 37, 40, and 34 components identified in the samples fermented by Y2, Y5, and Y6, respectively. Among these samples, undetected components in Y2 and Y5 were all four but were ethyl 9-decenoate, hexanoic acid, linalool and citronellol for the former, and were isoamyl acetate, decyl alcohol, decanoic acid and decanal for the latter. However, four undetected components in Y6’s were (E, E)-farnesol, 2,4-di-tert-butylphenol, 4-VG and (E)-nerolidol, respectively. 35 and 38 components were identified when Y2 co-cultured with Y5 and Y6, respectively. 11 and 9 volatiles were undetected in Y2 + Y5 and Y2 + Y6, respectively, which were once identified in the respective single strain, while 3 and 5 volatiles were newly detected. Six volatiles were undetected in both samples which involved 2-hydroxyethyl hexanoate, 1-hexadecanol, 2,6-di-tert-buty-4-sec-butyphenol, decyl alcohol, isoamyl acetate, and (E, E)-farnesol. Newly detected volatile was only diethyl succinate in both of the samples.
When three different strains belonged to Saccharomyces co-cultured with T. delbrueckii (Y7), volatiles species was unchanged for Y2 + Y7 and Y6 + Y7, undetected, and newly examined volatiles were all nine and ten contrast to the samples of Y2 and Y6, respectively. While undetected volatiles were eight, but new examined volatiles were five in Y5 + Y7 compared with Y5′s. New-detected components were 9,12-octadecadienoic acid ethyl ester, ethyl oleate, and tetradecanoic acid among three kinds of co-cultured samples, as well as nonanoic acid in Y5 + Y7 and Y6 + Y7. Only α-terpineol was undetected in all co-cultured samples, decyl alcohol was unexamined in addition to Y5 + Y7, while diisobutyl adipate and ethyl decanoate were undetected in Y5 + Y7 and Y6 + Y7. Compared with the samples inoculated simultaneously, the volatiles species were changed in respective sequential inoculated ones. For example, the undetected species were 10, 8, and 13, while new examined species were 6, 9, and 5, respectively, in Y2 + Y7, Y5 + Y7 and Y6 + Y7, which were fermented by sequential inoculated pattern. And then, 9,12-octadecadienoic acid ethyl ester, diisobutyl phthalate, and tetradecanoic acid were unexamined in sequential inoculated samples, while 9-decenoic acid and methyl hexadecanoate were unexamined, and diethyl succinate was newly analyzed in Y2 + Y7 and Y6 + Y7, acetic acid, dibutyl phthalate, and linalool were undetectable, diethyl succinate and methyl isohexenyl ketone were newly examined in Y2 + Y7 and Y5 + Y7′s.
The volatiles quality of Y5 was slightly higher than the other two strain’s. Phenethyl alcohol, 2,4-di-tert-butylphenol were dominant. When Y2 co-cultured with Y5 and Y6, the volatiles content was almost unchanged, but lower than Y5′s. The abundance of phenethyl alcohol also decreased than Y5′s while was a little increased than the other two respective strains. When Saccharomyces co-cultured with Y7, the volatiles content in Y5 + Y7 and Y6 + Y7 decreased. The contents of volatiles in Y2 + Y7 and Y5 + Y7, both were inoculated sequentially, were lower than the samples inoculated simultaneously. As shown in Fig. 5, dominant groups involved in alcohols, esters, acids, and phenols, accounted for 94.10% to 96.66%. The results suggested that the proportion of four dominant volatiles class depended on the interactive relationship between two strains and their characteristics. When Y2 co-cultured with Y5 or Y6, the result was different, the proportion of esters and phenol in the former sample was closer to Y2′s, which was closer to that of Y6′s; although, the proportion of acids increased in both co-cultured samples. Due to the simultaneous inoculated sample for Y2 co-cultured with Y7, the proportion of esters and acids increased compared with the respective strains. However, sequential inoculated sample was the opposite, especially, the proportion of acids induced to 7.63%. When Y5 or Y6 was co-cultured with Y7, respectively, the proportion of esters, acids, and phenols in the sequential inoculated samples increased, while the proportion of esters in the simultaneous inoculated samples decreased. It was reported that the contents of esters were increased by co-culture [33].
By coculturing, among Saccharomyces strains, the dominant components (abundance ≥ 0.05) have five of the same components which were ethyl octanoate, isoamyl alcohol, phenethyl alcohol, octanoic acid and 2,4-di-tert-butylphenol. Besides, Y5 + Y7 still have phenethyl acetate. Of them, the proportion of phenethyl alcohol accounted for 25.02% to 50.21%. Compared with pure culture, the content of these components was not changed significantly. Among them, the proportion of phenethyl alcohol increased, but the proportion of octanoic acid and ethyl octanoate decreased notably. For Y5, the proportion of phenethyl alcohol and phenethyl acetate decreased and others increased, resulting in the sum proportion of decrease of six dominant components. In addition, the sum proportion of five dominant components significantly increased by forming of 2,4-di-tert-butyphenol newly although ones of phenethyl alcohol decreased in sequential inoculated samples. The dominant components species were the same among these samples fermented by co-cultured among Saccharomyces strains, and their proportion was similar to Y2’s. These results were consistent with the previous document on brewing Riesling wines by coculturing of yeast strain [34]. The content of phenylethyl alcohol in the wines sequentially inoculated with S. cerevisiae and T. delbrueckii was higher than that of the control [35]. Non-conventional yeast has been reported to have a desirable and negative impact on flavor profile [36]. In addition to simultaneous (Y2 + Y7) inoculation, the ketone content increased significantly after co-culture, and the improvement in ketone was mainly due to the increase of geranyl acetane which contribute the fruity and fresh aroma to the flavor profile of the wine. Besides, co-culture has little effect on the content of phenols and terpenes.
Characterizing the flavor of the co-cultured samples
The detected volatile compounds were used for PLS-DA analysis to better understand the relationship between different inoculation modes of kiwi wine and corresponding flavor compounds, which 33.40% of the total variance was explained. As the results showed in Fig. 6, kiwi wines were distributed in different locations depending on the pattern of inoculation. Heptyl alcohol, decyl alcohol, (E)-nerolidol, 1-hexadecanol, dodecanol, isobutanol, phenylethyl alcohol in alcohol were closely related to single fermentation. Meanwhile, fatty acids of hexanoic acid, octanoic acid, decanoic acid, and dodecanoic acid, ethyl esters of ethyl decanoate, ethyl octanoate, ethyl lactate, and ethyl 2-furoate have a greater contribution to co-culture among Saccharomyces. Ethyl esters of ethyl 9-decenoate, ethyl dodecanoate, ethyl hexadecanoate, ethyl oleate, 9,12-octadecadienoic acid ethyl ester and ethyl phenylacetate, acetate esters of isoamyl acetate, hexyl acetate and phenylethyl acetate, ketones of geranyl acetane and methyl isohexenyl ketone, acids of 9-decenoic acid, tetradecanoic acid and benzoic acid have a greater contribution to the flavor of co-culture with T. delbrueckii.
Biplot for PLS-DA of aroma compounds in kiwi wine fermented by pure and co-cultured among Saccharomyces, as well as Saccharomyces with T. delbrueckii. Aroma compounds used for analysis were listed in Table 1
As shown in Fig. 7, heatmap analysis of volatiles indicated that Y2, Y5, and simultaneous inoculated co-culture of Y5 and Y7 were grouped into a cluster due to higher concentrations of heptanol, 1-dodecanol, (E)-nerolidol, (E, E)-farnesol, and acetic acid. Another cluster which were composed of single strains Y6, sequence inoculated co-culture samples as well as the rest simultaneous inoculated co-culture samples of them, Y6, Y2 co-cultured with Y5 or Y6, were grouped into one sub cluster since these sample, the concentrations of α-terpineol, terpinen-4-ol, linalool, and citronellol were higher. When Y7 co-cultured with Y2 by simultaneous inoculated, as well as co-cultured Y5 or Y6 by sequence inoculated, which have a higher content of isoamyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl 9-decenoate and 4-VG, so that fell into the same sub cluster. In addition, the rest of the samples inoculated were grouped in another sub cluster. As shown in Fig. 8, there were six volatile compounds of OAV > 1 in kiwi wine, including ethyl octanoate, decanal, terpinen-4-ol, ethyl hexanoate, linalool, and phenylethyl acetate. The difference of component’ OAV resulted in very different contributing to kiwi wine flavor feature. The co-culture of different strains among Saccharomyces endowed to orange peel and menthol aroma since OAV’s values of decanal (12.64) and terpinen-4-ol (10.07) were higher. Different strain of Saccharomyces co-cultured with T. delbrueckii endued a fruity and floral aroma as that of ethyl octanoate (184.65) and ethyl hexanoate (8.15) were higher.
Heatmap represents for volatile profile of kiwi wine fermented by pure and co-cultured among Saccharomyces, as well as Saccharomyces with T. delbrueckii. Aroma compounds used for heatmap analysis were listed in Table 1
Conclusions
The contribution of yeast to physicochemical properties and flavor of kiwi wine varied with the strains, either species or family. It was mainly changed by co-culturing among strains that the flavor characteristics of kiwi wine, while no significant difference of their physiochemical properties was observed in present research. Co-culture pattern not only affected the volatile content, but also affected identified species and the flavor profile. The volatile content in these samples fermented by co-culture was slightly decreased compared with that in the respective single ferment samples. It was interesting that the contribution of unique aroma component to kiwi wine was enhanced by co-culture fermentation. The OAV of ethyl octanoate and ethyl hexanoate was increased by co-culturing strains in Saccharomyces with T. delbrueckii, while that of decanal and terpinen-4-ol was enhanced by co-culturing of strains among Saccharomyces. Meanwhile, the effect was affected by the inoculation sequence. Results suggested that it was an excepting process to obtain high quality of kiwi wine by co-culturing technology of yeasts, and was very effective to optimize the process by polyphase analysis approaches.
References
Giudici P, Zambonelli C, Passarelli P, Castellari L (1995) Improvement of wine composition with cryotolerant Saccharomyces strains. Am J Enol Viticult 46:143–147
Castellari L, Pacchioli G, Zambonelli C, Tini V, Grazia I (1992) Isolation and initial characterization of cryotolerant Saccharomyces strains. Ital J Food Sci 3:179–186
Muratore G, Asmundo CN, Lanza CM, Caggia C, Licciardello F, Restuccia C (2007) Influence of Saccharomyces uvarum on volatile acidity, aromatic and sensory profile of Malvasia delle Lipari wine. Food Technol Biotech 45:101–106
Eglinton JM, Mcwilliam SJ, Fogarty MW, Francis IL, Kwiatkowski MJ, HØJ PB, Henschke PA (2000) The effect of Saccharomyces bayanus-mediated fermentation on the chemical composition and aroma profile of Chardonnay wine. Aust J Grape Wine R 6:190–196
Loira I, Vejarano R, Bañuelos MA, Morata A, Tesfaye W, Uthurry C, Villa A, Cintora I, Suárez-Lepe JA (2014) Influence of sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on wine quality. LWT-Food Sci Technol 59:915–922
Sanoppa K, Huang T-C, Wu M-C (2019) Effects of Saccharomyces cerevisiae in association with Torulaspora delbrueckii on the aroma and amino acids in longan wines. Food Sci Nutr 7:2817–2826
Chen D, Liu SQ (2016) Impact of simultaneous and sequential fermentation with Torulaspora delbrueckii and Saccharomyces cerevisiae on non-volatiles and volatiles of lychee wines. LWT-Food Sci Technol 65:53–61
Sun SY, Gong HS, Zhao YP, Liu WL, Jin CW (2016) Sequential culture with Torulaspora delbrueckii and Saccharomyces cerevisiae and management of fermentation temperature to improve cherry wine quality. J Sci Food Agr 96:1880–1887
Benito S (2018) The impact of Torulaspora delbrueckii yeast in winemaking. Appl Microbiol Biotechnol 102:3081–3094
Visintina S, Ramob L, Batista N, Dolci P, Schwan F, Cocolin L (2017) Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int J Food Microbiol 257:31–40
Liu S, Laaksonen O, Kortesniemi M, Kalpio M, Yang B (2018) Chemical composition of bilberry wine fermented with non-Saccharomyces yeasts (Torulaspora delbrueckii and Schizosaccharomyces pombe) and Saccharomyces cerevisiae in pure, sequential and mixed fermentations. Food Chem 266:262–274
Sadineni V, Kondapalli N, Obulam VSR (2012) Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann Microbiol 62:1353–1360
Lu Y, Chua J-Y, Huang D, Lee P-R, Liu S-Q (2016) Biotransformation of chemical constituents of durian wine with simultaneous alcoholic fermentation by Torulaspora delbrueckii and malolactic fermentation by Oenococcus oeni. Appl Microbiol Biotechnol 100:8877–8888
Garcia CV, Quek S-Y, Stevenson RJ, Winz RA (2012) Kiwifruit flavour: A review. Trends Food Sci Tech 24:82–91
López-Vázquez C, García-Llobodanin L, Pérez-Correa JR, López F, Blanco P, Orriols I (2012) Aromatic characterization of pot distilled kiwi spirits. J Agr Food Chem 60:2242–2247
Xi H (1999) China fruit wine. China light industry, Beijing
Li X, Xing Y, Cao L, Xu Q, Li S, Wang R, Jiang Z, Che Z, Lin H (2017) Effects of six commercial Saccharomyces cerevisiae strains on phenolic attributes, antioxidant activity, and aroma of kiwi (Actinidia deliciosa, cv.) wine. Biomed Res Int 2017:1–10
Soufleros EH, Pissa I, Petridis D, Lygerakis M, Mermelas K, Boukouvalas G, Tsimitakis E (2001) Instrumental analysis of volatile and other compounds of Greek kiwi wine; sensory evaluation and optimisation of its composition. Food Chem 75:487–500
SAC (2006) Analytical methods of wine and fruit wine. Standardization administration of the People's Republic of China, Beijing
Bimpilas A, Panagopoulou M, Tsimogiannis D, Oreopoulou V (2016) Anthocyanin copigmentation and color of wine: The effect of naturally obtained hydroxycinnamic acids as cofactors. Food Chem 197:39–46
Wang X, Xie K, Zhuang H, Ye R, Fang Z, Feng T (2015) Volatile flavor compounds, total polyphenolic contents and antioxidant activities of a China gingko wine. Food Chem 182:41–46
Liang R, Huang J, Wu X, Xu Y, Fan J, Wu C, Jin Y, Zhou R (2019) Characterizing the metabolites and the microbial communities of the soy sauce mash affected by temperature and hydrostatic pressure. Food Res Int 123:801–808
Niu M, Huang J, Jin Y, Wu C, Zhou R (2017) Volatiles and antioxidant activity of fermented Goji (Lycium Chinese) wine: effect of different oak matrix (barrel, shavings and chips). Int J Food Prop 20:S2057–S2069
Canonico L, Solomon M, Comitini F, Ciani M, Varela C (2019) Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol 84:103247
Hayasaka Y, Birse M, Eglinton J, Herderich M (2007) The effect of Saccharomyces cerevisiae and Saccharomyces bayanus yeast on colour properties and pigment profiles of a Cabernet Sauvignon red wine. Aust J Grape Wine R 13:176–185
Magyar I, Tóth T (2011) Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol 28:94–100
Caridi A (2007) New perspectives in safety and quality enhancement of wine through selection of yeasts based on the parietal adsorption activity. Int J Food Microbio 120:167–172
Dobrowolska-Iwanek J, Gąstol M, Wanat A, Krośniak M, Jancik M, Zagrodzki P (2014) Wine of cool-climate areas in south Poland. S Afr J Enol Vitic 35:1–9
Zhong W, Li X, Yang H, Li E (2019) A novel, effective, and feasible method for deacidifying kiwifruit wine by weakly basic ion exchange resins. J Food Process Eng 42:e12969
Sun SY, Che CY, Sun TF, Lv ZZ, He SX, Gu HN, Shen WJ, Chi DC, Gao Y (2013) Evaluation of sequential inoculation of Saccharomyces cerevisiae and Oenococcus oeni strains on the chemical and aromatic profiles of cherry wines. Food Chem 138:2233–2241
Duarte WF, Dias DR, Oliveira JM, Vilanova M, Teixeira JA, Silva JB, Schwan RF (2010) Raspberry (Rubus idaeus L.) wine: Yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res Int 43:2303–2314
Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacón J-J, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H (2012) Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol 32:243–253
Andorrà I, Berradre M, Rozès N, Mas A, Guillamón JM, Esteve-Zarzoso B (2010) Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur Food Res Technol 231:215–224
Benito S, Hofmann T, Laier M, Lochbühler B, Schüttler A, Ebert K, Fritsch S, Röcker J, Rauhut D (2015) Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur Food Res Technol 241:707–717
Azzolini M, Tosi E, Lorenzini M, Finato F, Zapparoli G (2015) Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J Microb Biot 31:277–293
Viana F, Gil JV, Genovés S, Valles S, Manzanares P (2008) Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol 25:778–785
Acknowledgements
This research was financially supported by the Sichuan University–Luzhoulaojiao Company Fund (Grant No. 17H1039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, J., Liu, M., Ye, P. et al. Characterization of major properties and aroma profile of kiwi wine co-cultured by Saccharomyces yeast (S. cerevisiae, S. bayanus, S. uvarum) and T. delbrueckii. Eur Food Res Technol 246, 807–820 (2020). https://doi.org/10.1007/s00217-020-03439-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03439-6