Abstract
The effect of vacuum is an emerging experimental parameter to consider during optimization of a variety of headspace microextraction methodologies. The positive effect of vacuum was initially demonstrated for headspace solid-phase microextraction and was recently expanded to single-drop microextraction and higher capacity sorbents i.e. stir bar sorptive extraction. In all cases, sampling under vacuum greatly accelerated the extraction kinetics of analytes exhibiting long equilibration times under atmospheric pressure. At the same time, the extraction of analytes that reached equilibrium fast was not affected. In all optimized methods, extraction times were greatly reduced and/or sampling temperatures were lower to those reported with the standard methodology under atmospheric pressure. This work succinctly overviews the effect of vacuum on the different headspace microextraction technologies reported so far. The fundamental concepts describing the pressure dependence of each methodology are pulled together and presented in a simplified manner. The latest findings on the combined effects of vacuum and several selected experimental parameters typically examined during method optimization are then presented and the practical aspects of past outcomes are highlighted. The discussion also includes the air-evacuation step and the analysis of complex matrices. This article is intended for readers who are either new to the field of vacuum headspace microextraction sampling or its use and want to exploit this powerful approach.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microextraction techniques are equilibrium non-exhaustive methods, developed to address the need for a reduction in solvent use to facilitate rapid and convenient sample preparation [1]. Depending on the type of extracting phase used, microextraction methods are commonly categorized as solid- and liquid-phase techniques. In solid-phase techniques, the extracting phase can be referred to as sorbent and includes certain solid-supported liquid phases like polydimethylsiloxane (PDMS) [2]. The most common and commercially available solid-based microextraction technique is solid-phase microextraction (SPME) that uses a thin fused silica fiber which is coated with a polymeric film to extract target analytes [1, 3]. Stir bar sorptive extraction (SBSE) is another method of choice and a scaled-up version of SPME that uses a higher volume sorbent and therefore has a higher analyte capacity [2, 4]. Liquid-phase techniques, generally known as solvent microextraction techniques or liquid-phase microextraction techniques, use a liquid phase as the extracting phase [5]. Single-drop microextraction was the first such reported method that used a water-immiscible organic solvent in the form of a microdrop to extract target analytes from the sample [6].
The two most basic sampling modes in microextraction are direct and headspace extraction [1, 2]. In the direct sampling mode, the liquid or solid extracting phase is immersed into the sample and analytes are extracted directly from the sample matrix. In the headspace sampling mode, the extracting phase is exposed to the headspace above the sample and the analytes need to be transported through the barrier of air before they can reach the extracting phase [1]. This modification serves primarily to protect the extraction phase from hostile matrices and prevent interaction with matrix interferences.
In headspace microextraction, the time needed to reach equilibrium depends on the properties of the target analyte, matrix, and extracting phase. To this end, past reports conclude that headspace microextraction of volatile analytes occurs faster than that of semi-volatiles [1, 7]. This is because semi-volatiles must be transported through the gaseous barrier to reach the coating, but their low affinity for the gas-phase results in small extraction rates and long equilibration times. It is noted, however, that relatively long equilibration times can be recorded even for analytes having a large affinity for the headspace provided that their affinity for the extracting phase is also high or that high capacity sorbents are used. This is because for these analytes, the large amounts to be extracted at equilibrium also require more time to approach this condition [1, 8].
Different strategies exist to reduce equilibration times during headspace microextraction, the most common being heating the sample [3]. Despite its widespread use, this approach is not always efficient as it can result in sample decomposition and/or the creation of other components or artifacts. Moreover, increasing the sampling temperature may decrease partitioning of target analytes and favor the gas phase over the extracting phase [1]. An alternative approach to reduce equilibration times is to perform headspace sampling under reduced pressure conditions. Vacuum headspace microextraction sampling does not affect the final analyte amount extracted at equilibrium, but greatly accelerates the extraction kinetics of analytes having long equilibration times under regular atmospheric pressure. The effect of vacuum was first studied for headspace SPME (HS-SPME) by Brunton et al. [9] and later confirmed by Darrouzes et al. [10] and Groenewold et al. [11]. In 2012, Psillakis et al. presented the theoretical model describing the pressure dependence of the so-called vacuum-assisted HS-SPME (Vac-HS-SPME) for water [12, 13], and in 2015, for solid matrices [14]. Vac-HS-SPME has been successfully applied to a variety of analytes and matrices including solids [15,16,17] and complex food matrices like wine [18], dairy products [19, 20], and extra virgin olive oil [21]. In 2017, the applicability of the approach was expanded by Trujillo-Rodríguez et al. to headspace SDME (HS-SDME) and the resulting method, termed vacuum-assisted HS-SDME (Vac-HS-SDME), yielded shorter equilibration times for short-chain free fatty acids compared with atmospheric pressure sampling [22]. In a later report, Psillakis et al. [23] proposed a numerical model for Vac-HS-SDME that quantified the pressure dependence of extraction rates, and related the theory to experimental data. In 2020, Solomou et al. [24] applied and formulated the vacuum approach to headspace sampling using SBSE, and the method was termed vacuum-assisted headspace sorptive extraction (Vac-HSSE). Headspace sampling under vacuum was also used in some vacuum-controlled sorbent traps called sorbent pens, which favor exhaustive extraction as they are packed with a large quantity of extraction material (approximately 10 times the volume typically used for SBSE and approximately 500 times the volume typically used for SPME). The technique called vacuum-assisted sorbent extraction (VASE) is commercialized, and despite the advantages of the low sampling pressure, only two VASE applications were recently published [25, 26]. In another recent report, the effect of vacuum was successfully applied to dynamic headspace in-tube extraction and the method was termed dynamic headspace vacuum transfer in trap extraction (DHS-VTT) [27]. The authors made a simple modification on a commercial hardware and achieved extraction of volatiles at shorter extraction times and lower temperatures compared with regular pressure. Although VASE and DHS-VTT reported the positive effect of vacuum on headspace sampling, both techniques are exhaustive, and for this reason they are not discussed in this work. It is also noted that although SBSE has a high opportunity to achieve exhaustive extraction, the method is not usually operated as an exhaustive extraction procedure [2], and for this reason the headspace sampling mode of SBSE (i.e., HSSE) is treated as a microextraction method and is included in this article.
The objective of this contribution is to review the latest advancements in vacuum-assisted headspace microextraction. For the first time, the positive effect of vacuum is discussed for different microextraction methodologies, namely, HS-SPME, HS-SDME, and HSSE. The fundamental aspects of each method are combined and presented in a simplified manner. Building on this knowledge and knowledge gained from past applications, the effects of selected important experimental parameters examined during method optimization are discussed and updated, and the practical aspects are highlighted. The current work overviews the effect of vacuum on different headspace microextraction methods and two detailed reviews are recommended to readers wishing to gain in-depth view of Vac-HS-SPME [28] and the environmental applications of Vac-HS-SPME [29].
Theoretical considerations
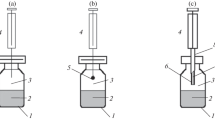
Headspace microextraction sampling under vacuum can only affect the extraction efficiency of analytes at the pre-equilibrium stage [12] and the theoretical aspects of this pressure dependence have been reported for HS-SPME [12,13,14, 19, 21, 28, 30], HS-SDME [23], and HSSE [24] and for different matrix types. This section presents the most important fundamental concepts applying in headspace microextraction sampling under vacuum, and succinctly pulls together the reported theoretical aspects of Vac-HS-SPME, Vac-HS-SDME, and Vac-HSSE. To better understand the pre-equilibrium aspects of headspace microextraction, the system is decoupled into two interfacial ones as depicted in Fig. 1: (a) the volatilization/evaporation step (sample/headspace system), and (b) the analyte uptake by the extracting phase (headspace/extracting phase system). Each step can be fast or slow depending on the properties of the analyte, sample matrix, and/or extracting phase and the slowest step will determine the overall speed of the extraction process. To gain a rich insight into the effect of vacuum on headspace microextraction and elucidate the distinct characteristics involved, each system will be discussed separately.
Representation of the two interfacial systems involved in headspace microextraction: (a) the evaporation/volatilization step (sample/headspace system), and (b) the analyte uptake by the extracting phase (headspace/extracting phase system). The different types of resistances are annotated based on the two-film theory formulation (when applicable), where (s), (g), and (e) are the sample, gas, and extracting phases
The effect of vacuum on the volatization step
The pressure dependence of the pre-equilibrium stage during evaporation from liquid and liquid-contained samples was initially explained using the classic two-film or resistance concept Fig. 1a [12, 13, 21]. In a recent report, Zhakupbekova et al. [29] used the second Fick’s law of diffusion to formulate the evaporation step. This approach has been successful in describing the pressure dependence, but less enlightening in connecting system’s properties with the effect of vacuum.
A simple criterion was proposed to predict the effect of vacuum on the evaporation rates (sample/headspace system), which, assuming fast analyte uptake by the extracting phase, could also be used to predict the effect of low pressure on the overall extraction kinetics [13]. According to this criterion, the extraction of analytes with a Henry’s Law constant KH ≤ 1.6 × 10−4 atm m3 mol−1 will be faster when sampling under vacuum compared with atmospheric pressure, whereas, for analytes with KH ≥ 1.6 × 10−4 atm m3 mol−1, vacuum conditions will not affect their extraction rates. This criterion was first formulated for HS-SPME and assumed fast equilibration of analytes between the headspace and SPME fiber coating [13]. Subsequent reports on HS-SDME and HSSE suggested that the KH criterion must be used with caution for higher capacity sorbents, since the assumption of fast equilibration at the headspace/extracting phase may not be fulfilled [23, 24]. As will be discussed in more details in the following section, this practically means that analyte uptake by higher capacity sorbents can be the rate-limiting step in extraction, and improvements in extraction efficiencies when lowering the total pressure can be recorded even for analytes with KH ≥ 1.6 × 10−4 atm m3 mol−1, reflecting accelerations at the headspace/extracting phase and not at the sample/headspace system.
The two-film theory was also used to describe the pressure dependence of the volatilization of analytes from non-aqueous liquid samples, namely, oily samples [21], and partitioning between air and olive oil as solvent was considered at the sample/headspace system. For such viscous samples, the viscosity values were taken into consideration, as they increased liquid-phase resistance and delayed analyte diffusion into the oil phase. It is noted that the establishment of a criterion that predicts the effect of vacuum when sampling oily matrices was not possible. Olive oil is a complex mixture of compounds that may vary in composition depending on the origin of the olives, and as such a limited number of air-olive oil partitioning data exists in the literature.
For solid samples, the model used to describe the pressure dependence of the volatilization process during pre-equilibrium HS-SPME sampling also took into account the tortuosity factor and increased path length of diffusing gas molecules in soil [14]. This formulation predicted that reducing the total pressure during pre-equilibrium headspace sampling would increase the vapor flux of chemicals at the soil surface when compared with atmospheric pressure conditions and result in a faster overall headspace microextraction process.
The effect of vacuum on the analyte uptake step
During HS-SPME, the evaporation of semi-volatiles from the sample to the headspace is the slow step in extraction whereas analyte uptake by the SPME fiber is considered a relatively fast process. However, depending on the properties and geometry of the extracting phase as well as the properties of the target compounds, analyte uptake can be the slow step in the overall extraction kinetics and result in long equilibration times. This can be the case for analytes with a high affinity for the extracting phase or when high capacity extracting phases are used [8] or when mass transfer inside the extracting phase is slow [23, 31]. In the first two cases, the high amount of analyte molecules to be extracted at equilibrium will require long times to reach this state. This can be more easily understood by considering the relationship used to express the time to effective equilibrium (t95%), defined as the time needed for the analyte’s concentration in the PDMS phase to reach 95% of its true equilibrium value [32]. This formula shows a proportional relationship between t95% and the partition coefficient between the gas phase and the extracting phase i.e. large partition coefficients will result in large t95%. This relationship also shows an inverse proportionality between t95% and the surface area to volume ratio of the sorbent coating under investigation. For example, the surface area to volume ratio of the PDMS coating in SBSE is four times lower to that of a PDMS-coated SPME fiber, and yields longer equilibration times for SBSE [24, 32]. Accordingly, for a given analyte, analyte uptake by the PDMS SPME fiber can be a relatively fast process, whereas uptake by the PDMS-coated stir bar can be the slow step in extraction and determine the overall extraction kinetics [24].
Two models were used in the past to describe the pressure dependence of analyte uptake by a liquid (e.g., organic solvent) or liquid-like (PDMS) extracting phase during pre-equilibrium headspace microextraction [23, 24]. In the model used for HS-SDME, gas uptake at the headspace-microdrop system was formulated in terms of the resistance model, an approach commonly used to describe gas uptake by droplets in atmospheric processes [23]. Calculations using naphthalene, acenaphthene, and pyrene as model analytes confirmed that gas-phase resistance was largely eliminated (> 96%) when reducing the sampling pressure from 1 to 0.04 atm; an effect nearly independent of analyte molecular mass. Although sampling under vacuum practically removed almost all gas-sided resistance, the impact of this improvement was dependent on the magnitude of the liquid-phase constraints within the microdrop. Essentially, if diffusion constraints inside the microrop are negligible, then reducing the sampling pressure will improve analyte uptake by the droplet. On the contrary, if liquid constraints dominate analyte uptake, then the reduction in gas-phase resistance induced by lowering the total pressure will not affect the kinetics of analyte uptake. In the case of HSSE, the two-film approach (Fig. 1b) was used to formulate analyte uptake of low-molecular weight PAHs model compounds (naphthalene, acenaphthene, and fluoranthene) by the PDMS-coated stir bars [24]. Similarly to HS-SDME, analyte uptake by the stir bar was accelerated when sampling under vacuum and the experimental data confirmed the net improvement in extraction efficiencies and resulted in shorter sampling times compared with regular pressure. An important outcome from both Vac-HS-SDME and Vac-HSSE investigations were related to the results recorded for naphthalene [23, 24]. The relatively high KH value of naphthalene suggested a facile evaporation from the water phase and as such, a negligible effect of vacuum in the evaporation step. Nonetheless, Vac-HS-SDME and Vac-HSSE sampling improved the extraction efficiency of naphthalene compared with the standard methodology and experimentally confirmed that vacuum sampling can accelerate analyte uptake. Based on this finding, the KH criterion, established to predict the effect of low sampling pressure on the evaporation step, should be used with caution as the assumption of fast equilibration between the headspace and extracting phase may not be satisfied. This implies that in cases where analyte uptake by the extracting phase is the slow step in extraction, vacuum sampling may be beneficial even if the analyte does not fulfill the KH criterion [23, 24]. It is noted that analytes satisfying the KH criterion may benefit from additional acceleration during analyte uptake and this will depend on analyte partitioning between the headspace and extracting phase [23, 24].
Selected parameters studied during method optimization
In the practical performance, the effect of vacuum can be successfully coupled to the effects of other experimental parameters typically considered during method optimization. The selection of optimum conditions depends on the nature and complexity of the sample to be analyzed and on the properties of the target analytes and extracting phase. This section discusses a selection of important parameters which are common to the microextraction technologies discussed here, and for which new findings were recently reported. Regarding Vac-HS-SPME, information on other experimental parameters can be found in two past review reports [28, 29]. It is noted that the discussion of the effect of the type of extracting phase is discussed within the different experimental parameters considered here.
The air-evacuation step
Adopting the vacuum approach during headspace microextraction is easy as the only extra step needed is that of air-evacuating the sample container before headspace sampling. In principle, air evacuation can proceed by hand, using a gastight syringe to remove the air out of the vial [11, 20, 33]. This approach has proven practical for Vac-HS-SPME of alkylphosphonates from solid surfaces [11] and in vivo Vac-HS-SPME sampling of volatiles emitted from fruits [33]. Nonetheless, some disadvantages are connected to this approach, including that septa are punctured multiple times (ideally, the air has to be removed over and over again), and that this approach cannot be as effective as the use of vacuum pump [20]. For these reasons, the use of a vacuum pump is recommended. Ideally, a vacuum gauge connected to the vacuum line can help monitor the pressure to which vials are evacuated, save time, and give indications on leaks from the cap or septum.
For liquid samples, air evacuation can proceed before or after introducing the sample [24, 34, 35]. On the other hand, for solid samples, the air is typically removed in the presence of the sample [14, 36], unless if a specially designed vessel is used [37, 38]. Moreover, depending on the microextraction method used, the air is removed with the extracting phase exposed to the gas phase (e.g., the coated stir bar in Vac-HSSE), and this setup was not found to affect extraction [24]. Removing the air in the presence of the sample should not affect the extraction of less volatile analytes but can lead to losses of the more volatile analytes due to aspiration [14, 17]. This drawback can be effectively overcome if an appropriate strategy is followed. The first thing to consider is that the air-evacuation time does not have to be long, and depending on the pump used and extraction vessel volume used, air-evacuation times should not exceed 1 min. This step can be optimized if a vacuum gauge is connected to the vacuum line and the minimum evacuation time can be determined. Another point to consider is to freeze the sample before air evacuation [17, 30]. This will decrease analyte concentration in the headspace and minimize the portion of volatile analytes aspired during air evacuation. It is noted that freezing of the sample should be done immediately after introducing the sample, so that analytes do not have enough time to equilibrate with the headspace. Recently, Capetti et al. reported that increasing the sample amount next to freezing a solid sample is an effective strategy to minimize aspiration of volatile analytes [17]. In their work, the authors concluded that problems with analyte losses recorded with 5 mg of frankincense sample could be overcome when using 100 mg of sample instead. Moreover, at this sample amount, an improvement in extraction efficiencies for semi-volatiles was confirmed with Vac-HS-SPME over regular HS-SPME.
The effect of sample temperature
Sampling temperature is one of the most important parameters to control in headspace microextraction methods [1]. At room temperature, only volatile analytes are effectively transported through the headspace to the extracting phase, whereas for semi-volatiles, heating the sample is an effective approach to increase their headspace concentrations and reduce their equilibration times. According to the theory, heating the sample under reduced pressure conditions should improve further the extraction kinetics [12]. During Vac-HS-SPME, this was not always experimentally verified and higher sampling temperatures under vacuum were found to reduce extraction efficiencies compared with atmospheric pressure (Fig. 2(i)). The effect was related to the increased levels of humidity when heating the sample and was more pronounced when an absorbent type of SPME fiber (i.e., PDMS) was used [39]. Subsequent studies on Vac-HSSE revealed that when a higher PDMS volume and layer thickness is used (such as in the case of PDMS-coated stir bar), successful coupling of sample heating and vacuum sampling was possible even at elevated temperatures (Fig. 2(ii)) [24]. This finding suggested that the non-ideal performance of PDMS when using a SPME fiber at higher sampling temperatures was related to the physical characteristics of the small thickness coating used in SPME fibers.
Effect of temperature on the extraction of selected PAHs using PDMS as the extracting phase in HS-SPME (a) under vacuum and (b) regular pressure conditions [39] and in HSSE (c) under vacuum and (d) regular pressure conditions [24]. Nap, Ace, and Flu stand for naphthalene, acenaphthene, and fluoranthene respectively
Regardless of the effect of temperature, it should be emphasized that adopting the vacuum approach can eliminate the need for heating the sample. In most optimized procedures, extraction efficiencies obtained under vacuum and at a mild sampling temperature were similar to those under regular pressure and at a much higher sample temperature (e.g., [18, 35]). It should also be noted here that heating the sample at elevated temperatures might reduce the effect of vacuum. The vapor pressure of analytes increases exponentially with temperature and heating the sample will increase the total pressure inside the sample container [18, 39].
The effect of extraction time
Vacuum headspace microextraction sampling greatly accelerates the extraction kinetics of analytes exhibiting long equilibration times under atmospheric pressure. This is important not only for analytes having a low affinity for the headspace but also for microextraction methods using a high capacity sorbent such as the coated stir bar in HSSE where the small surface area to volume ratio results in longer equilibration times compared with the SPME fiber format [24]. In these cases, optimum sampling times with any of the reported vacuum headspace microextraction methodologies are typically halved compared with regular procedure. In all past reports and for a given sampling temperature, extraction kinetics were always improved compared with the standard methodology, maximizing the number of extracted samples and saving laboratory time (e.g., in [10, 18, 19, 24]).
In cases where an adsorbent-type extracting phase is used, it is advised to keep extraction times short so as to minimize the effect of competitive adsorption [19]. This is because the faster uptake of analytes occurring under vacuum was found to intensify the competitive displacement of the lower molecular weight and more volatile analytes present in multicomponent solutions. This intensification in analyte uptake may result in extraction time profiles with an adsorption maximum that will occur faster and will be lower to that recorded under normal pressure conditions [19].
The effect of matrix
The vacuum approach has been successfully applied to a variety of matrices and the challenges associated were similar to those reported with the standard methodology. In all cases, extraction was faster and/or proceeded at a lower temperature under vacuum compared with regular pressure sampling. So far, all applications dealing with complex matrices report the performance of Vac-HS-SPME, reflecting the fact that this technology is more explored and exploited than any other vacuum-assisted headspace microextraction method.
Soil samples were the first type of complex matrix studied with Vac-HS-SPME and matrix effects could be successfully controlled using internal standards [14], multiple extractions [36], and/or addition of a solvent as modifier [14, 36]. In particular, adding water as a modifier and creating a slurry was found an effective approach to improve the release of the target analytes into the headspace, since the competition between nonionic organic compounds and polar water molecules for the surface sites of the solid sample enhanced the release of the organics into the aqueous phase [14]. It should be reminded here that in this four-phase system, analytes’ desorption rates from the solid matrix to water may be slower than evaporation from water and this may delay replenishment of analyte molecules in the headspace and as such the overall extraction rate [14, 28].
Another type of complex solid matrix studied with Vac-HS-SPME was that of frankincense resins, a natural plant raw material [17]. During optimization, the authors studied for the first time the air-evacuation step in the presence of the solid sample and overcame problems of analyte losses by freezing the sample and increasing the sample amount. The authors combined vacuum sampling and fast gas chromatography and achieved more than halving the total analysis time of 51 markers (50 min total analysis time compared with 120 min reported in the past).
Another interesting application was that reporting the successful Vac-HS-SPME sampling of haloanisoles from wine samples [18]. The wine matrix represented a challenging type of matrix to examine the behavior and performance of Vac-HS-SPME. The high ethanol content and other volatile wine components of wine were expected to induce changes in analytes’ solubilities and as such, partitioning with the headspace [40, 41]. Moreover, ethanol and other wine volatiles were expected to compete with haloanisoles for binding sites in the porous SPME fiber and this effect was expected to be intensified under vacuum conditions [19, 42]. By far, the biggest challenge for Vac-HS-SPME was the increase in total pressure due to the presence of ethanol and other wine volatiles that could reduce the positive effect of vacuum on HS-SPME. Nonetheless, the results from this study showed that the changes induced by the wine matrix were not sufficient to prevent the positive effect of vacuum, and Vac-HS-SPME sampling for 30 min at 25 °C yielded lower detection limits than those with regular HS-SPME sampling for 30 min at 55 °C.
The Vac-HS-SPME approach was also successful in analyzing and improving the information gained in the aroma profiling of olive oil, a complex and entirely lipidic matrix [21]. The use of vacuum avoided heating the sample to high temperatures (~ 80 °C reported in the past) where olive oil degradation can be accelerated and analytical challenges related to this matrix are more accentuated. With Vac-HS-SPME, the extraction efficiencies of early eluting analytes were not affected compared with regular pressure since these analytes were extracted at equilibrium (Fig. 3). For the rest, extraction efficiencies were improved with Vac-HS-SPME and a much richer volatile profile could be obtained compared with regular HS-SPME (Fig. 3) An important point to consider was the high viscosity value of the olive oil matrix that increased liquid-phase resistance and delayed extraction. The authors concluded that applying mild heating (i.e., reducing the viscosity of the oily sample and increasing headspace concentrations) next to reducing the total pressure was the best analytical HS-SPME strategy for obtaining fast a rich volatile profile from the olive oil samples [21].
Comparison of the total ion chromatograms obtained at 30 °C using regular (bottom chromatogram) and Vac-HS-SPME (upper chromatogram) sampling from extra virgin olive oil. For identifying the 33 markers (v1-v33), the reader is referred to the original publication [21]
Recently, Rice et al. [33] reported a breakthrough Vac-HS-SPME application, where on site and non-destructive HS-SPME sampling of the biogenic volatiles emitted from a single grape berry took place under low place pressure conditions. In their report, a modified glass vial supporting SPME sampling of individual berries in vivo and on site was used as enclosure and negative pressure was created with a syringe. The authors concluded that Vac-HS-SPME was found feasible to detect volatile organic compounds (39 compounds) emitted in vivo from single grape berries.
Outlook
The effect of vacuum is an emerging experimental parameter to evaluate next to other parameters typically examined during the method optimization step (e.g., sampling temperature and extraction time). Headspace microextraction sampling under vacuum is not “exclusive” to SPME and the approach was recently expanded to HS-SDME and HSSE. It is anticipated that vacuum sampling can be extended to any other microextraction method that can be performed in the headspace sampling mode.
Vacuum sampling offers many advantages over the standard methodology (Table 1). Adopting the vacuum approach is easy, and the only extra step required was that of air-evacuating the sample container before or after sample introduction. The effect of vacuum is particularly beneficial for analytes having long equilibration times and the optimum sampling times are typically halved compared with the standard methodology at a given temperature. Next to speed, vacuum headspace microextraction sampling results in better intra-day precision and minimizes the need for heating the sample [19]. The latter holds an immense potential for areas like food and beverages as it allows preserving the sample character and excludes sample decomposition and formation of other compounds. The vacuum approach was also successful in sampling complex matrices and the challenges associated were similar to those seen when using the standard methodology. In the case of complex oil samples, the richer volatile profile obtained under vacuum compared with regular pressure also resulted in partial co-elution of analytes [21]. This points towards future opportunities in using comprehensive two-dimensional gas chromatography mass spectrometry and exploring untargeted analysis of complex samples.
The vacuum approach was first proposed in 2001 [9]. Despite the net advantages of adopting this approach, up until in 2017, the method was still at an early stage of exploration and exploitation, with only 11 reports existing in the field that mainly covered the theoretical aspects and environmental applications of Vac-HS-SPME [28]. Today, 21 journal publications cover different features, applications, and methodologies. Despite the net advantages of adopting the vacuum approach, research in the area is slowly expanding and the involvement of more research groups and routine analysts is necessary. Two problems were previously assigned as the cause of this slow development [29], the first one being limitations in automation when using Vac-HS-SPME. The recent vial closure developed by E. Psillakis addressed this issue as it guarantees gastight conditions for extended times and is fully compatible with any commercial multi-purpose autosampler having a SPME module [43]. For HS-SDME and HSSE, automation is not an issue, since high throughput sample preparation is achieved off-line by extracting many samples in parallel. The second problem that impedes widespread application of vacuum headspace microextraction sampling is that the methods are not commercialized and as such, potential users do not have access to commercially available gastight vial closures [43]. Currently, vacuum headspace microextraction is in the transition process from development to commercialization and critical action is needed to make this transition happen and bring the technology closer to researchers and routine analysts wanting to exploit the immense potential of vacuum headspace microextraction.
References
Pawliszyn J. Handbook of solid phase microextraction. First ed. London: Elsevier Inc.; 2012.
Mitra S. In: Mitra S, editor. Sample preparation techniques in analytical chemistry. Hoboken: Wiley; 2003.
Risticevic S, Lord H, Górecki T, Arthur CL, Pawliszyn J. Protocol for solid-phase microextraction method development. Nat Protoc. 2010;5:122–39.
Baltussen E, Sandra P, David F, Cramers C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: theory and principles. J Microcolumn Sep. 1999;11:737–47.
Psillakis E, Kalogerakis N. Developments in liquid-phase microextraction. TrAC Trends Anal Chem. 2003;22:565–74.
Jeannot MA, Cantwell FF. Solvent microextraction into a single drop. Anal Chem. 1996;68:2236–40.
Górecki T, Pawliszyn J. Effect of sample volume on quantitative analysis by solid-phase microextraction part 1. Theoretical considerations. Analyst. 1997;122:1079–86.
Schnobrich CR, Jeannot MA. Steady-state kinetic model for headspace solvent microextraction. J Chromatogr A. 2008;1215:30–6.
Brunton NP, Cronin DA, Monahan FJ. The effects of temperature and pressure on the performance of carboxen/PDMS fibres during solid phase microextraction (SPME) of headspace volatiles from cooked and raw Turkey breast. Flavour Frag J. 2001;16:294–302.
Darrouzès J, Bueno M, Pécheyran C, Holeman M, Potin-Gautier M. New approach of solid-phase microextraction improving the extraction yield of butyl and phenyltin compounds by combining the effects of pressure and type of agitation. J Chromatogr A. 2005;1072:19–27.
Groenewold GS, Scott JR, Rae C. Recovery of phosphonate surface contaminants from glass using a simple vacuum extractor with a solid-phase microextraction fiber. Anal Chim Acta Elsevier. 2011;697:38–47.
Psillakis E, Yiantzi E, Sanchez-Prado L, Kalogerakis N. Vacuum-assisted headspace solid phase microextraction: improved extraction of semivolatiles by non-equilibrium headspace sampling under reduced pressure conditions. Anal Chim Acta. 2012;742:30–6.
Psillakis E, Mousouraki A, Yiantzi E, Kalogerakis N. Effect of Henry’s law constant and operating parameters on vacuum-assisted headspace solid phase microextraction. J Chromatogr A. 2012;1244:55–60.
Yiantzi E, Kalogerakis N, Psillakis E. Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples. Anal Chim Acta. 2015;890:108–16.
Orazbayeva D, Kenessov B, Zhakupbekova A. Quantification of transformation products of unsymmetrical dimethylhydrazine in aqueous extracts from soil based on vacuum-assisted headspace solid-phase microextraction. Chem Bull Kaz Nat Univ. 2018:4–11.
Ghiasvand A, Zarghami F, Beiranvand M. Ultrasensitive direct determination of BTEX in polluted soils using a simple and novel pressure-controlled solid-phase microextraction setup. J Iran Chem Soc. 2018;15:1051–9.
Capetti F, Rubiolo P, Bicchi C, Marengo A, Sgorbini B, Cagliero C. Exploiting the versatility of vacuum-assisted headspace solid-phase microextraction in combination with the selectivity of ionic liquid-based GC stationary phases to discriminate Boswellia spp. resins through their volatile and semivolatile fractions. J Sep Sci. 2020:1–11.
Vakinti M, Mela SM, Fernández E, Psillakis E. Room temperature and sensitive determination of haloanisoles in wine using vacuum-assisted headspace solid-phase microextraction. J Chromatogr A. 1602;2019:142–9.
Trujillo-Rodríguez MJ, Pino V, Psillakis E, Anderson JL, Ayala JH, Yiantzi E, et al. Vacuum-assisted headspace-solid phase microextraction for determining volatile free fatty acids and phenols. Investigations on the effect of pressure on competitive adsorption phenomena in a multicomponent system. Anal Chim Acta. 2017;962:41–51.
Sýkora M, Vítová E, Jeleń HH. Application of vacuum solid-phase microextraction for the analysis of semi-hard cheese volatiles. Eur Food Res Technol. 2020;246:573–80.
Mascrez S, Psillakis E, Purcaro G. A multifaceted investigation on the effect of vacuum on the headspace solid-phase microextraction of extra-virgin olive oil. Anal Chim Acta. 2020;1103:106–14.
Trujillo-Rodríguez MJ, Pino V, Anderson JL. Magnetic ionic liquids as extraction solvents in vacuum headspace single-drop microextraction. Talanta. 2017;172:86–94.
Psillakis E, Koutela N, Colussi AJ. Vacuum-assisted headspace single-drop microextraction: eliminating interfacial gas-phase limitations. Anal Chim Acta. 2019;1092:9–16.
Solomou N, Bicchi C, Sgorbini B, Psillakis E. Vacuum-assisted headspace sorptive extraction: theoretical considerations and proof-of-concept extraction of polycyclic aromatic hydrocarbons from water samples. Anal Chim Acta. 2020;1096:100–7.
Jeleń HH, Gaca A, Marcinkowska M. Use of sorbent-based vacuum extraction for determination of volatile phenols in beer. Food Anal Methods. 2018;11:3089–94.
Trujillo-Rodríguez MJ, Anderson JL, Dunham SJB, Noad VL, Cardin DB. Vacuum-assisted sorbent extraction: an analytical methodology for the determination of ultraviolet filters in environmental samples. Talanta. 2020;208:120390.
Fuchsmann P, Tena Stern M, Bischoff P, Badertscher R, Breme K, Walther B. Development and performance evaluation of a novel dynamic headspace vacuum transfer “in trap” extraction method for volatile compounds and comparison with headspace solid-phase microextraction and headspace in-tube extraction. J Chromatogr A. 1601;2019:60–70.
Psillakis E. Vacuum-assisted headspace solid-phase microextraction: a tutorial review. Anal Chim Acta. 2017;986:12–24.
Zhakupbekova A, Baimatova N, Kenessov B. A critical review of vacuum-assisted headspace solid-phase microextraction for environmental analysis. Trends Environ Anal Chem. 2019;22:e00065.
Zhakupbekova A, Baimatova N, Kenessov B. Unpublihsed results.
Kenessov B, Derbissalin M, Koziel JA, Kosyakov DS. Modeling solid-phase microextraction of volatile organic compounds by porous coatings using finite element analysis. Anal Chim Acta. Elsevier Ltd. 2019;1076:73–81.
Qin Z, Bragg L, Ouyang G, Pawliszyn J. Comparison of thin-film microextraction and stir bar sorptive extraction for the analysis of polycyclic aromatic hydrocarbons in aqueous samples with controlled agitation conditions. J Chromatogr A. 2008.
Rice S, Maurer D, Fennell A, Dharmadhikari M, Koziel J. Evaluation of volatile metabolites emitted in-vivo from cold-hardy grapes during ripening using SPME and GC-MS: a proof-of-concept. Molecules. 2019;24:536.
Yiantzi E, Kalogerakis N, Psillakis E. Design and testing of a new sampler for simplified vacuum-assisted headspace solid-phase microextraction. Anal Chim Acta. Elsevier Ltd. 2016;927:46–54.
Glykioti M-L, Yiantzi E, Psillakis E. Room temperature determination of earthy-musty odor compounds in water using vacuum-assisted headspace solid-phase microextraction. Anal Methods. 2016;8:8065–71.
Orazbayeva D, Kenessov B, Psillakis E, Nassyrova D, Bektassov M. Determination of transformation products of unsymmetrical dimethylhydrazine in water using vacuum-assisted headspace solid-phase microextraction. J Chromatogr A. 2018;1555:30–6.
Ghiasvand A, Koonani S, Yazdankhah F, Farhadi S. A comparison study on a sulfonated graphene-polyaniline nanocomposite coated fiber for analysis of nicotine in solid samples through the traditional and vacuum-assisted HS-SPME. J Pharm Biomed Anal. 2018;149:271–7.
Beiranvand M, Ghiasvand A. Simple, low-cost and reliable device for vacuum-assisted headspace solid-phase microextraction of volatile and semivolatile compounds from complex solid samples. Chromatographia. 2017;80:1771–80.
Psillakis E, Yiantzi E, Kalogerakis N. Downsizing vacuum-assisted headspace solid phase microextraction. J Chromatogr A. 2013;1300:119–26.
Câmara JS, Alves MA, Marques JC. Development of headspace solid-phase microextraction-gas chromatography–mass spectrometry methodology for analysis of terpenoids in Madeira wines. Anal Chim Acta. 2006;555:191–200.
Robinson AL, Ebeler SE, Heymann H, Boss PK, Solomon PS, Trengove RD. Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning. J Agric Food Chem. 2009;57:10313–22.
Vlachos P, Kampioti A, Kornaros M, Lyberatos G. Matrix effect during the application of a rapid method using HS-SPME followed by GC–ECD for the analysis of 2,4,6-TCA in wine and cork soaks. Food Chem. 2007;105:681–90.
Psillakis E. Methods and vial closures for headspace extraction under vacuum. 2019. p. US patent 62/939,359.
Acknowledgments
This article is based upon work from the Sample Preparation Task Force and Network, supported by the Division of Analytical Chemistry of the European Chemical Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Female Role Models in Analytical Chemistry.
Rights and permissions
About this article
Cite this article
Psillakis, E. The effect of vacuum: an emerging experimental parameter to consider during headspace microextraction sampling. Anal Bioanal Chem 412, 5989–5997 (2020). https://doi.org/10.1007/s00216-020-02738-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02738-x