Abstract
A pressure-controlled headspace solid-phase microextraction (PC-HS-SPME) setup was developed, by reconsidering the strengths and weaknesses points of the similar reported systems. The new setup was coupled with gas chromatography–flame ionization detection (GC–FID) for direct analysis of benzene, toluene, ethylbenzene and xylene (BTEX) in contaminated soils, without any sample preparation step. The important experimental factors, affecting the performance of the method, including volumes of extraction and vacuum vials, type of SPME fiber, extraction time and temperature, moisture content of the sample, and sonication time were studied and optimized. Under the optimal conditions, good linearity of the calibration curves (R2 > 0.997) was obtained in the concentration range of 0.1–20,000 ng g−1. The limits of detections were found to be 0.001–0.08 ng g−1. The relative standard deviations, for six repetitive analyses of 100 ng g−1 BTEX, were obtained to be 5.7–12.3%. The PC-HS-SPME–GC–FID procedure was successfully applied for the extraction and determination of BTEX in the polluted soil samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, solvent-free microscale quantitative and qualitative analysis methods, based on principles of green chemistry, have attracted great attention because of their green features [1]. Consequently, design of sustainable and green microextraction strategies is currently a hot investigating topic in a multidisciplinary area including analytical chemistry, environmental monitoring, biology, medicine, pharmacy and agriculture [2]. In this way, one of the most effective efforts was carried out by introducing the solid-phase microextraction (SPME) method in 1989 [3]. SPME is a solvent-free sample preparation method which reduces the steps, expense, waste and time of analyzes. Additionally, it can be easily automated and applied in biological studies [4]. On the other hand, a lot of researches have been done to improve its performance and applications, during recent years [5]. Electrochemically enhanced SPME [6], microwave-assisted SPME [7], ultrasonic-assisted SPME [8], solvent-assisted SPME [9], total-vaporization SPME [10], micelle-assisted thin-film SPME [11], electromembrane-assisted SPME [12], purge-assisted headspace SPME [13], as well as vortex-assisted magnetic dispersive SPME [14], are new approaches that have recently been used to enhance the efficiency of SPME.
The headspace sampling is the most common and useful mode of SPME, which extracts analytes from the upper atmosphere of sample, without contact to sample matrix. HS-SPME has been widely used for the extraction of different volatile and semi-volatile analytes from complex matrices [15]. It occurs through a multi-step process including partitioning of analytes between sample and headspace and between the headspace and fiber. For most analytes, transfer of analytes from sample into the headspace is the rate-limiting step, which causes equilibrium process to be slow [16]. Agitation, sonication, microwave irradiation and heating of the sample matrix are some of the proposed strategies to decrease the equilibrium duration in HS-SPME. One of the successful innovations accomplished to promote the SPME performance was cooling-assisted SPME, which is very efficient in complicated matrices such as soil, sludge and clay, with analytes tightly attached to their active sites [17,18,19,20]. Another efficient approach to reduce the equilibrium time and reinforce the extraction efficiency is reduced-pressure headspace SPME, which was firstly introduced in thin 2001 [21]. The second study was carried out in 2005 [22], by evaluating of HS-SPME on the extraction of organotin compounds by combining the effects of pressure and agitation procedure. These studies were abandoned until 2011 that a new HS-SPME report released about the recovery of phosphonate surface contaminants from glass using a vacuum extractor [23]. This study showed that reduced pressure reduces the boundary layer around the SPME fiber, which reinforces analytes to trap on the SPME fiber. A research entitled “vacuum-assisted headspace SPME” was reported in 2012, in which the effects of Henry’s law constant on PC-HS-SPME of polycyclic aromatic hydrocarbons (PAHs) have been studied [24]. It demonstrated that vacuum sampling significantly improves the extraction kinetics, especially for analytes with low Henry’s law constant (KH). The PC-HS-SPME studies were continued by the extraction of chlorophenols, as model of semi-volatile organic compounds [25]. In another study, the PC-HS-SPME setup was downsized and used to extract low molecular weight PAHs using commercial fibers [26]. It was showed that humidity content of sample matrix decreases the extracted amounts of PAHs with low or intermediate KH, especially at elevated sampling temperatures. In a recent research, a field vacuum extractor, coupled with a portable fast-duty cycle gas chromatography–mass spectrometry (GC–MS), was used for analyzing of organophosphonate compounds in vinyl floor tile. The enhancement effect of reduced-pressure on sensitivity of HS-SPME has also been evaluated by the extraction of aroma compounds from solid and liquid samples [27]. In a new research, the PC-HS-SPME procedure was used to extract PAHs from solid matrices [28]. In another SPME research, temperature-controlled HS-SPME was coupled to PC-HS-SPME for the extraction of PAHs in sediment samples [29]. It should be noticed that in all aforementioned studies [24,25,26,27,28,29,30], relatively the same PC-HS-SPME setups have been used. This system suffers from a serious drawback, i.e, sample has to be directly exposed to the vacuum condition, during the evacuation process. This phenomenon impairs the extraction process by sucking off the liquid sample or solid particles inside the vacuum system, which causes serious errors in the results. To compensate this effect, solid samples were necessary to be mixed with water and taken as slurry mixtures, while water may interfere with the extraction process by increasing the number of competing molecules. Moreover, after each extraction, the vacuum vial must be removed and cleaned, which increases the number of steps and time of the extraction. Therefore, it is vital to design simple and easy-to-use PC-HS-SPME systems, without these defects.
The aim of this study was to design, fabricate and evaluate a new simple, low-cost PC-HS-SPME setup that can prevent the sample to be exposed vacuum, during the pressure reduction period. The developed setup is very simple, operator-friendly and low-cost, with the possibility of analyzing of solid and liquid sample, without the water addition and slurry. Benzene, toluene, ethylbenzene, and xylene (BTEX), which are among the most carcinogenic and mutagenic species found in environment [31], were used as the model VOCs analytes to evaluate the new proposed setup. The PC-HS-SPME setup was coupled to gas chromatography–flame ionization detection (GC–FID) and applied for direct extraction and measurement of BTEX in contaminated soils, without any sample preparation step.
Experimental
Chemicals and supplies
Pure benzene (≥ 99.9%), toluene (≥ 99.8%), ethylbenzene (≥ 99.0%), and three isomers of xylene, i.e, meta- (≥ 99.0%), para- (≥ 99.0%), and ortho-xylene (≥ 99.5%) were purchased from Merck (Darmstadt, Germany). All of the used organic solvents and slats were of the analytical reagent grade, provided by Merck or Fluka. The standard stock solution (1000 μg mL−1) was prepared by dissolution of BTEX in ethanol. Fresh working solutions were prepared daily by diluting the stock solution in ethanol. The stock and working standard solutions were kept at 4 °C. The standard sand sample was provided by the National Water Research Institute of Canada (NWRI, Burlington, Canada). Commercial polydimethylsiloxane (PDMS), carboxen/polydimethylsiloxane (CAR/PDMS), and carboxen/divinylbenzene/polydimethylsiloxane (CAR/DVB/PDMS) SPME fibers were obtained from Supelco (Bellefonte, PA, USA). All SPME fibers were conditioned according to the manufacturer’s recommendation prior to the first use. The SPME experiments were performed using a manual fiber holder supplied by Supelco. Glass SPME extraction vials (10, 20 and 40 m) with screw cap and silicone–PTFE septa were provided by Supelco. For the accurate transfer of small volumes of solvents and solutions, 10-, 50-, 100- and 500-μL microsyringes (Hamilton, Reno, NV, USA) were employed.
Instruments
Chromatographic separations and determinations were carried out using a GC-2010 Plus AF Shimadzu gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a split/splitless injector (SPL-2010 Plus), a flame ionization detector (FID-2010 Plus) and a GC solution software (version 2.4). The separations were performed using a BP5 fused-silica capillary column (30 m × 0.32 mm × 0.25 µm). Ultrasonic irradiation of the samples was conducted using an 18 kHz, 450 W ultrasonicator (PFO100 5RS Series, Sonica, Italy), equipped with a temperature-controlled water bath. A JB DV-42505 vacuum pump (J/B Industries Inc., USA), with 6 mbar ultimate vacuum power, was used for evacuation of the vacuum chamber.
Fabrication of the PC-HS-SPME setup
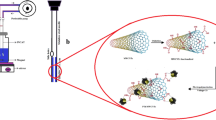
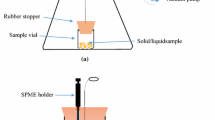
A 250-mL vacuum Erlenmeyer flask was selected as the vacuum chamber (Fig. 1). It was fitted with a silicone stopper, which had a proper hole in its center (suitable for a septum compatible with the needle of the SPME fibers). A 10-mL SPME extraction vial was fixed at the bottom of vacuum chamber using silicon adhesive, as the sample container. Opening and closing of the sample container’s cap was done by a stainless steel wire, which had been passed through the stopper. Lateral exit tube of the vacuum chamber was connected to the vacuum pump using a glass valve. Prior to the first use, the vacuum chamber was purged with dry nitrogen for 1 h to remove any possible contamination.
PC-HS-SPME procedure
For analysis of BTEX in a solid sample using the PC-HS-SPME–GC–FID method, 2 g sample was placed into the sample vial and its cap was closed. Then, the stopper of the vacuum chamber was closed. The vacuum valve was opened and the pump turned on to evacuate the chamber, while the sample vial was closed and remained at atmospheric pressure. After the complete air evacuation, the pump was turned off. After that, the sample vial cap was opened using the stainless steel wire. In this way, the pressure gradient caused the analytes to effectively release from the sample matrix and evaporated into the vacuum chamber. Thereafter, the fiber’s protecting needle was injected into the vacuum chamber and the fiber exposed to the headspace of the sample, for 10 min at 25 °C. Finally, the fiber was retracted and immediately injected into the GC–FID injection port for quantitation of the analytes.
The main component of sand is silica that contains different amounts of metal oxides. Therefore, it is very similar to natural soil and can be used as the model matrix for optimization of soil analysis studies [17]. Accordingly, to optimize the affecting experimental parameters, 2 g of standard sand was placed into the sample vial and its cap was closed. A proper volume of BTEX standard solution was spiked onto the sand sample (to obtain the desired concentration) using a microsyringe. Then, the sample was subjected to the PC-HS-SPME–GC–FID strategy.
GC–FID analysis
For separation and quantification of BTEX using the GC–FID instrument, temperatures of the injector and detector were set at 250 and 280 °C, respectively. Nitrogen (purity > 99.999%) was used as the carrier gas at a flow rate of 1 mL min−1. The flow rates of FID gases (zero air and hydrogen) and make-up gas (nitrogen) were set at 300, 30 and 30 mL min−1, respectively. The GC temperature programming was started at 40 °C, ramped to 100 °C with a rate of 10 °C min−1 and held constant for 1 min. Then, temperature was raised to 250 °C at a rate of 50 °C min−1. So, the total GC run time was 10 min. Quantification of the analytes was performed using the external calibration curves (R2 > 0.99) obtained by direct injection of BTEX standard solutions with different concentration into the GC–FID system.
Results and discussion
Type of fiber’s coating
To obtain the optimized extraction conditions, the important experimental variables including fiber’s type, volumes of the sample vial and vacuum chamber, extraction temperature and time, and sonication time were evaluated. Selection of fiber coating is generally the most important stage in SPME studies, because a proper choice can improve both the sensitivity and selectivity of the extraction. Three different SPME fibers with PDMS, CAR/PDMS and CAR/DVB/PDMS extraction phases were used to extract BTEX from solid samples. The CAR/DVB/PDMS fiber resulted in the highest overall sensitivity and therefore was chosen to continue the study.
The effect of the extraction vial and vacuum chamber volumes
To investigate the effects of extraction volume, 10-, 20- and 40-mL vials were evaluated. Another experiment was also done without the extraction vial, in which the sample has been placed at bottom of the vacuum chamber. As the results show (Fig. 2a), the extraction efficiency varies inversely with decreasing of the extraction vial’s volume. This fact can be explained by considering the compensation effect of sample vial’s volume on the applied vacuum. Each sample vial entraps some air in it at atmospheric pressure. After the evacuation and opening the sample vial, this air releases into the vacuum chamber and decreases the vacuum level. Therefore, the best extraction efficiency is obtained by the least volume of the extraction sample (i.e., with no sample vial), while use of sample vial is vital. If the sample is placed directly in bottom of the vacuum chamber, the analytes or sample will be pulled out of the chamber during the evacuation. In other words, accurate analysis of solid samples without the use of sample vial is not possible. Thus, 10-mL SPME vial was chosen as the best choice for sample vial for further studies.
a The effect of sample vial volume on the extraction efficiency of PC-HS-SPME procedure (sample: 2 g sand containing 0.5 μg g−1 of each BTEX; vacuum chamber: 500 mL; extraction temperature: 25 °C; extraction time: 10 min) and b the extracted amounts of BTEX related to volume of the vacuum chamber (sample: 2 g sand containing 0.5 μg g−1 of each BTEX; sample vial: 10 mL; extraction temperature: 25 °C; extraction time: 10 min)
The effect of the vacuum chamber volume on the extraction efficiency was also studied by using 250-, 500- and 1000-mL vacuum flasks for PC-HS-SPME of BTEX. The results show that the extraction efficiency increases with decreasing of the chamber volume (Fig. 2b). This variable may be considered as the actual volume of the sample headspace. Therefore, any decrease in its volume will increase the concentration of analytes and consequently the extraction efficiency. These results are in agreement with those previously reported for the conventional HS-SPME studies [32].
Effect of extraction temperature and time
Extraction temperature has a bilateral effect on the efficiency of HS-SPME experiments. Higher extraction temperatures thermodynamically result in higher headspace concentration of analyte due to increasing of its partial vapor pressure. On the other hand, higher sample temperatures decrease the affinity of the fiber coating to adsorb analytes. Therefore, the extraction temperature profiles of conventional HS-SPME methods usually have an optimum point [33, 34]. This optimal temperature is usually not enough to obtain reasonable extraction efficiencies especially in solid samples, with their analytes tightly adsorbed to their native matrix. The reduced-pressure condition was anticipated to positively affect this trend, because of its same effect as the temperature raise. Therefore, different PC-HS-SPME experiments were performed with varying temperatures over the range of 25–125 °C (Fig. 3). A significant decrease in the sensitivity was observed with increasing of sample temperature from 25 to 125 °C. These observations are on general agreement with the aforementioned descriptions. In PC-HS-SPME, the interfering air molecules are evacuated from the headspace and at the same time releasing of analytes form the sample tissue is enhanced. These two simultaneous phenomena significantly improve the release of analytes from the sample matrix and their effective adsorption by the SPME fiber. Therefore, raising sample temperature cannot further increase the analytes’ release from the matrix. On the other hand, higher temperatures can reduce the partition coefficients of analytes between the headspace and the fiber. This effect will be possibly smaller, in the case of heavier and less-volatile analytes such as PAHs compared with BTEX. Accordingly, 25 °C was chosen as the optimal extraction temperature for further studies.
The exposure time of the fiber to the headspace was also evaluated by using different extraction times (1–60 min). The results revealed that the extracted amounts of BTEX increased with increasing of extraction time up to 10 min and then remained constant (Fig. S-1). Therefore, 10 min was selected as the extraction time for further PC-HS-SPME experiments.
Comparison of the PC-HS-SPME procedure with conventional HS-SPME
In order to provide the experimental evidences on improvement of the HS-SPME efficiency, under the reduced-pressure condition, different samples containing varying amounts of BTEX were analyzed using the developed method in both atmospheric- and reduced-pressure conditions (Fig. 4). The results show that amounts of the extracted analytes using PC-HS-SPME are on average nearly two times higher than those obtained by the conventional HS-SPME method.
Analytical performances
To evaluate the quantitative figures of merit of the PC-HS-SPME–GC–FID method, linear dynamic ranges (LDRs), limits of detection (LODs) and relative standard deviations (RSDs) were investigated under the optimized experimental conditions (Table 1). RSDs for six replicate analyses (100 ng g−1 of BTEX) were found 5.7–12.3%. LDRs and LODs were obtained to be 0.1–20,000 (R2 > 0.99.) and 0.001–0.08 ng g−1, respectively. Fig. S-2 shows the calibration curves for extraction and determination of BTEX using the PC-HS-SPME–GC–FID method.
Analysis of contaminated soil real samples
The developed setup was applied for the extraction and determination of BTEX in three contaminated soil samples. The samples were collected from the area of a gas station in Khorramabad City (Lorestan, Iran). The results were also compared with those obtained by an validated ultrasonic-solvent extraction method coupled to GC–FID (USE-GC–FID) [35]. The statistical tests showed that the results of the PC-HS-SPME–GC–FID procedure are in agreement with those achieved by the USE-GC–FID method (Table 2). A sample GC–FID chromatogram of BTEX, extracted from a contaminated soil sample (real sample No. 2) using the PC-HS-SPME procedure, is shown in Fig. 5.
Conclusion and future remarks
By reconsidering the strengths and weaknesses points of the reported systems, a simple, low-cost, and effective PC-HS-SPME setup was fabricated. It was coupled to GC–FID and evaluated for the direct extraction and ultrasensitive determination of BTEX in soil samples, without any sample pretreatment step. On the contrary to the developed PC-HS-SPME systems, the new device is able to analyze both liquid and solid samples, without loss during the evacuation process. It may be easily coupled with different SPME configuration such as fiber SPME with commercial fibers or handmade nanofibers, needle trap device (NTD), inside needle capillary adsorption trap (INCAT) device, fiber-in-needle SPME, as well as different liquid-phase microextraction (LPME) modes. The PC-HS-SPME–GC–FID setup provides a reliable and reproducible ultrasensitive method for the extraction and determination of VOCs in complicated solid samples. However, the results showed relatively high RSDs for methodology. It was successfully applied to measure BTEX in polluted soil samples and the results showed good agreement, compared with those obtained by a validated USE-GC–FID procedure.
References
F. Chemat, M.A. Vian, G. Cravotto, Green extraction of natural products: concept and principles. Int. J. Mol. Sci. 13, 8615–8627 (2012). https://doi.org/10.3390/ijms13078615
X. Hu, J. Li, Q. Chen, Z. Lin, D. Yin, Combined effects of aqueous suspensions of fullerene and humic acid on the availability of polycyclic aromatic hydrocarbons: evaluated with negligible depletion solid-phase microextraction. Sci. Total Environ. 493, 12–21 (2014). https://doi.org/10.1016/j.scitotenv.2014.05.107
R.P. Belardi, J.B. Pawliszyn, The application of chemically modified fused silica fibers in the extraction of organics from water matrix samples and their rapid transfer to capillary columns. Water Qual. Res. J. Can. 24, 179–191 (1989)
A.R. Ghiasvand, L. Setkova, J. Pawliszyn, Determination of flavour profile in Iranian fragrant rice samples using cold-fibre SPME-GC-TOF-MS. Flav. Frag. J. 22, 377–391 (2007). https://doi.org/10.1002/ffj.1809
W. Wardencki, M. Michulec, J. Curyło, A review of theoretical and practical aspects of solid-phase microextraction in food analysis. Int. J. Food Sci. Technol. 39, 703–717 (2004). https://doi.org/10.1111/j.1365-2621.2004.00839.x
J. Zeng, J. Chen, X. Song, Y. Wang, J. Ha, X. Chen, X. Wang, An electrochemically enhanced solid-phase microextraction approach based on a multi-walled carbon nanotubes/Nafion composite coating. J. Chromatogr. A 1217, 1735–1741 (2010). https://doi.org/10.1016/j.aca.2012.03.054
P.-C. Hsieh, C.-L. Lee, J.-F. Jen, K.-C. Chang, Complexation-flocculation combined with microwave-assisted headspace solid-phase microextraction in determining the binding constants of hydrophobic organic pollutants to dissolved humic substances. Analyst 140, 1275–1280 (2015). https://doi.org/10.1039/C4AN01923G
A. Ghiasvand, S. Shadabi, S. Hajipour, A. Nasirian, M. Borzouei, E. Hassani-Moghadam, P. Hashemi, Comparison of ultrasound-assisted headspace solid-phase microextraction and hydrodistillation for the identification of major constituents in two species of Hypericum. J. Chromatogr. Sci. 54, 264–270 (2016). https://doi.org/10.1093/chromsci/bmv136
Y. Xu, W. Fan, M.C. Qian, Characterization of aroma compounds in apple cider using solvent-assisted flavor evaporation and headspace solid-phase microextraction. J. Agric. Food Chem. 55, 3051–3057 (2007). https://doi.org/10.1021/jf0631732
C.L. Rainey, D.E. Bors, J.V. Goodpaster, Design and optimization of a total vaporization technique coupled to solid-phase microextraction. Anal. Chem. 86, 11319–11325 (2014). https://doi.org/10.1021/ac5030528
E. Boyac, J. Pawliszyn, Micelle assisted thin-film solid phase microextraction: a new approach for determination of quaternary ammonium compounds in environmental samples. Anal. Chem. 86, 8916–8921 (2014). https://doi.org/10.1021/ac5015673
A.R. Fakhari, A. Sahragard, H. Ahmar, H. Tabani, A novel platform sensing based on combination of electromembrane-assisted solid phase microextraction with linear sweep voltammetry for the determination of tramadol. J. Electroanal. Chem. 747, 12–19 (2015). https://doi.org/10.1016/j.jelechem.2015.01.032
C.-H. Hung, H.-P. Ho, M.-T. Lin, C.-Y. Chen, Y.-Y. Shu, M.-R. Lee, Purge-assisted headspace solid-phase microextraction combined with gas chromatography/mass spectrometry for the determination of trace nitrated polycyclic aromatic hydrocarbons in aqueous samples. J. Chromatogr. A 1265, 1–6 (2012). https://doi.org/10.1016/j.chroma.2008.10.056
X. Cheng, H. Yan, X. Wang, N. Sun, X. Qiao, Vortex-assisted magnetic dispersive solid-phase microextraction for rapid screening and recognition of dicofol residues in tea products. Food Chem. 162, 104–109 (2014). https://doi.org/10.1016/j.foodchem.2014.04.023
A. Ghiasvand, S. Dowlatshah, N. Nouraei, N. Heidari, F. Yazdankhah, A solid-phase microextraction platinized stainless steel fiber coated with a multiwalled carbon nanotube-polyaniline nanocomposite film for the extraction of thymol and carvacrol in medicinal plants and honey. J. Chromatogr. A 1406, 87–93 (2015). https://doi.org/10.1016/j.chroma.2015.06.052
S.H. Haddadi, V.H. Niri, J. Pawliszyn, Study of desorption kinetics of polycyclic aromatic hydrocarbons (PAHs) from solid matrices using internally cooled coated fiber. Anal. Chim. Acta 652, 224–230 (2009). https://doi.org/10.1016/j.aca.2009.05.026
A.R. Ghiasvand, S. Hosseinzadeh, J. Pawliszyn, New cold-fiber headspace solid-phase microextraction device for quantitative extraction of polycyclic aromatic hydrocarbons in sediment. J. Chromatogr. A 1124, 35–42 (2006). https://doi.org/10.1016/j.chroma.2006.04.088
A.R. Ghiasvand, M. Pirdadeh-Beiranvand, Cooling/heating-assisted headspace solid-phase microextraction of polycyclic aromatic hydrocarbons from contaminated soils. Anal. Chim. Acta 900, 56–66 (2015). https://doi.org/10.1016/j.aca.2015.10.016
A.R. Ghiasvand, S. Hajipour, N. Heidari, Cooling-assisted microextraction: comparison of techniques and applications. Trends Anal. Chem. 77, 54–65 (2016). https://doi.org/10.1016/j.trac.2015.12.008
M. Behfar, A.R. Ghiasvand, F. Yazdankhah, Reinforced microextraction of polycyclic aromatic hydrocarbons from polluted soil samples using an in-needle coated fiber with polypyrrole/graphene oxide nanocomposite. J. Sep. Sci. 40, 2975–2983 (2017). https://doi.org/10.1002/jssc.201700244
N.P. Brunton, D.A. Cronin, F.J. Monahan, The effects of temperature and pressure on the performance of carboxen/PDMS fibres during solid phase microextraction (SPME) of headspace volatiles from cooked and raw turkey breast. Flav. Frag. J. 16, 294–302 (2001). https://doi.org/10.1002/ffj.1000
J. Darrouzès, M. Bueno, C. Pécheyran, M. Holeman, M.P. Gautier, New approach of solid-phase microextraction improving the extraction yield of butyl and phenyltin compounds by combining the effects of pressure and type of agitation. J. Chromatogr. A 2005, 19–27 (1072). https://doi.org/10.1016/j.chroma.2005.02.026
G.S. Groenewold, J.R. Scott, C. Rae, Recovery of phosphonate surface contaminants from glass using a simple vacuum extractor with a solid-phase microextraction fiber. Anal. Chim. Acta 697, 38–47 (2011). https://doi.org/10.1016/j.aca.2011.04.034
E. Psillakis, A. Mousouraki, E. Yiantzi, N. Kalogerakis, Effect of Henry’s law constant and operating parameters on vacuum-assisted headspace solid phase microextraction. J. Chromatogr. A 1244, 55–60 (2012). https://doi.org/10.1016/j.chroma.2012.05.006
E. Psillakis, E. Yiantzi, L. Sanchez-Prado, N. Kalogerakis, Vacuum-assisted headspace solid phase microextraction: improved extraction of semivolatiles by non-equilibrium headspace sampling under reduced pressure conditions. Anal. Chim. Acta 742, 30–36 (2012). https://doi.org/10.1016/j.aca.2012.01.019
E. Psillakis, E. Yiantzi, N. Kalogerakis, Downsizing vacuum-assisted headspace solid phase microextraction. J. Chromatogr. A 1300, 119–126 (2013). https://doi.org/10.1016/j.chroma.2013.02.009
C. Lee, Y. Lee, J.G. Lee, A.J. Buglass, Development of a reduced pressure headspace solid-phase microextraction-gas chromatography/mass spectrometric (rpHSSPME-GC/MS) method and application to aroma analysis. Anal. Methods 7, 6504–6513 (2015). https://doi.org/10.1039/C5AY00980D
E. Yiantzi, N. Kalogerakis, E. Psillakis, Vacuum-assisted headspace solid phase microextraction of polycyclic aromatic hydrocarbons in solid samples. Anal. Chim. Acta 890, 108–116 (2015). https://doi.org/10.1016/j.aca.2015.05.047
S. Xu, Q. Shuai, J. Pawliszyn, Determination of polycyclic romatic hydrocarbons in sediment by pressure-balanced cold fiber solid phase microextraction. Anal. Chem. 88, 8936–8941 (2016). https://doi.org/10.1021/acs.analchem.6b01944
F. Xu, Á. García-Bermejo, G. Malarvannan, B. Gómara, H. Neels, A. Covaci, Multi-contaminant analysis of organophosphate and halogenated flame retardants in food matrices using ultrasonication and vacuum assisted extraction, multi-stage cleanup and gas chromatography–mass spectrometry. J. Chromatogr. A 1401, 33–41 (2015). https://doi.org/10.1016/j.chroma.2015.05.001
J.N. Bianchin, G. Nardini, J. Merib, A.N. Dias, E. Martendal, E. Carasek, Simultaneous determination of polycyclic aromatic hydrocarbons and benzene, toluene, ethylbenzene and xylene in water samples using a new sampling strategy combining different extraction modes and temperatures in a single extraction solid-phase microextraction-gas chromatography–mass spectrometry procedure. J. Chromatogr. A 1233, 22–29 (2012). https://doi.org/10.1016/j.chroma.2012.02.022
T. Gorecki, Effect of sample volume on quantitative analysis by solid-phase microextraction. Part 1. Theoretical considerations. Analyst 122, 1079–1086 (1997). https://doi.org/10.1039/A701303E
A.R. Ghiasvand, N. Heidari, Cooling-assisted headspace hollow fiber-based liquid-phase microextraction setup for direct determination of PAHs in solid samples by using volatile solvents. Chromatographia 79, 1187–1195 (2016). https://doi.org/10.1007/s10337-016-3133-x
A.R. Ghiasvand, F. Yazdankhah, S. Hajipour, Use of volatile organic solvents in headspace liquid-phase microextraction by direct cooling of the organic drop using a simple cooling capsule. J. Sep. Sci. 39, 3011–3018 (2016). https://doi.org/10.1002/jssc.201600142
H.S. Sin, O.S. Gwon, The simultaneous analysis of benzene, toluene, ethylbenzene, o, m, p-xylenes and total petroleum hydrocarbons in soil by GC–FID after ultra-sonication. Bull. Korean Chem. Soc. 21, 1101–1105 (2000)
Acknowledgements
The authors sincerely acknowledge Lorestan University’ Vice Chancellor of Research and Technology, for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghiasvand, A., Zarghami, F. & Beiranvand, M. Ultrasensitive direct determination of BTEX in polluted soils using a simple and novel pressure-controlled solid-phase microextraction setup. J IRAN CHEM SOC 15, 1051–1059 (2018). https://doi.org/10.1007/s13738-018-1302-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1302-6