Abstract
Moisture sorption isotherms and glass transition temperature (T g) of paprika oleoresin microcapsules were evaluated. Microcapsules were produced by spray drying using modified starch (Capsul®) as encapsulating agent. The differential and integral thermodynamic functions of enthalpy and entropy were estimated from the sorption data for paprika oleoresin microcapsules. T g of microcapsules conditioned at various water activities (a w’s) were determined by modulated differential scanning calorimetry. The critical water content or water activity (RHc) was estimated from T g values. Both a w and T g were used to determine the critical conditions for microcapsules storage. The GAB model provided a good fit to the experimental data. The point of maximum stability according to the minimum integral entropy (ΔS int)T was found at a w = 0.241 at 35 °C, and at this point the system was within the glassy state. RHc was founded at a w = 0.789 at 35 °C; all microcapsules stored at a w’s ≤ 0.627 were able to maintain their structural integrity without caking and stickiness occurring. The lowest carotenoid degradation of microcapsules during storage at 35 °C proceeded at a w = 0.742, which was near to RHc; however, microcapsules stored at this a w showed incipient caking and agglomeration.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paprika oleoresin is obtained by solvent extraction of dehydrated red pepper fruits, a process that generates oil with high carotenoids concentration; it has several applications as a natural colorant in the food industry mainly to correct or even reinforce color in foodstuffs, or to provide some flavoring [1]. The lipophilic extract is valuable because of its pigment profile, composed by a wide variety of carotenoids; all of these contain the same polyene chain with alternated double and single bonds [2]. The carotenoid content in the oleoresin depends on quality of raw material employed and processing conditions, which could decrease the initial value, but total pigment content between 30 and 90 g/kg are normally achieved [3]. Carotenoid profile of this product provides or reinforces food color, increases its original antioxidant value and provides provitamin A value. In spite of the recognition of the beneficial role of carotenoids in human health, they are not considered as essential nutrients and as such do not have a dietary reference intake (DRI) value assigned to them [4]. Nevertheless, according to Food and Agriculture Organization and the World Health Organization (FAO/WHO) a dietary intake of 300–1300 µg/day of retinol activity equivalents is recommended (this interval considers age, sex, pregnancy, lactation, etc.) [5], and paprika oleoresin may provide important contributions to that amount since retinol equivalent values between 500 and 2000 µg/g have been reported in this oleoresins [3]. Thus, paprika oleoresins may join to the carotenoid supplements nowadays offered.
On the other hand, polyenic structure of carotenoids pigments is responsible for their colorant properties, antioxidant activities and biological functions. However, it is also makes them very sensitive to heat, light and pro-oxidant conditions; these facts provoke isomerization and oxidation reactions, which results in the loss of carotenoid properties.
To prevent carotenoid degradation in food additives, microencapsulation is often applied [6–9]. Microencapsulation involves a solid, liquid or gaseous component in a wall material, in order to form a particle that may offer protection against oxygen, heat, humidity and light [10]. Therefore, microencapsulation could be used to avoid or delay paprika oleoresin degradation and stabilize it during storage before use.
Spray drying is the most common and cheapest technique to produce microencapsulated food materials. Equipment is readily available, and production costs are lower than most other methods. Additionally, spray drying is quite suitable in the encapsulation of oils and oleoresins [11].
In the food industry, the main carriers used for oil encapsulation are polysaccharides, starches, cellulose and its derivatives, gums and proteins [12]. Starches are abundant and cheap encapsulating agents that protect encapsulated ingredients from oxidation. Native starches have several limitations, which restrict their use as encapsulating agents. As a result, modified starches have been used to address these functionality problems [9].
In order to ensure commercial life, microcapsules must remain stable during long periods of time. This is crucial as paprika oleoresin is not immediately used for industrial purposes, but is stored.
The temperature at which an amorphous system changes from the glassy to the rubbery state defines the glass transition temperature (T g). A phase transition from glassy to rubbery results in drastic changes in molecular mobility of food polymers, which has been linked to changes in product quality and may result in stability losses in low-moisture amorphous foods [13, 14]. Theoretically, the occurrence of diffusion-controlled reactions is not allowed by the high viscosity of the matrix in the glassy state [15, 16]. However, some diffusion-controlled reactions, such as oxidation and nonenzymatic browning, may occur, even at the glassy state as it was demonstrated by some authors [17–19]. T g values change as a w (and moisture content) changes. The influence of moisture on T g can be easily examined by performing thermal analysis on samples that have been equilibrated to different water activities, and hence different moisture contents [13, 14]. Multiple studies have shown a good correlation between a w and T g. This correlation is fairly linear over a a w range of 0.1–0.7 [20–22].
Conversely, the relationship between equilibrium moisture content of products and water activity a w at a certain temperature can be described by the moisture sorption isotherm [23, 24]. Thermodynamic parameters are readily derived from sorption isotherms at different temperatures. Parameters such as enthalpy, entropy and Gibbs free energy are useful to explain reactions and phenomena at molecular level in materials [25]. Changes in some thermodynamic properties with respect to moisture content can provide a good description of the sorption mechanisms and can be used to estimate points of transition between mechanisms [26].
Integral entropy describes the degree of disorder, or randomness of motion, of the water molecules. It quantifies the mobility of the adsorbed water molecules and indicates the degree to which the water–substrate interaction exceeds that of the water molecules [27, 28]. There are many studies in the literature that use the point of minimum integral entropy as a useful tool to predict maximum stability point of dehydrated food [8, 26, 29, 30].
Several authors have coupled the concepts related to water activity (thermodynamic properties) with those of glass transition temperature (T g) in order to evaluate food stability, thus providing an integrated approach to the role of water in food [22, 31–34]. Critical water content or water activity (RHc) is normally used to estimate the most stable storage conditions, which is obtained by relating T g and water content data [15].
The water activity and glass transition concepts, which are complementary, have their respective limits: Water activity is a thermodynamical property linked to water availability defined at equilibrium. Foods, on the other hand, are mostly heterogeneous in composition and may not be in a state of equilibrium, while glass transition temperature relates to the relative molecular mobility of water between the glassy and rubbery states [16].
The objective of this work was to relate the point of minimum integral entropy with glass transition temperature (T g) to establish the best storage conditions for paprika oleoresin microcapsules in order to protect its carotenoid profile.
Materials and methods
Material, chemicals and reagents
Paprika oleoresin was obtained from AMCO (Mexico City); modified starch Capsul® (lot: LDA-510) was supplied by National Starch Food Innovation (Mexico City). Acetone and water were all of HPLC grade from Baker.
Preparation of emulsions
Emulsions were prepared by mixing paprika oleoresin into a suspension of wall material in deionized water at a rate of 1:4 (g of paprika oleoresin/g of wall material), and 0.3 g/L total solids of Capsul®. The crude emulsion was then re-circulated through a twin-stage valve homogenizer (APV-1000) at 30,000 kPa.
Preparation of microcapsules by spray drying
Emulsion was spray dried in a Büchi 290 mini spray dryer (Flawil, Switzerland). The dryer was equipped with 0.5-mm-diameter nozzle. The operating conditions for the dryer were as follows: inlet air temperature of 180 ± 5 °C and outlet air temperature of 100 ± 5 °C. Microcapsules were recovered from the collection chamber. These powders were stored in a desiccator with vacuum containing P2O5 to prevent moisture absorption prior to further studies.
Carotenoid determination
Carotenoid contain was determined through a spectrophotometric method proposed by Hornero and Mínguez [35]. For oleoresin capsules, approximately 0.025 g were dissolved in a volumetric flask containing 100 mL of acetone and then filtered and absorbance measurements were made in a diode array spectrophotometer (Agilent model 8453) at 472 and 508 nm. In order to obtain both isochromic carotenoid and total carotenoid fractions, the absorbance values obtained were introduced in the following equations:
where C R represents the red isochromatic fraction content, C Y represents the yellow isochromatic fraction content, and C T represents total carotenoid content.
All carotenoid determinations were carried out in triplicate during storage.
Vapor sorption isotherms
Samples of the spray-dried encapsulated paprika oleoresin were placed in desiccators with vacuum (13 kPa) containing P2O5 for 15 days at room temperature (25 °C). The moisture sorption data were obtained using the gravimetric method described by Lang et al. [36]. One to two grams of samples were weighed in triplicate into standard weighing dishes with a circular section on the bottom. Samples were placed in separate desiccators containing saturated salt slurries in the range of water activity from 0.102 to 0.85 using the a w values reported by Labuza et al. [37]. The samples were held at 25, 35 and 45 °C until equilibrium was reached. Values of water activity were generated using equations reported in the same paper. Equilibrium was assumed when the difference between two consecutive weightings was less than 1 mg/g of solids. The time to reach the equilibrium varied from 45 to 55 days. The Guggenheim–Anderson–de Boer (GAB) equation was used in modeling water sorption [38]:
where a w is water activity, M is moisture content of the sample on dry basis, M 0 is the monolayer moisture content, C is the Guggenheim constant, given by \( C = c^{'} \exp \left( {h_{\text{m}} - h_{\text{n}} } \right)/RT \), where c′ is the equation constant, h m is the heat of sorption of the first layer, h n is the heat of sorption of the multilayer, R is the gas constant, T is the absolute temperature, and k is the constant that accounts for properties of multilayer molecules with respect to bulk liquid and given by \( k = k^{'} \exp (h_{1} - h_{n} )/RT \), where k′ is the equation constant and h 1 is the heat of condensation of pure water. The parameter values of GAB equation (M 0, C and k) were estimated by fitting the mathematical model to the experimental data, using nonlinear regression Kaleidagraph 4.0 package (Synergy Software, 2457 Perkiomen Avenue Reading, PA 19606-2049, USA).
Goodness of fit was evaluated using the average of the relative percentage difference between the experimental and predicted values of the moisture content or mean relative deviation modulus (P) defined by the following equation [39]:
where M i is the moisture content at observation i, M pi is the predicted moisture content at that observations, and N is the number of observations. It is generally assumed that a good fit is obtained when P < 0.5.
Determination of thermodynamic parameters
The free energy for water adsorption (ΔG) was calculated using the equation of Gibbs:
where R (J/mol K) is the universal gas constant, T(K) is the sorption isotherm temperature, and a w is the water activity.
The respective differential and integral entropies are obtained from their differential and integral heats, respectively. The usual entropy discussed qualitatively or quantitatively (statistical mechanics) in terms of order–disorder of the adsorbed molecules is the integral entropy and not the differential entropy [40, 41].
Differential properties
Changes in differential enthalpy at the water–solid interface at different stages of the adsorption process were determined using Othmer’s equation [42]:
where P v is vapor pressure of water in the food, \( P_{\text{v}}^{\text{o}} \) is vapor pressure of pure water at the same temperature, H v(T) is isosteric heat for water adsorption, \( H_{\text{v}}^{\text{o}} (T) \) is heat of condensation of pure water, M is moisture, and C is adsorption constant.
A plot of ln P v against ln \( P_{\text{v}}^{\text{o}} \) generates a straight line if the ratio \( H_{\text{v}} \left( T \right)/H_{\text{v}}^{\text{o}} \left( T \right) \) is maintained constant in the range of temperatures studied.
The net isosteric heat of adsorption or differential enthalpy is defined by:
by calculating \( H_{\text{v}} \left( T \right)/H_{\text{v}}^{\text{o}} (T) \) with Othmer’s equation and substituting into last Eq., it is possible to estimate the net isosteric heat of adsorption at different temperatures using steam tables. With values obtained for enthalpy changes, the variation in the molar differential entropy (ΔS dif) T may be estimated using:
where \( S_{1} = \left( {\partial S/\partial N_{1} } \right)_{\text{TiP}} \) is the molar differential entropy of water adsorbed in the food, S L is molar entropy of pure water in equilibrium with the vapor, S is total entropy of water adsorbed in the food, N 1 is the number of moles of water adsorbed in the food, R is the universal gas constant, a w is the water activity, and T is the temperature (K).
Integral properties
Molar integral enthalpy is calculated using an expression similar to that for differential enthalpy, maintaining diffusion pressure constant:
where H vi(T) is the integral molar heat of water adsorbed in food and ϕ can be found by [43].
where ϕ is the diffusion pressure or surface potential of the food, μ a is the chemical potential of the adsorbent in the condensed phase, μ ap is the chemical potential of the pure adsorbent, W ap is the molecular weight of the adsorbent, W v is the molecular weight of water, and ϕ/α 1 constant is similar to a process at ϕ constant. When values for (ΔH int) are obtained, changes in molar integral entropy can be calculated using differential enthalpy equation:
where S S = S/N is integral entropy of water adsorbed in the food.
Storage stability
Fourteen samples containing ca. 1 g of microcapsules were placed in desiccators containing saturated solutions of LiCl, MgCl2, Mg(NO3)2 and NaCl for 35 days at 35 °C. The water activities of the desiccants were 0.108, 0.318, 0.515 and 0.743, respectively. Two samples of each wall material were withdrawn every 5 days for spectrophotometric measurement. The samples were put into the desiccants immediately after they were spray dried, and these were taken as the zero time samples.
Degradation reaction rate calculation
For each isochromatic fraction of paprika oleoresin encapsulated in Capsul® and for all water activities (0.108, 0.318, 0.515 and 0.743), the change in concentration was related to treatment time. The degradation reaction rate (k v) was calculated as the percentage of color retained with time in hours (t). The system assayed followed theoretical zero and first-order kinetics. The result showing the best multiple correlation coefficient (R) was selected. For all microcapsules and water activities assayed, 16 regression lines were obtained for the degradation rate of red and yellow pigments.
Glass transition
The glass transition temperature (T g) was determinate by a modulated differential scanning calorimeter (DSC Q-2000, TA Instruments, New Castle, DE, USA), equipped with a refrigerated cooling accessory. The Thermal Solutions Instrument Control and Universal Analysis Software were used (TA Instruments, New Castle, DE, USA). The samples (5 ± 0.1 mg) were transferred to aluminum pans, sealed hermetically, and weighed. The calorimeter was calibrated whit indium (melting point 156.98 °C). Three replicates were carried out for each analysis. Samples were cooled at 20 °C/min to a temperature that was −80 °C; then, a isothermal was performed by 10 min and finally samples were heated at 3 °C/min using an amplitude of 0.636 °C and a period of 40 s. Glass transition temperature was determined as the onset point of the step change on the heat flow curve.
Structure analysis
Structure of spray-dried microcapsules equilibrated at different water activities (0.108, 0.515, 0.743 and 0.967) were evaluated with a scanning electron microscope (SEM), Jeol model JSM-5600lv. The microcapsules were attached to SEM stubs of 2.54 cm diameter using two-sided adhesive tape. The specimens were coated with gold–palladium (plasma deposition method) and examined on the SEM at 15 kV.
Results and discussion
Thermodynamic parameters
Water activity of foods can be related to stability and the rates of deteriorative reactions. The parameters from the GAB model fitting were calculated (Table 1). The GAB model was found to fit very well with the experimental data over the whole measured a w range, as the values of the relative mean deviation (P) were <3 % at 25, 35 and 45 °C.
Monolayer moisture content of the material (M 0) indicates the amount of water that is strongly adsorbed to specific sites, and it has been considered as the critical water content at which a dehydrated foods are more stable [44]. The M 0 values obtained for paprika oleoresin microcapsules are shown in Table 1; it can be noted that M 0 decreased as temperature increased, which is due to the fact that adsorption is an exothermic process. Additionally, the value of C, which is associated with the chemical potential differences between the monolayer and superior layers, showed a clear trend with temperature changes. It is assumed that strong adsorbent–adsorbate interactions, which are exothermic, produce temperature lowering and increases in C.
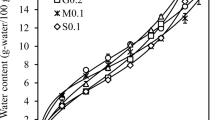
Differential and integral entropies changes with respect to moisture content at 35 °C for paprika oleoresin encapsulated with Capsul® are showed in Fig. 1. The integral entropy indicates the order–disorder grade with the water molecules are absorbed in the surface of the dehydrated food. As the microcapsules adsorbed moisture the integral entropy diminished to a minimum point. At this point, strong bonds between adsorbate and adsorbent occur, which make water less available to participate in degradation reactions, and therefore, maximum stability can be assumed. The intersection of the curves is found at the minimum integral entropy, and in this investigation, the point of maximum stability against carotenoid degradation was found at 6.441 g water/100 g soluble solids for paprika oleoresin microcapsules, corresponding to a water activity close to 0.244. Nevertheless, paprika oleoresin microcapsules showed a small zone where minimum integral entropy had no big changes. This zone begins at moisture contents of 5.888 g water/100 g soluble solids (a w = 0.191) and ends at 6.944 g water/100 g soluble solids (a w = 0.286) (Fig. 1). The monolayer value calculated for the GAB equation fell into the minimum entropy zone; similar results have been reported by Domínguez et al. [29].
Microcapsules stability based on water activity and glass transition
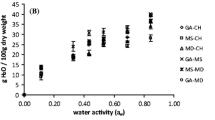
The temperature at which microcapsules have their glass transition depends strongly on the water content. Moisture is the most common plasticizer in food materials. Glass transition temperature (T g) decreased with increasing water content caused by the plasticizing effect of water, as can be observed in Fig. 2. The same trend was observed by several authors for dehydrated foods [15, 33, 45, 46].
The water activity value that decreases the T g to room temperature has been identified as RHc (the critical water content and water activity), and like T g, RHc is unique to each material type [14, 47, 48]. For amorphous glassy materials, such as oleoresin microcapsules stored and exposed to ambient relative humidity, a rise in ambient humidity above the RHc will result in a glass transition, and the powder will become susceptible to deteriorative changes like stickiness, caking and collapse, resulting in quality loss. Hence, in order to obtain the critical conditions for paprika oleoresin microcapsules storage, sorption isotherm and T g data were plotted as a function of a w (Fig. 2) and the critical values of water activity and moisture were obtained considering a room temperature of 35 °C. This plot depicts a linear relationship between water activity and glass transition temperature. GAB monolayer and minimum entropy zone values were included in order to relate with RHc.
RHc was found at a water activity of 0.789, over this point microcapsules turn into a rubbery state. GAB monolayer and minimum integral entropy values correspond to water activities of 0.238 and 0.244, respectively, consequently, microcapsules were in glassy state, and at these a w no difference between T g values were founded (77.85 °C).
Degradation of total carotenoids content during storage of paprika oleoresin encapsulated in Capsul® is shown in Fig. 3. Paprika oleoresin microcapsules exposed to a w = 0.742 showed the lowest carotenoid degradation; this a w was very close to RHc, which indicates that microcapsules were near to the transition point between glassy and rubbery states. This may be the cause for microcapsules to develop visual structural changes and the water gain led to a change in their flow properties as a result of caking and agglomeration. For this reason, these conditions cannot be taken as the best stability against carotenoid degradation.
Table 2 shows degradation rate constants (k v’s) of both carotenoid fractions present in paprika oleoresin microcapsules. An increase in storage water activity from 0.108 to 0.515 led to an increase in k v’s, but microcapsules were still within the glassy state. Based on the properties associated with the glassy amorphous state, where food materials exist in a metastable condition and remain stable for extended periods of time [13], it would be expected that no further degradation of the carotenoid content of the microcapsules should arise at all. However, the rate of oxidation of the paprika oleoresin microcapsules, occurred rapidly within the glassy state. Carotenoid degradation is dependent upon a proper matrix formation as well as exclusion of oxygen from the matrix. Glassy characteristics may delay oxidation only if the oxygen is suitable to be entrapped by the molecular structure of the glassy matrix.
Similar results were reported by Beristain et al. [18], who claim that the major determinant of the microcapsules shelf life is the porosity to oxygen diffusion of the dried matrix, regardless of the supercooled liquid state of the matrix.
On the other hand, all treatments showed that degradation particularly affects the yellow pigments, while the pigments making up the red fractions are degraded more slowly during storage at 35 °C. This behavior is attributed to the existence of an isokinetic temperature (T isok), which has been previously reported by Perez-Gálvez et al. [49]; it is approximately 82.8 °C; at temperatures below this T isok, degradation is preferentially toward the yellow fraction, while at higher temperatures, it is toward the red fraction.
The lowest carotenoid degradation rates took place in microcapsules stored at a water activity of 0.108, whereas they remain in glassy state. Therefore, the best stability against carotenoid degradation of paprika oleoresin microcapsules occurs at a water activity near to the zone of minimum integral entropy.
Morphology of paprika oleoresin microcapsules
SEM micrographs of the capsules stored demonstrated that microcapsules had a rounded outer surface with the formation of teeth or concavities and they varied in size. The appearance of teeth on the surface is attributed to rapid evaporation of drops of liquid during the drying process in the atomizer [50]. Similar morphologies were founded by Buffo [51], Finotelli and Rocha-Leao [52] and Rocha [50], in orange essential oil, ascorbic acid and lycopene microcapsules obtained by spray drying using Capsul® as wall material, respectively.
Changes on the physical characteristics of the paprika oleoresin microcapsules stored at 35 °C at different water activities could be observed. SEM micrographs reveal that microcapsules at all water activities proved during storage were able to keep their structural integrity, an attribute that is essential to ensure low gas permeability, better protection and carotenoid retention. When microcapsules were stored at water activities of 0.627 or lower, they remained as a free-flowing powder, but at a w = 0.742 they started showing agglomeration, and at water activities higher than 0.742 microstructural changes were observed: Microcapsules became unable to keep their structural integrity and complete agglomeration of all material occurred, with the subsequent formation of hard and dark blocks. Similar findings were reported by Tonon et al. [15] for spray-dried Açai juice; these authors concluded that this behavior was a result from the compaction, and advanced stage in caking associated with a pronounced loss of system integrity that was caused by thickening of interparticle bridges due to flow, reduction of interparticle spaces and deformation of particle clumps under pressure. At water activities of 0.821 and higher, most of the microcapsules disappeared, leading to a highly sticky mass (Fig. 4).
Conclusions
Microencapsulation by spray drying resulted to be a convenient strategy to prevent carotenoid degradation avoiding oxygen-mediated auto-oxidation reactions, besides this technique is not only efficient but also economic due to production costs are lower than those associated with most other methods of encapsulation. The GAB equation was useful for modeling moisture sorption of paprika oleoresin microcapsules in all a w range studied. Minimum integral entropy was found at 6.441 g water/100 g soluble solids, corresponding to a water activity close to 0.244. Microcapsules had the slowest kv at low water activities, carotenoid degradation rate increased as storage water activity increased, even when microcapsules remained in the glassy state. Carotenoid degradation particularly affected the yellow pigments due to the existence of T isok. RHc was found at a water activity of 0.789; however, high kv’s were found bellow this a w. Results obtained in this work suggest that knowing of RHc value is not enough to establish maximum stability conditions, but relating RHc with ΔSint min provided a useful tool to predict the best stability against carotenoid oxidation.
References
Pérez Gálvez A, Hornero-Méndez D, Mínguez-Mosquera MI (2009) Stability of paprika without supplementary antioxidants during storage under industrial controlled conditions. J Agric Food Chem 57:4718–4723
Pérez A, Mínguez MI (2004) Degradation, under non-oxygen-mediated autooxidation, of carotenoid profile present in paprika oleoresins with lipid substrates of different fatty acid composition. J Agric Food Chem 52:632–637
Pérez-Gálvez M, Jarén-Galán MI Mínguez-Mosquera (2005) Impact of the increased thermal processing on retinol equivalent values of paprika oleoresins. J Food Eng 71:379–385
Raoa AV, Rao LG (2007) Carotenoids and human health. Pharmacol Res 55:207–216
Zempleni J, Suttie JW, Gregory JF III, Stover PJ (2014) Handbook of vitamins. CRC Press, New York
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM, Argüelles-Monal W (2004) Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr Polym 56:41–45
Laos K, Lõugas T, Mandamets A, Vokk R (2007) Encapsulation of β-carotene from sea buckthorn (Hippophae rhamnoides L.) juice in furcellaran beads. Innov Food Sci Emerg 8:395–398
Rascón MP, Beristain CI, García HS, Salgado MA (2011) Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and soy protein isolate as wall materials. LWT Food Sci Technol 44:549–557
Corralo Spada J, Zapata Norena CP, Ferreira Marczaka LD (2012) Study on the stability of β-carotene microencapsulated with pinhão. Carbohydr Polym 89:1166–1173
Frascareli EC, Silva VM, Tonon RV, Hubinger MD (2012) Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod Process 90:413–424
Gharsallaoui A, Roudaut G, Chambin O, Voilley A, Saurel R (2007) Applications of spray-drying in microencapsulation of food ingredients: an overview. Food Res Int 40:1107–1121
Turchiuli C, Jimenez Munguia MT, Hernandez Sanchez M, Cortes Ferre H, Dumoulin E (2013) Use of different supports for oil encapsulation in powder by spray drying. Powder Technol 255:103–108
Roos YH (2007) Water activity and glass transition. In: Barbosa-Canovas G, Fontana AJ, Schmidt SJ, Labuza TP (eds) Water activity in foods, 1st edn. Blackwell Publishing and IFT Press, Ames, pp 29–46
Carter BP, Schmidt SJ (2012) Developments in glass transition determination in foods using moisture sorption isotherms. Food Chem 132:1693–1698
Tonon RV, Baroni AF, Brabet C, Gibert O, Pallet D, Hubinger MD (2009) Water sorption and glass transition temperature of spray dried acai (Euterpe oleracea Mart.) juice. J Food Eng 94:215–221
Djendoubi Mrad N, Bonazzi C, Courtois F, Kechaou N, Boudhrioua Mihoubi N (2013) Moisture desorption isotherms and glass transition temperatures of osmo-dehydrated apple and pear. Food Bioprod Process 9(1):121–128
Schebor C, Buera MP, Karel M, Chirife J (1999) Color formation due to nonenzymatic browning in amorphous, glassy, anhydrous, model systems. Food Chem 65(4):427–432
Beristain CI, Azuara E, Vernon-Carter EJ (2002) Effect of water activity on the stability to oxidation of spray-dried encapsulated orange peel oil using mesquite gum (Prosopis juliflora) as wall material. J Food Sci 67:206–211
Miao S, Roos YH (2004) Comparison of nonenzymatic browning kinetics in spray-dried and freeze-dried carbohydrate-based food model systems. J Food Sci 69(7):222–331
Aguilera JM, Levi G, Karel M (1993) Effect of water content on the glass transition and caking of fish protein hydrolyzates. Biotechnol Prog 9:651–654
Roos YH, Karel M (1991) Applying state diagrams to food processing and development. Food Technol 45:66–71
Shrestha AK, Howes T, Adhikari BP, Bhandari BR (2007) Water sorption and glass transition properties of spray dried lactose hydrolysed skim milk powder. LWT-Food Sci Technol 40(9):1593–1600
Quirijns EJ, van Boxtel AJB, van Loon WKP, van Straten G (2005) Sorption isotherms, GAB parameters and isosteric heat of sorption. J Sci Food Agric 85(11):1805–1814
Xiao Q, Tong Q (2013) Thermodynamic properties of moisture sorption in pullulan–sodium alginate based edible films. Food Res Int 54:605–1612
Cano-Higuita DM, Villa-Vélez HA, Telis-Romero J, Váquiro HA, Nicoletti Telis VR (2013) Influence of alternative drying aids on water sorption of spray dried mango mix powders: a thermodynamic approach. Food Bioprod Process. doi:10.1016/j.fbp.2013.10.005
Viganó J, Azuara E, Telis VRN, Beristain CI, Jiménez M, Telis-Romero J (2012) Role of enthalpy and entropy in moisture sorption behavior of pineapple pulp powder produced by different drying methods. Thermochim Acta 528:63–71
Mazza G, Le Maguer M (1978) Water sorption properties of yellow globe onion (Allium cepa L.). Can Inst Food Sci Technol J 11(4):189–193
Lago CC, Liendo-Cárdenas M, Zapata Noreña CP (2013) Thermodynamic sorption properties of potato and sweet potato flakes. Food Bioprod Process 91(4):389–395
Domínguez IL, Azuara E, Vernon-Carter EJ, Beristain CI (2007) Thermodynamic analysis of the effect of water activity on the stability of macadamia nut. J Food Eng 81:566–571
Bonilla E, Azuara E, Beristain CI, Vernon-Carter EJ (2010) Predicting suitable storage conditions for spray-dried microcapsules formed with different biopolymer matrices. Food Hydrocolloid 24:633–640
Sablani SS, Kasapis S, Rahman MS, Al-Jabri A, Al-Habsi N (2004) Sorption isotherms and state diagram for evaluating stability criteria of abalone. Food Res Int 37:915–924
Moraga G, Martínez-Navarrete N, Chiralt A (2006) Water sorption isotherms and phase transitions in kiwi fruit. J Food Eng 72(2):147–156
Kurozawa LE, Park KJ, Hubinger MD (2009) Effect of maltodextrin and gum Arabic on water sorption and glass transition temperature of spray dried chicken meat hydrolysate protein. J Food Eng 91(2):287–296
Symaladevi RM, Sablani SS, Tang J, Powers J, Swanson BG (2009) State diagram and water adsorption isotherm of raspberry (Rubus idaeus). J Food Eng 91(3):460–467
Hornero D, Mínguez MI (2001) Rapid spectrophotometric determination of red and yellow isochromic carotenoid fractions in paprika y red pepper oleoresins. J Agric Food Chem 49:3584–3588
Lang KW, McCune TD, Steinberg MP (1981) Proximity equilibration cell for rapid determination of sorption isotherms. J Food Sci 46(3):936–938
Labuza TP, Kaanane A, Chen JY (1985) Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J Food Sci 50:385–391
Weisser H (1985) Influence of temperature on sorption equilibria. In: Simato D, Multon JL (eds) Properties of water in foods. Martinus Nijhoff Publishers, Dordrecht, pp 133–151
Lomauro CJ, Bakshi AS, Labuza TP (1985) Evaluation of food moisture sorption isotherm equations. Part I. Fruit, vegetable and meat products. LWT-Food Sci Technol 28:111–117
Hill TL, Emmett PH, Joyner LG (1951) Calculation of thermodynamic functions of adsorbed molecules from adsorption isotherm measurements: nitrogen on graphon. J Am Chem Soc 73:5102–5107
Rizvi SSH, Benado AL (1984) Thermodynamic properties of dehydrated foods. Food Technol 38(3):83–92
Othmer DF (1940) Correlating vapor pressure and latent heat data. A new plot. Ind Eng Chem 32:841–856
Nunes RV, Rotstein E (1991) Thermodynamics of the water-foodstuff equilibrium. Dry Technol 9(1):113–117
Goneli ALD, Corrêa PC, Oliveira GHH, Afonso PC Jr (2013) Water sorption properties of coffee fruits, pulped and green coffee. LWT-Food Sci Technol 50:386–391
Goula AM, Karapantsios TD, Achilias DS, Adamopoulos KG (2008) Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J Food Eng 85(1):73–83
Wang S, Chen CT, Sciarappa W, Wang CY, Camp MJ (2008) Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J Agric Food Chem 56:5788–5794
Yuan X, Carter BP, Schmidt SJ (2011) Determining the critical relative humidity at which the glassy to rubbery transition occurs in polydextrose using an automatic water vapor sorption instrument. J Food Sci 76:78–89
Sillick M, Gregson CM (2010) Critical water activity of disaccharide/maltodextrin blends. Carbohydr Polym 79:1028–1033
Pérez-Gálvez A, Jarén-Galán M, Mínguez-Mosquera MI (2000) Effect of high-temperature degradative processes on ketocarotenoids present in paprika oleoresins. J Agric Food Chem 48:2966–2971
Rocha GA, Fávaro-Trindade CS, Ferreira Grosso CR (2012) Microencapsulation of lycopene by spray drying: characterization, stability and application of microcapsules food and bioproducts processing. Food Bioprod Process 90:37–42
Buffo RA, Probst K, Zehentbauer G, Reineccius GA (2002) Effects of agglomeration on the properties of spray-dried encapsulated flavours. Flavour Frag J 17:292–299
Finotelli PV, Rocha-leão MHM (2005) Microencapsulation of ascorbic acid in maltodextrin and capsul using spray-drying. Anais do 4◦ Mercosur congress on process systems engineering. Innov Food Sci Emerg 8:395–398
Acknowledgments
The authors wish to thank the Consejo Nacional de Ciencia y Tecnología (CONACyT) for the support received through Project 124229.
Conflict of interest
We certify that no actual or potential conflict of interest in relation to this article exists.
Compliance with Ethics requirement
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rascón, M.P., Bonilla, E., García, H.S. et al. T g and a w as criteria for the oxidative stability of spray-dried encapsulated paprika oleoresin. Eur Food Res Technol 241, 217–225 (2015). https://doi.org/10.1007/s00217-015-2446-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-015-2446-6