Abstract
A new simple, low-cost, reliable vacuum-assisted headspace solid-phase microextraction (VA-HS-SPME) device was fabricated and evaluated considering the strengths and weaknesses of previously reported systems. The device can be applied for analysis of solid and liquid samples without sample loss or vacuum loss during the evacuation process, in contrast to similar setups. Additionally, it is simpler, lower cost, and more operator friendly for direct extraction of volatiles and semivolatiles from complicated solid matrices. It was coupled with gas chromatography-flame ionization detection (GC-FID) and applied for direct extraction and determination of polycyclic aromatic hydrocarbons (PAHs) in polluted soil samples, without any sample preparation steps. Parameters affecting the performance of the developed method, such as extraction temperature and time, vacuum level, volumes of vacuum chamber and sample vial, and desorption condition, were investigated and optimized. Under the optimal conditions, calibration curves were linear over the range of 0.01–2 μg g−1 (R 2 > 0.996). The limits of detection (LODs) were found to lie in the range of 0.3–0.8 ng g−1, while the relative standard deviations (RSDs) for six replicate analyses were 5.3–7.1%. The developed VA-HS-SPME/GC-FID procedure was used for ultrasensitive determination of PAHs in contaminated soil samples; the results were statistically in agreement with those obtained using a validated ultrasonic solvent extraction (USE) method.
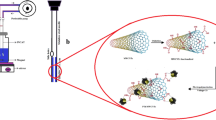
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a known class of ubiquitous carcinogenic pollutants, generated primarily by incomplete combustion of petrol, coal, and wood [1]. A part of environmental PAHs originate from natural sources such as open burning petroleum or coal deposits, forest fires, and volcanic eruptions. However, major anthropogenic sources of PAHs include petroleum, coal, and aluminum industries, residential heating, and motor vehicle exhaust [2]. PAHs in the atmosphere are portioned between gas phase and aerosols, which significantly affects their fate and how they enter soil, water, and the human body [3].
Nowadays, chemical analysis is directed by green chemistry principles, so microscale solvent-free separation procedures have attracted great attention from scientists [4]. Consequently, design and development of sustainable and green microanalysis strategies is currently a hot multidisciplinary research topic in a broad range of scientific fields, including analytical chemistry, environmental assessment and monitoring, biochemistry, pharmacology, and agriculture. In this regard, the most effective step was the introduction of solid-phase microextraction (SPME) in 1990 [5], as a solvent-free sample preparation method, which reduces the number of steps, cost, waste, and time of analysis. Additionally, this method can be easily automated and used for in vivo biological studies [6]. Moreover, fiber-SPME has been promoted and its limitations addressed during recent years [7], leading to many improvements in its performance and applications [8]. Agitation, sonication, heating, and microwave irradiation of the sample matrix have been proposed as strategies to decrease the equilibration time in the SPME technique [9]. During recent years, cooling-assisted SPME (CA-SPME) [10,11,12], electrochemically enhanced SPME [13], microwave-assisted headspace SPME [14], ultrasonic-assisted headspace SPME [15], solvent-assisted SPME [16], total-vaporization SPME [17], micelle-assisted SPME [18], electromembrane-assisted SPME [19], purge-assisted headspace SPME [20], and vortex-assisted magnetic dispersive SPME [21] have been applied to enhance the efficiency of SPME.
Headspace (HS) sampling is one of the most useful and widely used modes of SPME, in which analytes are extracted from the headspace of the sample without contact with the sample matrix [22]. In this sampling mode, extraction occurs during a multistep equilibrium including partitioning of analytes between the sample/headspace and headspace/fiber coating. Usually, the transfer of analytes from the sample into the headspace is the rate-limiting step, especially for solid matrices, resulting in longer extraction times [23]. Among the aforementioned strategies for improving the efficiency, simultaneous heating of the sample matrix and cooling of the fiber coating is among the most successful suggestions [24, 25]. Another efficient approach to reduce the equilibrium time and enhance the extraction efficiency is reduced-pressure HS-SPME, introduced in 2001 [26]. In that study, the effect of pressure and temperature on the efficiency of HS-SPME analysis of volatile organic compounds (VOCs) was evaluated. The results revealed that reduction of the headspace pressure significantly improved the amount and number of VOCs extracted. Another study carried out in 2005 [27] evaluated the effects of pressure and agitation on the HS-SPME strategy. Another report in 2011 described recovery of semivolatile organic contaminants by HS-SPME using a vacuum extractor [28]; the results showed that use of reduced pressure sped up the release of analytes from the sample matrix and their partitioning into the headspace. It was also revealed that reduced pressure reduced the boundary layer around the sorbent and reinforced trapping of analytes on the SPME fiber. The term “vacuum-assisted HS-SPME” (VA-HS-SPME) was first used by Psillakis et al. [29], who studied the effect of the Henry’s law constant on VA-HS-SPME of PAHs from aqueous samples. That study demonstrated that vacuum sampling significantly improved the extraction kinetics, especially for analytes with low Henry’s law constant (K H). The evaporation rate of such species is mainly controlled by the mass transfer rate in the thin gas boundary layer adjacent to the headspace/sample interface. Further work on VA-HS-SPME investigated extraction of chlorophenols under reduced pressure conditions [30], with the formulation of a theoretical model for the pressure dependence of the sampling under nonequilibrium conditions. Moreover, in other research [31], the VA-HS-SPME setup was downsized and used to extract low-molecular-weight PAHs using commercial SPME fibers. It was demonstrated that humidity in the sample matrix decreased the extracted amounts of PAHs with low or intermediate K H, especially at elevated sampling temperature with polydimethylsiloxane (PDMS) fiber. In different research, a field vacuum extractor, coupled with portable fast-duty-cycle gas chromatography-mass spectrometry (GC–MS), was used to analyze organophosphonate compounds in vinyl floor tiles [32]. The enhancement effect of vacuum on the sensitivity of HS-SPME was evaluated by extraction of aroma compounds from solid (tobacco leaf) and liquid (black mulberry juice) samples [33]. In another report, the previously mentioned VA-HS-SPME system was used to extract PAHs from solid matrices [34]. The vacuum effect has also been coupled to ultrasonic-assisted extraction (UAE) of organophosphate and halogenated flame retardants in food samples, before GC–MS measurement [35]. The vacuum extractor setup was improved and employed for HS-SPME of polychlorinated biphenyls (PCBs) from spiked river water samples, using a polydimethylsiloxane/divinylbenzene (PDMS/DVB) commercial SPME fiber [36]. In a different study, Pawliszyn et al. [37] compared the amounts of PAHs extracted (from sand samples) using regular cold-fiber SPME (CF-SPME) with results obtained by pressure-balanced CF-SPME. In further research, VA-HS-SPME was coupled to gas chromatography with flame ionization detection (GC-FID) for extraction of volatile free fatty acids and phenols, and the results compared with those obtained using commercial and polymeric ionic liquid-based SPME fibers [38]. The VA-HS-SPME strategy was recently applied for analysis of 2-methylisoborneol and geosmin in water, at room temperature [39]. In recently published research, the influence of surface sampling factors on the recovery of dimethyl methylphosphonate, spiked onto painted wallboard surfaces, was evaluated by reduced-pressure SPME and solvent extraction, using an accelerated diffusion sampler [40]. Recently, headspace single-drop microextraction (HS-SDME) was carried out, under vacuum condition, for extraction of short-chain free fatty acids [41]. A tutorial review describing and summarizing all reported vacuum-assisted methods has also been published [42].
To summarize the cited literature, in early work on reduced-pressure SPME [26, 27], the sample had to be exposed directly to vacuum condition. This can interfere with accurate measurements, by sucking off the analyte and/or sample into the vacuum system. A handmade syringe was also used to evacuate the vacuum extractor [28, 32, 37], limiting the vacuum level to that obtainable using human hand force. Additionally, this compartment was prone to loss of analytes and vacuum level reduction, due to the moving parts in this system. An amended version of this setup was also prepared, in which the vacuum was applied more effectively using a hand-screw clamp [33]. The same issues also apply for the accelerated diffusion sampler [40]. All new versions of the VA-HS-SPME setup [29,30,31, 38, 39, 41] were designed to analyze liquid samples, and some of them still used a handmade syringe instead of a vacuum pump for evacuation. In these setups, solid samples must be mixed with water and taken as slurry mixtures. However, presence of water can also impair extraction by increasing the number of molecules competing with analytes. Moreover, the vacuum chamber must be removed and cleaned (washed) after each extraction. This increases the number of steps and also the time of analysis. A VA-HS-SPME setup developed to analyze solid samples [34] also suffers from the mentioned limitations, despite its ability to analyze solid samples. Therefore, it is vital to design a new VA-HS-SPME system in which these problems are solved.
The aim of this work is to introduce a low-cost, simple, reliable VA-HS-SPME setup for all types of sample, considering all the strengths and weaknesses of previously reported setups. The new system avoids exposure of the sample to vacuum during the evacuation period. It also enables analysis of any type of solid or liquid sample, with no need to make slurry. To the best of the authors’ knowledge, such a system has never been reported. The new VA-HS-SPME setup was evaluated using extraction of PAHs (as model compounds) from contaminated soil samples, followed by GC-FID determination.
Materials and Methods
Chemicals
Naphthalene (Nap), acenaphthene (Ace), fluorene (Fln), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), and pyrene (Pyr), all > 99.0% analytical standards, were purchased from Sigma-Aldrich (Germany). All other chemicals were of analytical reagent grade and provided by Sigma-Aldrich or Merck (Darmstadt, Germany). Standard stock solution (1000 μg mL−1) of a mixture of the seven PAHs was prepared by dissolution of appropriate amounts in methanol. Standard working solutions were prepared daily by appropriate dilutions of the stock. All standard stock and working solutions were stored at 4 °C. PDMS commercial fibers were obtained from Supelco (Bellefonte, PA, USA) and preconditioned according to manufacturer’s recommendation, prior to first use. SPME experiments were performed using a manual fiber holder supplied by Supelco and glass SPME extraction vials (10, 20, and 40 mL), sealed with aluminum caps and Teflon-coated silicone septa. For accurate transfer of low volumes of solvents and solutions, 10-, 50-, 100-, and 500-μL microsyringes (Hamilton, Reno, NV, USA) were used. Real sample solutions were filtered using 0.45-μm cellulose acetate filters (Sartorius, Göttingen, Germany) prior to analysis using the ultrasound-assisted solvent extraction (UA-SE) method. A standard sand sample was kindly provided by the National Water Research Institute of Canada (NWRI, Burlington, Canada).
Instruments
Chromatographic separations and determinations were performed using a DANI Master GC-FID (Milan, Italy) system, equipped with a Clarity workstation (version 3.0.02.244) and a CP-Sil PONA CB fused-silica capillary column (50 m × 0.25 mm I.D. × 0.5 µm film thickness) from Varian (Lake Forest, CA, USA). Nitrogen and hydrogen gases with purity of 99.999% were purchased from Pars-Havaye Alborz Company (Tehran, Iran). A diaphragm MD4CNT vacuum pump (Vacuubrand GmbH and Co. KG, Wertheim, Germany), with 1.5 mbar ultimate vacuum, was used to evacuate air. The sample matrices were heated using a Stuart CD162 hotplate-stirrer (Staffordshire, UK).
GC-FID Separation and Determination of PAHs
To determine the best condition for separation and quantification of PAHs, different GC-FID temperature programs were applied and the optimal program selected. The optimum program started at 100 °C and remained constant for 1 min, then the temperature was raised to 265 °C at rate of 25 °C min−1 and held constant for 13 min. Hence, the total GC run time was 20.6 min. GC runs were conducted in splitless mode with the injector and detector set at 280 and 300 °C, respectively. High-purity (99.999%) nitrogen was employed as carrier at constant flow rate of 0.8 mL min−1. The flow rates of N2 (makeup gas), H2 and air (FID gases) were adjusted at 25, 40, and 350 mL min−1, respectively.
Fabrication of VA-HS-SPME Setup
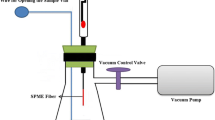
To fabricate a proper VA-HS-SPME setup that could compensate for or eliminate the limitations of previously reported setups and prevent exposure of the sample to the headspace during the evacuation process, various innovative strategies were examined. Preliminary tests were carried out to investigate the reliability, simplicity, and construction costs of the designed setups. Finally, a 250-mL vacuum Erlenmeyer flask was selected as the vacuum chamber (Fig. 1). It was fit with a silicone stopper, with a proper septum hole in its center for injection of the SPME fiber needle. Then, a 10-mL SPME vial was fixed at the bottom of the vacuum chamber using silicone glue, as the sample container. The cap of the sample container was opened and closed using a stainless-steel rod, which had been passed through the stopper. The lateral exit tube of the vacuum chamber was connected to a vacuum pump through a proper glass valve. The vacuum chamber was purged using hot dry nitrogen for 24 h to remove any possible contamination and glue volatiles. The vacuum chamber and its connections were sealed based on Swagelok, USA qualitative standard, to avoid any possible leak or vacuum loss.
VA-HS-SPME Procedure
To optimize the experimental variables for VA-HS-SPME of PAHs from contaminated soil samples, standard sand was used as model matrix. The main constituent of sand is silica. It also contains varying amounts of different metal oxides. Therefore, it is very similar to natural soil and can be used as a model matrix for soil analysis studies [43]. Thus, 5 g of standard sand sample was poured into the sample container; after closing the cap, it was fortified with 50 μL standard solution of PAHs to obtain 100 μg mL−1 concentration, followed by proper mixing. Then, the system was connected to the vacuum pump. After complete air evacuation (60 s), the glass valve was closed and the vacuum pump turned off. Thereupon, the sample vial cap was opened using the stainless-steel rod. Thereby, the analytes were easily released from the sample matrix and rapidly dispersed in the vacuum chamber. Then, the SPME fiber was injected into the vacuum chamber and exposed to the headspace of the sample for 20 min at 60 °C. Finally, the SPME fiber was retracted and immediately injected into the GC-FID injector for separation and determination of PAHs. The fiber was held in the GC injector for 60 s at 280 °C, for complete desorption of extracted PAHs.
Results and Discussion
After selection of the proper design and fabrication of the VA-HS-SPME system, important experimental variables affecting the efficiency of the VA-HS-SPME method were evaluated and optimized. The experimental parameters studied to determine the best extraction conditions included extraction temperature, extraction time, desorption time, evacuation time, vacuum level, and volumes of extraction vial and vacuum chamber. Based on previously published reports [34, 43], PDMS was selected as the best type of fiber coating for extraction of PAHs.
Effect of Extraction Temperature
Extraction temperature has a bilateral effect in conventional HS-SPME analysis. Thermodynamically, higher extraction temperature results in higher headspace concentration of analytes, by increasing their partial vapor pressure and Henry’s law constant. On the other hand, higher sample temperature decreases the tendency of the fiber coating to adsorb analytes. Therefore, there is generally an optimum extraction temperature for HS-SPME [10], which is usually not high enough for significant enhancement of the extraction efficiency, especially from solid samples with analytes that are tightly attached to their native matrix. However, use of the reduced-pressure condition can compensate for these temperature-related problems in the HS-SPME procedure. To study the effect of temperature on the extracted amounts of PAHs, different VA-HS-SPME experiments were performed, varying the temperature in the range of 25–80 °C. The results (Fig. 2) demonstrated that the optimum temperature for phenanthrene, fluoranthene, and pyrene (with high boiling points) was 70 °C. In contrast, a decrease in sensitivity was observed for naphthalene, acenaphthene, and fluorene (with lower boiling points) with increasing sample temperature above 50 °C, while this effect occurred for anthracene (with a medium boiling point) above 60 °C. Use of high temperature could reduce the partition coefficients of analytes between the headspace and fiber, because adsorption of PAHs on the fiber surface is an exothermic process. Accordingly, 60 °C was chosen as the optimum extraction temperature for further studies.
Effect of Extraction Time
The effect of the exposure time of the SPME fiber to the headspace was evaluated by using different extraction times in the range of 5–40 min (Fig. 3). The results showed that the extracted amounts increased with increasing time up to 15 min for naphthalene and acenaphthene, 20 min for fluorene and anthracene, and 30 min for fluoranthene and pyrene, then remained constant. These results show that the equilibrium times required for complete extraction of the PAHs increased with their boiling point. To choose a proper extraction time, both volatile and semivolatile analytes should be considered. Consequently, 20 min was selected as the optimal value, suitable for all seven PAHs with different volatilities.
Effect of Desorption Conditions
To assess the best conditions for complete desorption of extracted PAHs from the SPME fiber, we investigated desorption time in the range of 10–600 s and desorption temperatures in the range of 260–300 °C. The results revealed that 60 s at 280 °C was a suitable condition for complete release of all analytes from the fiber.
Effect of Vacuum Level and Sample Vial Volume
The power of the vacuum pump was constant and could not be controlled at different levels, but the evacuation time was controllable. Therefore, to determine the optimal vacuum level, we studied different evacuation times over the range of 10–300 s. The results showed that the vacuum chamber reached the maximum vacuum level after 30 s of pump operation. However, to obtain more reliable results, 60 s was considered to be the best evacuation time.
To investigate the effect of the extraction vial volume, sample vials with volume of 10, 20 and 40 mL were evaluated for use in VA-HS-SPME of PAHs from sand samples. The results demonstrated that the extraction efficiency was inversely proportional to the volume of the extraction vial, with the 10-mL vial showing the highest extraction efficiency for all analytes. This effect can be explained base on the effect of the sample volume on the vacuum level. Each sample vial contains some air, which remains unaffected during the evacuation because the cap of the sample vial is closed. After opening the cap of the sample vial, this air (depending on the volume of the vial) enters the vacuum chamber, decreasing the level of vacuum. Thus, the 10-mL SPME vial was selected as the best choice for the extraction vial. Similar studies were conducted to investigate the effect of the vacuum chamber volume. The results showed that the VA-HS-SPME efficiency varied in the order: 250 > 500 > 1000 ml. Therefore, 250 mL was chosen as the best vacuum chamber volume. It is clear that decreasing the vacuum chamber volume (which can be considered to be the headspace volume) will increase the extraction efficiency, similar to in traditional HS-SPME. However, it was necessary to use a vacuum chamber to prevent exposure of the sample to vacuum during the evacuation period. Additionally, vacuum chambers with lower volume (< 25 mL) suffer from mechanical limitations when fixing the extraction vial within. Therefore, a 250-mL flask was selected as the best vacuum chamber.
Analytical Figures of Merit
To evaluate the analytical performance of the VA-HS-SPME/GC-FID method, linear dynamic ranges (LDRs), limits of detection (LODs), and relative standard deviations (RSDs) for extraction of PAHs from solid samples were investigated, using the optimized conditions (Table 1). The RSDs for six replicate analyses of seven PAHs (1 μg g−1) were found to be 5.3–7.1%. The LDRs were found to lie in the range of 0.9–2000 ng g−1, with determination coefficients (R 2) higher than 0.996. The LODs, corresponding to the amounts of analyte for which the signal-to-noise ratio is equal to 3, were found to lie in the range of 0.3–0.8 ng g−1 for the examined PAHs [44]. These results demonstrate the good performance, acceptable precision, and high sensitivity of the VA-HS-SPME/GC-FID procedure for extraction and determination of PAHs in complex solid samples.
Analysis of PAHs in Real Soil Samples
As the most critical part of the study and to assess the applicability of the VA-HS-SPME/GC-FID strategy for analysis of complicated solid matrices, it was applied for extraction and determination of PAHs in real soil samples. The results were also compared with those obtained using a validated UA-SE procedure [45]. The samples were collected from different areas of a fuel station in Lorestan Petrochemical Company in Khorramabad (located in the west of Iran). The results, which are summarized in Table 2, showed that the concentrations of PAHs obtained using the VA-HS-SPME/GC-FID method were statistically in agreement with those obtained using the UA-SE/GC-FID procedure. To compare the results of the proposed VA-HS-SPME method and those obtained by the UA-SE procedure, t test was applied. Based on the values of t critical for n = 3, significant differences were not observed between the results. This demonstrates that UA-SE is a powerful procedure for release and extraction of VOCs from solid matrices. However, in the VA-HS-SPME method, interfering air molecules are evacuated (removed) form the headspace of the sample and at the same time release of analytes from the sample matrix is enhanced by the vacuum. These two simultaneous phenomena significantly improve release of analytes from sample, their quick diffusion into the headspace, and consequent sorption by the SPME fiber.
To demonstrate the superiority of the proposed strategy over the traditional HS-SPME/GC-FID method, the two procedures were applied for analysis of PAHs in a real soil sample. The results showed that the VA-HS-SPME/GC-FID method was more sensitive, precise, and accurate. Additionally, the peaks of the PAHs obtained by the VA-HS-SPME/GC-FID procedure had higher area and better resolution and were sharper. These features are clearly observable in the chromatogram, in comparison with that obtained using the conventional HS-SPME/GC-FID method (Fig. 4). Therefore, it is concluded that the proposed VA-HS-SPME/GC-FID methodology can be successfully applied for direct analysis of PAHs in complicated solid samples, without any sample preparation steps.
Conclusions and Future Prospects
A simple, low-cost, reliable VA-HS-SPME device was fabricated and evaluated for direct extraction and sensitive determination of PAHs in complex solid samples. It can be easily applied to analyze solid and liquid samples without sample/analyte loss during the evacuation process. Also, it does not require a slurry to be made for analysis of solid samples, in contrast to previously reported methods. The evacuation process is conducted using a pump with high evacuation power and high reproducibility. Additionally, the possibility of vacuum and sample loss during the evacuation and extraction process is nearly zero. The reduced-pressure condition enhances release of analytes from the sample matrix while simultaneously improving their adsorption by the extraction phase. This effective analyte preconcentration enables low LODs, providing a powerful and reliable ultrasensitive method for extraction and determination of VOCs in contaminated solid samples.
References
Chen J-W, Wang S-L, Hsieh DPH, Yang H-H, Lee H-L (2012) Carcinogenic potencies of polycyclic aromatic hydrocarbons for back-door neighbors of restaurants with cooking emissions. Sci Total Environ 417:68–75. doi:10.1016/j.scitotenv.2011.12.012
Abdel-Shafy HI, Mansour MSM (2016) A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Petrol 25:107–123. doi:10.1016/j.ejpe.2015.03.011
de Boer J, Wagelmans M (2016) Polycyclic aromatic hydrocarbons in soil-practical options for remediation. Clean 44:648–653. doi:10.1002/clen.201500199
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13:8615–8627. doi:10.3390/ijms13078615
Arthur CL, Pawliszyn J (1990) Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. doi:10.1021/ac00218a019
Ghiasvand AR, Setkova L, Pawliszyn J (2007) Determination of flavour profile in Iranian fragrant rice samples using cold-fibre SPME–GC-TOF-MS. Flav Frag J 22:377–391. doi:10.1002/ffj.1809
Behfar M, Ghiasvand AR, Yazdankhah F (2017) Reinforced microextraction of polycyclic aromatic hydrocarbons from polluted soil samples using an in-needle coated fiber with polypyrrole/graphene oxide nanocomposite. J Sep Sci 40:2975–2983. doi:10.1002/jssc.201700244
Souza-Silva ÉA, Jiang R, Rodríguez-Lafuente A, Gionfriddo E, Pawliszyn J (2015) A critical review of the state of the art of solid-phase microextraction of complex matrices I. Environmental analysis. Trends Anal Chem 71:224–235. doi:10.1016/j.trac.2015.04.016
Ghiasvand AR, Nasseri M, Farsizaeh S, Meshkatalsadat MH, Sadeghi-Sarabi R, Shadabi S, Borzoei M (2011) Chemical characterization of cultivated Tagetes minuta L. by use of ultrasound-assisted head space SPME and GC-MS. Chromatographia 73:1031–1035. doi:10.1007/s10337-011-1955-0
Ghiasvand AR, Hajipour S, Heidari N (2016) Cooling-assisted microextraction: comparison of techniques and applications. Trends Anal Chem 77:54–65. doi:10.1016/j.trac.2015.12.008
Ghiasvand AR, Heidari N (2016) Cooling-assisted headspace hollow fiber-based liquid-phase microextraction setup for direct determination of PAHs in solid samples by using volatile solvents. Chromatographia 79:1187–1195. doi:10.1007/s10337-016-3133-x
Ghiasvand AR, Pirdadeh-Beiranvand M (2015) Cooling/heating-assisted headspace solid-phase microextraction of polycyclic aromatic hydrocarbons from contaminated soils. Anal Chim Acta 900:56–66. doi:10.1016/j.aca.2015.10.016
Zeng J, Chen J, Song X, Wang Y, Ha J, Chen X, Wang X (2010) An electrochemically enhanced solid-phase microextraction approach based on a multi-walled carbon nanotubes/Nafion composite coating. J Chromatogr A 1217:1735–1741. doi:10.1016/j.aca.2012.03.054
Hsieh P-C, Lee C-L, Jen J-F, Chang K-C (2015) Complexation-flocculation combined with microwave-assisted headspace solid-phase microextraction in determining the binding constants of hydrophobic organic pollutants to dissolved humic substances. Analyst 140:1275–1280. doi:10.1039/C4AN01923G
Ghiasvand A, Nasirian A, Koonani S, Nouriasl K (2017) Ultrasonic and cooling approaches for reinforcement of the microextraction methods. Anal Bioanal Chem Res 4:105–126. doi:10.22036/abcr.2016.59882.1114
Xu Y, Fan W, Qian MC (2007) Characterization of aroma compounds in apple cider using solvent-assisted flavor evaporation and headspace solid-phase microextraction. J Agric Food Chem 55:3051–3057. doi:10.1021/jf0631732
Rainey CL, Bors DE, Goodpaster JV (2014) Design and optimization of a total vaporization technique coupled to solid-phase microextraction. Anal Chem 86:11319–11325. doi:10.1021/ac5030528
Boyaci E, Pawliszyn J (2014) Micelle assisted thin-film solid phase microextraction: a new approach for determination of quaternary ammonium compounds in environmental samples. Anal Chem 86:8916–8921. doi:10.1021/ac5015673
Fakhari AR, Sahragard A, Ahmar H, Tabani H (2015) A novel platform sensing based on combination of electromembrane-assisted solid phase microextraction with linear sweep voltammetry for the determination of tramadol. J Electroanal Chem 747:12–19. doi:10.1016/j.jelechem.2015.01.032
Hung C-H, Ho H-P, Lin M-T, Chen C-Y, Shu Y-Y, Lee M-R (2012) Purge-assisted headspace solid-phase microextraction combined with gas chromatography/mass spectrometry for the determination of trace nitrated polycyclic aromatic hydrocarbons in aqueous samples. J Chromatogr A 1265:1–6. doi:10.1016/j.chroma.2008.10.056
Cheng X, Yan H, Wang X, Sun N, Qiao X (2014) Vortex-assisted magnetic dispersive solid-phase microextraction for rapid screening and recognition of dicofol residues in tea products. Food Chem 162:104–109. doi:10.1016/j.foodchem.2014.04.023
Zhang Z, Pawliszyn J (1993) Headspace solid-phase microextraction. Anal Chem 65:1843–1852. doi:10.1021/ac00062a008
Haddadi SH, Pawliszyn J (2009) Cold fiber solid-phase microextraction device based on thermoelectric cooling of metal fiber. J Chromatogr A 1216:2783–2788. doi:10.1016/j.chroma.2008.09.005
Ghiasvand AR, Yazdankhah F (2017) Single-step reinforced microextraction of polycyclic aromatic hydrocarbons from soil samples using an inside needle capillary adsorption trap with electropolymerized aniline/multi-walled carbon nanotube sorbent. J Chromatogr A 1487:47–53. doi:10.1016/j.chroma.2017.01.056
Tajik L, Bahrami A, Ghiasvand A, Shahna FG (2017) Determination of BTEX in urine samples using cooling/heating-assisted headspace solid-phase microextraction. Chem Papers. In Press. doi:10.1007/s11696-017-0176-x
Brunton NP, Cronin DA, Monahan FJ (2001) The effects of temperature and pressure on the performance of carboxen/PDMS fibres during solid phase microextraction (SPME) of headspace volatiles from cooked and raw turkey breast. Flav Frag J 16:294–302. doi:10.1002/ffj.1000
Darrouzès J, Bueno M, Pécheyran C, Holeman M, Potin-Gautier M (2005) New approach of solid-phase microextraction improving the extraction yield of butyl and phenyltin compounds by combining the effects of pressure and type of agitation. J Chromatogr A 1072:19–27. doi:10.1016/j.chroma.2005.02.026
Groenewold GS, Scott JR, Rae C (2011) Recovery of phosphonate surface contaminants from glass using a simple vacuum extractor with a solid-phase microextraction fiber. Anal Chim Acta 697:38–47. doi:10.1016/j.aca.2011.04.034
Psillakis E, Mousouraki A, Yiantzi E, Kalogerakis N (2012) Effect of Henry’s law constant and operating parameters on vacuum-assisted headspace solid phase microextraction. J Chromatogr A 1244:55–60. doi:10.1016/j.chroma.2012.05.006
Psillakis E, Yiantzi E, Sanchez-Prado L, Kalogerakis N (2012) Vacuum-assisted headspace solid phase microextraction: improved extraction of semivolatiles by non-equilibrium headspace sampling under reduced pressure conditions. Anal Chim Acta 742:30–36. doi:10.1016/j.aca.2012.01.019
Psillakis E, Yiantzi E, Kalogerakis N (2013) Downsizing vacuum-assisted headspace solid phase microextraction. J Chromatogr A 1300:119–126. doi:10.1016/j.chroma.2013.02.009
Groenewold GS, Scott JR, Lee ED, Lammert SA (2013) Rapid analysis of organophosphonate compounds recovered from vinyl floor tile using vacuum extraction coupled with a fast-duty cycle GC/MS. Anal Methods 5:2227–2236. doi:10.1039/C3AY26280D
Lee C, Lee Y, Lee J-G, Buglass AJ (2015) Development of a reduced pressure headspace solid-phase microextraction-gas chromatography/mass spectrometric (rpHSSPME–GC/MS) method and application to aroma analysis. Anal Methods 7:6504–6513. doi:10.1039/C5AY00980D
Yiantzi E, Kalogerakis N, Psillakis E (2015) Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples. Anal Chim Acta 890:108–116. doi:10.1016/j.aca.2015.05.047
Xu F, García-Bermejo Á, Malarvannan G, Gómara B, Neels H, Covaci A (2015) Multi-contaminant analysis of organophosphate and halogenated flame retardants in food matrices using ultrasonication and vacuum assisted extraction, multi-stage cleanup and gas chromatography-mass spectrometry. J Chromatogr A 1401:33–41. doi:10.1016/j.chroma.2015.05.001
Yiantzi E, Kalogerakis N, Psillakis E (2016) Design and testing of a new sampler for simplified vacuum-assisted headspace solid-phase microextraction. Anal Chim Acta 927:46–54. doi:10.1016/j.aca.2016.05.001
Xu S, Shuai Q, Pawliszyn J (2016) Determination of polycyclic aromatic hydrocarbons in sediment by pressure-balanced cold fiber solid phase microextraction. Anal Chem 88:8936–8941. doi:10.1021/acs.analchem.6b01944
Trujillo-Rodríguez MJ, Pino V, Psillakis E, Anderson JL, Ayala JH, Yiantzi E, Afonso AM (2017) Vacuum-assisted headspace-solid phase microextraction for determining volatile free fatty acids and phenols. Investigations on the effect of pressure on competitive adsorption phenomena in a multicomponent system. Anal Chim Acta 962:41–51. doi:10.1016/j.aca.2017.01.056
Glykioti M-L, Yiantzi E, Psillakis E (2016) Room temperature determination of earthy–musty odor compounds in water using vacuum-assisted headspace solid-phase microextraction. Anal Methods 8:8065–8071. doi:10.1039/C6AY02210C
Mo K-F, Heredia-Langner A, Fraga CG (2017) Evaluating and modeling the effects of surface sampling factors on the recovery of organic chemical attribution signatures using the accelerated diffusion sampler and solvent extraction. Talanta 164:92–99. doi:10.1016/j.talanta.2016.11.016
Trujillo-Rodríguez MJ, Pino V, Anderson JL (2017) Magnetic ionic liquids as extraction solvents in vacuum headspace single-drop microextraction. Talanta 172:86–94. doi:10.1016/j.talanta.2017.05.021
Psillakis E (2017) Vacuum-assisted headspace solid-phase microextraction: a tutorial review. Anal Chim Acta 986:12–24. doi:10.1016/j.aca.2017.06.033
Ghiasvand AR, Hosseinzadeh S, Pawliszyn J (2006) New cold-fiber headspace solid-phase microextraction device for quantitative extraction of polycyclic aromatic hydrocarbons in sediment. J Chromatogr A 1124:35–42. doi:10.1016/j.chroma.2006.04.088
Vial J, Jardy A (1999) Experimental comparison of the different approaches to estimate LOD and LOQ of an HPLC method. Anal Chem 71:2672–2677. doi:10.1021/ac981179n
Banjoo DR, Nelson PK (2005) Improved ultrasonic extraction procedure for the determination of polycyclic aromatic hydrocarbons in sediments. J Chromatogr A 1066:9–18. doi:10.1016/j.chroma.2005.01.033
Acknowledgements
The authors would like to thank the managers of Lorestan Petrochemical Company for their help with fabrication of the VA-HS-SPME setup and for provision of the chromatographic laboratory to carry out the experiments. The authors are also grateful to Dr. Fereshteh Mousavi, official English translator and instructor, for editing this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any grants.
Conflict of interest
Mohammad Beiranvand and Ali Reza Ghiasvand have nothing to declare.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Beiranvand, M., Ghiasvand, A. Simple, Low-Cost and Reliable Device for Vacuum-Assisted Headspace Solid-Phase Microextraction of Volatile and Semivolatile Compounds from Complex Solid Samples. Chromatographia 80, 1771–1780 (2017). https://doi.org/10.1007/s10337-017-3422-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3422-z