Abstract
A rapid and reliable method based on magnetic solid-phase extraction (MSPE) and ultra-high performance liquid chromatography (UHPLC-FLD) analysis was developed and validated for the quantitative determination of seven polycyclic aromatic hydrocarbons (PAHs) in water samples. Hybrid composites made up of magnetic nanoparticles (MNPs) and carbon nanotubes (CNTs) were tested as adsorbent materials. The main factors influencing the extraction yields were optimized, namely the amount and type of adsorbent in the adsorption, and the solvent, the type of energy and the time in the desorption step. The selected composite was made up of Fe3O4 and multiwalled (MW)-CNTs. The desorption was carried out with sonication probe and tetrahydrofuran (THF); this solvent makes the method directly compatible with the chromatographic mobile phase and it reduces the analysis time. Under the optimized conditions, the LODs and LOQs achieved were in the range of 0.025–0.73 and 0.04–2.4 ng mL−1, respectively. The calibration curves were linear (R 2 ≥ 0.9936) over the concentration ranges from 1 to 500 ng mL−1. The recoveries of PAHs were from 76.4 ± 1.7 up to 106.5 ± 3.5%. The method was applied to synthetic and real (tap, dam, river and mineral) water samples with different characteristics to evaluate the performance under real conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds produced by incomplete combustion of organic matter at high temperature and pyrolysis processes from natural and anthropogenic sources. They can be easily spread and distributed into the different environmental compartments. Furthermore, these compounds are known to induce the formation of cancer after chronic exposure [1] and the US Environmental Protection Agency (USEPA) has highlighted 16 PAH congeners as priority pollutants due to their wide distribution and high toxicity. Therefore, PAHs are subjected to regulations in food and drinking water. Thus, the European Union has established maximum levels of some congeners in some foodstuffs at levels of micrograms per kilogram [2] and the World Health Organization (WHO) has set a total maximum concentration for six PAHs in drinking water at 0.2 ng mL−1 [3].
The monitoring of PAHs has traditionally been carried out by gas chromatography with either flame ionization (GC-FID) or mass spectrometry (GC-MS) detection, and liquid chromatography with visible/ultraviolet (LC-UV/vis) or fluorescence (LC-FLD) detectors. Recently, ultra-high performance liquid chromatography (UHPLC) has taken the lead as it provides enhanced peak resolutions in short times. As a result, the separation of 16 EPA PAHs takes place in 12 min [4, 5] while the run time for the same separation usually takes no less than 25 min in conventional LC [6, 7] and 40 min in GC [8, 9].
The levels of PAHs in water are typically in the range of nanograms per milliliter or lower and an adequate sample preparation to increase selectivity and/or sensitivity is necessary. Liquid-liquid extraction (LLE), liquid-liquid microextraction (LLME), or solid-phase extraction (SPE) have traditionally been the techniques of choice [10,11,12,13]. Although these techniques provide good accuracy and low limits of detection through preconcentration, they are often time consuming and some of them generate considerable solvent waste.
In the last years, nanostructured materials have been incorporated to sample preparation. Carbon nanotubes (CNTs), especially multiwalled (MWCNTs), have become most attractive as sorbent materials in analytical processes because they possess several interesting properties such as high surface area, a pore structure, high mechanical strength, and chemical stability [14]. They are also known to have hydrophobic and π–π interactions with PAHs [15, 16]. Multiwalled CNTs have been used as sorbents in ordinary SPE cartridges, in micro-columns, and packed inside a porous polypropylene membrane for the monitoring of PAHs in surface, tap, river, and sea water [9, 17, 18]. They have also been combined with polyvinyl alcohol cryogel (MWCNTs/PVA) to form composites to be used for extraction and preconcentration of PAHs in water samples [19]. However, the preparation of these CNT-supported materials is usually time consuming and technically complex with high costs. Moreover, the weakest point of these carbon nanostructures to be used in aqueous matrices is that they strongly agglomerate due to their high hydrophobic nature, hindering the PAH adsorption processes [16, 20].
A very interesting alternative is the synthesis of hybrid nanostructures with CNTs and magnetic nanoparticles (MNPs). With this combination, the analytes in solution are adsorbed onto the dispersed CNT-MNPs and then these are separated from the solution by an external magnet. These composites combine the unique properties of CNTs as sorbents for hydrophobic compounds, such as phthalate acid esters or estrogens [21, 22] with the ease of handling of magnetic solid-phase extraction (MSPE). Moreover, the time-consuming procedures of packing in a cartridge or mini-column and the subsequent need for complicated separation by centrifugation or filtration steps are avoided. As for the determination of PAHs, these CNT-MNP composites have been used in hydrophobic samples, i.e., oil [23] and grilled meat [16], but not in water samples up to present. In fact, other magnetic composites have been reported for these samples, for example with polydopamine, polypirrol, triphenylamine, or graphene [24,25,26,27], and only recently, modified CNTs have been used for this purpose. Thus, Bunkoed and Kanatharana [28] used a composite by caging Fe3O4 nanoparticles (NPs) and MWCNTs into calcium alginate beads. Menezes et al. [20] used amphiphilic magnetic CNTs made up of a completely hydrophobic tranche (undoped) and another more hydrophilic/polar made of N-doped CNT as MSPE sorbents for environmental waters because they disperse better than the pristine CNTs. The authors also reported a high difficulty for the congeners with high molecular weight to desorb from the CNTs of the composites.
The combination of magnetic CNTs as adsorbents for MSPE with UHPLC using a sub-2 μm column coupled to FLD meets the requirements for a fast, simple, selective, and sensitive method for the analysis of PAHs. To our best knowledge, this combination has never been used for the monitoring of PAHs in water. Therefore, the aim of this work is to develop and validate an analytical method for the monitoring of seven PAHs using this combination of techniques and its application in water samples with different characteristics. The PAHs selected were naphthalene (NAPH), acenaphtene (ACE), fluorene (FLU), phenanthrene (PHEN), anthracene (ANTH), fluoranthene (FLT), and pyrene (PYR) because of their favorable interaction with CNTs, especially in terms of desorption with organic solvents [20].
Experimental
Chemicals and materials
Analytical standards of NAPH, ACE, FLU, PHEN, ANTH, FLT, and PYR of 97.5% or higher purity were purchased from Sigma-Aldrich (Bellefonte, PA, USA). A stock standard solution was prepared in acetonitrile at 10 μg mL−1 of each analyte. Working standard solutions were made by appropriate dilution of the stock standard solutions with ultrapure water. Iron (II) chloride tetrahydrate, iron (III) chloride hexahydrate, sodium acetate (Sigma-Aldrich), and ethylene glycol pure (Panreac, Barcelona, Spain) were used to synthesize the magnetic nanoparticles. Single-walled (SW) CNTs 2–5-μm long and 1.2–1.5 nm of diameter (50–70% carbon basis) were purchased from Sigma-Aldrich. Multiwalled (MW) CNTs 5–20-μm long and 30 ± 15-nm thick (95% purity in MWCNTs) were purchased from Nano-Lab (Waltham, MA, USA). LC-MS grade water, methanol and acetonitrile (MeCN) for mobile phases, and HPLC grade tetrahydrofuran (THF) were purchased from Scharlab (Barcelona, Spain). Humic acid sodium salt was obtained from Sigma-Aldrich. Samples were filtered through 13 mm × 0.2 μm nylon filters (Agilent Captiva, Agilent). Ultrapure water (18.2 MΩ cm at 25 °C) was obtained from an Elga Purelab Ultra Analytic water purification system.
Instrumentation
An Agilent 1200 SL UHPLC system (Agilent Technologies Inc.) was used. The UHPLC equipment included a binary pump, a thermostated column compartment, and a UV-Visible and a fluorescence detector in line. The separation was conducted on a Zorbax Eclipse PAH column (2.1 × 50 mm; 1.8 μm) fitted with a guard cartridge (2.1 × 12.5 mm; 5 μm). Column and guard cartridges were purchased from Agilent. The mobile phase was filtered using a vacuum filtration system through 0.2-μm polyamide membrane filters (Sartorius Stedim Biotech GmbH, Göttingen, Germany). The flow rate was 0.42 mL min−1. Gradient separation was carried out using water and acetonitrile as A and B solvents, respectively. The linear gradient profile was as follows: B maintained at 40% for the first 0.45 min, then increased to 100% at t = 6 min and maintained for 3 min. The mobile phase was returned to initial conditions in 0.5 min and was kept for 1.5 min for a total run time of 11 min. Oven temperature was set at 25 °C. Data analysis was done using Agilent ChemStation software (Agilent Technologies). The analytes were determined using a fluorescence detector (FLD). The excitation wavelength was 260 nm and the emission wavelengths were 350 nm (NAPH, ACE, FLU, and PHEN); 420 nm (ANTH and PYR); and 500 nm (FLT).
A ZX3 vortex stirrer (Velp Scientifica, Usmate, Italy) was used for the adsorption. A Sonoplus ultrasonic homogenizer (Bandelin, Berlin, Germany) fitted with a HD generator 2200 was used for desorption of PAHs from the magnetic composites. The homogenizer was equipped with a titanium micro-tip of 3 mm diameter. The maximum power was 200 W and the frequency was 20 kHz. A conductivity meter (Crison, microCM 2200) was used for measuring the conductivity of both synthetic and real water samples.
Synthesis of CNT-MNPs
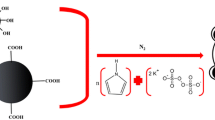
The magnetic MW or SW-CNT composites were prepared by in situ high-temperature decomposition of the magnetic precursor [29] with some modifications [30]. Briefly, 0.014 g of either FeCl2·4H2O or FeCl3·6H2O and 0.004 g of either SW or MWCNT were dispersed into 0.75 mL ethylene glycol in a glass vial. Then, 0.036 g of sodium acetate was added and dissolved. The solution was allowed to stand at room temperature for 30 min, after which the glass vial was heated in an oven at 200 °C for 24 h. After cooling, the product was washed with 1 mL of ultra pure water and the CNT-MNPs were recovered by applying a magnetic field via a magnet placed on the outer wall of the glass vial. This cleanup procedure was repeated five times. The CNT-MNPs thus obtained can be stored in ultrapure water (1 mL) or dried at 80 °C until needed. The synthesized material was characterized by transmission electron microscopy (TEM) to confirm that the CNT-MNP composite was obtained [30].
Magnetic SPE procedure
An amount of 5 mg of magnetic composite nanomaterials was put into a 10-mL vial. The first step was conditioning with 1 mL of acetonitrile and 3 mL of deionized water in this order. Then, 5 mL of sample were added. The mixture was vigorously vortexed for 5 min so that the adsorbents can disperse uniformly in solution. An external magnet was placed at the bottom of the vial and, after no more than 2 min, the separation of the supernatant from the composites had been achieved. The supernatant was decanted, filtered (0.2 μm), and injected into the UHPLC system to assess the degree of adsorption. Finally, the target compounds were desorbed with 5 mL of THF by sonication probe for 3 min at 20% power. After the solution was cleared by applying the external magnet, 1 mL of this solution was filtered (0.2 μm) and injected into the UHPLC system for analysis.
Water samples
Synthetic water samples containing different amounts of NaCl (0.1, 15 and 35 ‰) in the absence and in the presence of 1 mg L−1 of humic acids were prepared. To demonstrate the applicability of the method, tap, dam, and river water and three types of mineral water with different conductivity were selected. Tap water was sampled from our lab after allowing for 10 min to flow. Dam and river waters were obtained from Toledo province (Spain). In all cases, the sampling bottle was rinsed three times with water before it was filled up. Mineral waters were purchased in a local supermarket and used without any additional treatment.
All samples were analyzed in duplicate. They were also spiked at 20 ng mL−1 of each analyte and analyzed in duplicate.
Results and discussion
Chromatographic analysis by UHPLC-FLD
The performance declared by the column supplier was assessed using the same chromatographic conditions. Since some peaks overlapped partially, some minor modifications in the elution gradient proposed had to be carried out to get all the peaks base line resolved. The final conditions are given in the Experimental section. As an example, a chromatogram corresponding to a standard solution of 20 ng mL−1 is shown in Fig. 1. As it can be seen, the separation was achieved in 7 min.
UHPLC-FLD chromatogram of a standard solution of the PAHs containing naphthalene (NAPH), acenaphtene (ACE), fluorene (FLU), phenanthrene (PHEN), anthracene (ANTH), fluoranthene (FLT), and pyrene (PYR) at 20 ng mL−1 each, recorded at the maximum emission wavelengths of every analyte (350, 420, and 500 nm), after excitation at 260 nm. The chromatogram at 500 nm is referred to secondary y-axis
Optimization of the MSPE conditions
Adsorption
Composites made up of Fe (III), Fe (II), and 75:25 mixture of both combined with SW or MWCNTs were synthesized as explained above in the Experimental section and tested for adsorption. For the adsorption, 10 mg of composite were put in a vial, added 1 mL of a solution of the seven analytes at 1 mg L−1 resulting in a total loading of 0.7 mg g−1 of composite and vortexed for 5 min. The magnet was placed at the bottom of the vial and after 2 min, the solution was clear. The supernatant was filtered to get rid of possible agglomerates and injected into the chromatographic system. No peaks were obtained in any case, which means the adsorption was >99% regardless of the composite. Given that, and also because of stability to oxidation, the subsequent experiments were carried out with Fe (III) as magnetic precursor. Multiwalled CNTs were selected for subsequent experiments because they are described to be less prone to aggregation than SWCNTs [30].
Other experiments were carried out with different amounts of MWCNT-MNP composite (1, 3, and 5 mg) and concentration of PAHs (20, 100, and 500 ng mL−1) to test the adsorption capacity and vortexed for 5 min. Again, the adsorption was quantitative in all cases. Therefore, the adsorption capability of PAHs by the hybrid nanocomposite is fully demonstrated.
Desorption
The parameters affecting desorption were studied systematically after the adsorption of 5 mL of a standard solution of the analytes at 100 ng mL−1 onto 5 mg of MWCNT-MNP composites. The initial conditions for desorption were vortex mixing for 3 min in 5 mL of solvent.
The desorption solvent should be efficient and mobile phase compatible, so it has to be carefully selected. Organic solvents like hexane, acetone, toluene, dichloromethane, and mixtures of them have been used for desorbing PAHs. Moreover, these solvents are volatile, which is fine for GC-MS methods [9, 20] but they cannot mix with water, so they are not compatible with mobile phases used in LC methods. In these cases, the desorption solvents have to be evaporated to dryness and reconstituted, i.e., in mobile phase, before LC separation, which increases the sample preparation time. Several experiments were conducted using 5 mL of toluene as desorption solvent with recoveries below 24% after the required evaporation and reconstitution, so this not only increases the analysis time but it also hinders recoveries of PAHs due to their volatility, as described elsewhere [31]. Acetonitrile (MeCN) and tetrahydrofuran (THF) are polar and water-soluble solvents so they are compatible with LC mobile phase and that is why they were tested. Standard solutions previously adsorbed in MWCNT-MNP composites were desorbed in 5 mL of each one of the solvents as indicated above. The recoveries with MeCN were from 17 to 55% while those with THF were from 49 to 89%, so THF was selected for desorption in further experiments (Fig. 2). It seems the number of rings affect the recoveries in MeCN because PAHs with two or three rings were all around 50% recovery, but PAHs with four rings were below 30%. On contrary, this factor seems to have no influence in THF. The desorption of all analytes is higher in THF than in MeCN. The elution strength in Snyder eluotropic series (ε°) is 0.45 and 0.65 for THF and MeCN, respectively. This means that THF is less polar than MeCN, so PAHs are more easily desorbed in THF. Although the recoveries increased, other factors were studied in order to get quantitative results.
Comparison of the efficiency of desorption using 5 mL of either acetonitrile (MeCN) or tetrahydrofuran (THF) (vortex mixing for 3 min) after adsorption of 5 mL of 100 ng mL−1 of naphthalene (NAPH), acenaphtene (ACE), fluorene (FLU), phenanthrene (PHEN), anthracene (ANTH), fluoranthene (FLT), and pyrene (PYR) onto 5 mg of MWCNT-MNP composite
The influence of the type of CNT on desorption was also studied. Two magnetic composites made up of SWCNTs and MWCNTs each were used for this purpose. After the adsorption, the analytes were desorbed in 5 mL of THF by vortex mixing for 3 min. The recoveries were in the range of 49 to 89% for MWCNTs composites and of 44 to 91% for SWCNTs composites, that is, the recoveries were the same regardless of the type of CNT. Finally, MWCNT-MNPs were selected for the advantages indicated in the adsorption process.
Ultrasound energy applied either by ultrasonic bath or sonication probe was tested in order to increase the efficiency of desorption. Firstly, ultrasonic bath from 1 to 5 min was tested but the recoveries did not improve. Secondly, desorption was carried out with a sonication probe at different times (20 s to 3 min), power (20–90%), and cycles (1–6). The best recoveries, in the range of 76.4 ± 1.7 to 106.5 ± 3.5%, were obtained with 3 min at 20% power in 1 cycle. A comparison of the recoveries by sonication probe and vortex is shown in Fig. 3. As can be seen, the recoveries obtained with the sonication probe are better, so it was selected for desorption.
Comparison of the efficiency of desorption with 5 mL of tetrahydrofuran using vortex mixing (for 3 min) and sonication probe (for 3 min at 20% power) after adsorption of 5 mL of 100 ng mL−1 of naphthalene (NAPH), acenaphtene (ACE), fluorene (FLU), phenanthrene (PHEN), anthracene (ANTH), fluoranthene (FLT), and pyrene (PYR) onto 5 mg of MWCNT-MNPs
The possibility of reusing the nanocomposites was studied. The results showed that the adsorption/desorption cycle could be carried out up to three times in a row with no effect on the recoveries. Beyond this point, the nanocomposites began to lose magnetic properties, presumably due to the sonication, so they became useless for MSPE.
Performance of the CNT-MNPs UHPLC-FLD method
The parameters of the instrumental performance of the UHPLC-FLD separation are given in Table 1. Calibration curves were established for analytes in the range of 1–500 ng mL−1. The coefficients of determination, R 2, were over 0.9936 and the intercepts were negligible according to Student’s t test (p < 0.05). The instrumental limits of detection (LOD) and quantification (LOQ) were estimated for signal-to-noise ratios of 3 and 10, respectively. As observed in Table 1, the LODs and LOQs ranged from 0.025 to 0.73 ng mL−1 and from 0.04 to 2.4 ng mL−1, respectively.
The quantification was done by means of response factors obtained from standards injected before and after three samples. The accuracy was assessed by replicate analysis of 5 mL of a standard solution of the analytes at 5 ng mL−1 using 5 mg of each composite as explained above. The recoveries obtained ranged from 76.4 ± 1.7% (ANTH) to 106.5 ± 3.5% (NAPH) (n = 6). The precision of the method using composites synthesized in the same batch ranged from 0.91 (FLT) to 6.1 (NAPH) % RSD. The same solution was analyzed in duplicate using composites from three different synthetic batches. The results showed 5.1 (FLT) to 10 (NAPH) % RSD (n = 6).
Recovery studies involving preconcentration were carried out, too. Aliquots of 20 mL of standard solutions of the analytes at 1, 5, and 80 ng mL−1 resulting in a total loading of 0.028, 0.14 and 2.24 mg g−1 of composite, respectively, were analyzed as explained above. The recoveries obtained for 80 ng mL−1 were from 8% (ANTH) up to 57% (NAPH) lower than the ones obtained for 1 or 5 ng mL−1. This means that preconcentration seems to be limited by the amount of analytes adsorbed.
Analysis of water samples
Firstly, recovery studies were carried out in synthetic water samples. To do so, ultrapure water (S0) was added 0.1, 15, and 35‰ of NaCl in absence of humic acid (S1, S2, and S3, respectively) and in presence of 1 mg L−1 of humic acid (S4, S5, and S6, respectively) in order to mimic the salinity and organic matter content that may occur in real water samples. The conductivity was 225.3 ± 3.8; 26,025 ± 403; and 54,150 ± 506 μS cm−1 for 0.1, 15, and 35‰, respectively. The synthetic water samples were spiked with the PAHs at 20 ng mL−1. Thus, duplicate aliquots of 5 mL of each synthetic water sample were submitted to analysis and injected in the chromatographic system by triplicate. In all cases, the adsorption of the analytes was quantitative. Table 2 shows the recoveries of the analytes, expressed as mean value (n = 6), and the relative standard deviation. There is a slight decrease in the recoveries in the presence of either NaCl or humic acid. When both are present, the recoveries are similar to those obtained with the only presence of NaCl, so there seems to be no additive effect. In light of the results of the present work, there is a clear influence of the salinity and organic matter content in the accuracy of the method.
Secondly, tap, river, dam, and three mineral waters with conductivities from 47.8 ± 0.8 to 1588 ± 19 μS cm−1 were analyzed. Naphthalene was detected at 4.99 ± 0.05 and 4.03 ± 0.05 ng mL−1 in river and dam waters, respectively. The rest of the analytes were below the LODs in all samples. These levels of NAPH are consistent with the results reported in river [18] and lake water [20] samples, with NAPH around 3 ng mL−1 in both of them. In the case of the river water samples, NAPH was the most concentrated congener [18].
All these real samples were subsequently spiked with PAHs at 20 ng mL−1 to carry out recovery studies. Table 3 shows the recoveries of the analytes, expressed as mean value (n = 6), and the relative standard deviation. Again, the recoveries were lower than for standard solutions in ultrapure water (Table 2, S0 (0; 0)) in all analytes and samples. The decrease in the recoveries in all samples was similar to the decrease found in the synthetic waters.
In previous works, dealing with MSPE or CNTs as sorbents for PAHs, the recoveries obtained in spiked river and lake water samples were lower than in the optimization process. However, the authors either gave no explanation to this [17] or assumed the recoveries in the real samples would be the same as in spiked ultrapure water [20].
Conclusions
A simple, rapid, and environment-friendly method based on MSPE and UHPLC-FLD for the monitoring of PAHs in water was developed. The magnetic composites are made up of Fe (III) as magnetic precursor and MWCNTs. A comparison of the present method with previously reported methods using MSPE prior to HPLC for the determination of PAHs in water is shown in Table 4. The present method provides a number of advantages. As for analytical figures, the combination of UHPLC-FLD after MSPE provides instrumental LOD in the range of the ones reported in literature or even below them [24] and RSD in spiked samples close to the lowest [27]. The present method shows the lowest analysis time. It also shows one of the lowest times in the preparation of the composite because MWCNTs can be used without modification or functionalization. Moreover, the use of THF and sonication increases the efficiency of desorption, and, finally, it requires the least amount of sample and nanocomposite.
Since salinity and organic matter have significant effects on recoveries, both should be taken into account in the analysis of real samples of water. Future studies should cover the wide range of waters with different characteristics that can affect adsorption and desorption onto these composites. Likewise, the assessment of recoveries in spiked samples prior to analysis and/or the use of adequate internal standards can be suggested to improve the present method.
References
WHO. Health risks of persistent organic pollutants from long-range transboundary air pollution. World Health Organization: Copenhagen; 2003.
European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. 2006.
WHO. Polycyclic aromatic hydrocarbons in drinking-water. Background document for preparation of WHO guidelines for drinking-water quality. Geneva: World Health Organisation; 2003.
Purcaro G, Moret S, Bučar-Miklavčič M, Conte LS. Ultra-high performance liquid chromatographic method for the determination of polycyclic aromatic hydrocarbons in a passive environmental sampler. J Sep Sci. 2012;35:922–8.
Vinci G, Antonelli ML, Petri R. Rapid determination of polycyclic aromatic hydrocarbons in rainwater by liquid-liquid microextraction and LC with core-shell particles column and fluorescence detection. J Sep Sci. 2013;36(3):461–8.
Liaud C, Millet M, Le Calvé S. An analytical method coupling accelerated solvent extraction and HPLC-fluorescence for the quantification of particle-bound PAHs in indoor air sampled with a 3-stages cascade impactor. Talanta. 2015;131:386–94.
Zou Y, Chen Y, Yan Z, Chen C, Wang J, Yao S. Magnetic solid-phase extraction based on tetrabenzyl modified Fe3O4 nanoparticles for the analysis of trace polycyclic aromatic hydrocarbons in environmental water samples. Analyst. 2013;138:5904–12.
Pérez RA, Albero B, Tadeo JL, Fraile MV, Sánchez-Brunete C. Determination of PAHs in soil leachates by magnetic solid-phase extraction using nanoparticles and gas chromatography-tandem mass spectrometry. Anal Methods. 2014;6:1941–50.
Ma J, Xiao R, Li J, Yu J, Zhang Y, Chen L. Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography–mass spectrometry. J Chromatogr A. 2010;1217:5462–9.
Pensado L, Blanco E, Casais MC, Mejuto MC, Martinez E, Carro AM, et al. Strategic sample composition in the screening of polycyclic aromatic hydrocarbons in drinking water samples using liquid chromatography with fluorimetric detection. J Chromatogr A. 2004;1056:121–30.
Zoccolillo L, Amendola L, Tarallo GA. Halocarbons in Antarctic surface waters and snow. Int J Environ Anal Chem. 1996;63(2):91–8.
Martinez E, Gros M, Lacorte S, Barceló D. Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water, sediments and mussels. J Chromatogr A. 2004;1047:181–8.
Delhomme O, Rieb E, Millet M. Solid-phase extraction and LC with fluorescence detection for analysis of PAHs in rainwater. Chromatographia. 2006;65(3):163–71.
Socas-Rodríguez B, Herrera-Herrera AV, Asensio-Ramos M, Hernández-Borges J. Recent applications of carbon nanotube sorbents in analytical chemistry. J Chromatogr A. 2014;1357:110–46.
Wang WD, Huang YM, Shu WQ, Cao J. Multiwalled carbon nanotubes as adsorbents of solid-phase extraction for determination of polycyclic aromatic hydrocarbons in environmental waters coupled with high-performance liquid chromatography. J Chromatogr A. 2007;1173:27–36.
Moazzen M, Ahmadkhaniha R, Gorji ME, Yunesian M, Rastkari N. Magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in grilled meat samples. Talanta. 2013;115:957–65.
Wu H, Wang X, Liu B, Lu J, Du B, Zhang L, et al. Flow injection solid-phase extraction using multi-walled carbon nanotubes packed micro-column for the determination of polycyclic aromatic hydrocarbons in water by gas chromatography–mass spectrometry. J Chromatogr A. 2010;1217:2911–7.
Guo L, Lee HK. Development of multiwalled carbon nanotubes based micro-solid-phase extraction for the determination of trace levels of sixteen polycyclic aromatic hydrocarbons in environmental water samples. J Chromatogr A. 2011;1218:9321–7.
Kueseng P, Thammakhet C, Thavarungkul P, Kanatharana P. Multiwalled carbon nanotubes/cryogel composite, a new sorbent for determination of trace polycyclic aromatic hydrocarbons. Microchem J. 2010;96:317–23.
Menezes HC, Resende de Barcelos SM, Dias Macedo DF, Purceno AD, Machado BF, Carvalho Teixeira AP, et al. Magnetic N-doped carbon nanotubes: a versatile and efficient material for the determination of polycyclic aromatic hydrocarbons in environmental water samples. Anal Chim Acta. 2015;873:51–6.
Ding J, Gao Q, Li X-S, Huang W, Shi Z-G, Feng Y-Q. Magnetic solid-phase extraction based on magnetic carbon nanotube for the determination of estrogens in milk. J Sep Sci. 2011;34(18):2498–504.
Luo YB, Yu QW, Yuan BF, Feng YQ. Fast microextraction of phthalate acid esters from beverage, environmental water and perfume samples by magnetic multi-walled carbon nanotubes. Talanta. 2012;90:123–31.
Zhao Q, Wei F, Luo YB, Ding J, Xiao N, Feng YQ. Rapid magnetic solid-phase extraction based on magnetic multiwalled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in edible oils. J Agric Food Chem. 2011;59:12794–800.
Wang Y, Wang S, Niu H, Ma Y, Zeng T, Cai Y, et al. Preparation of polydopamine coated Fe3O4 nanoparticles and their application for enrichment of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A. 2013;1283:20–6.
Xu SN, Zhao Q, He HB, Yuan BF, Feng YQ, Yu QW. Rapid determination of polycyclic aromatic hydrocarbons in environmental water based on magnetite nanoparticles/polypyrrole magnetic solid-phase extraction. Anal Methods. 2014;6:7046–53.
Long Y, Chen Y, Yan F, Chen C, Pan D, Cai Q, et al. Triphenylamine-functionalized magnetic microparticles as a new adsorbent coupled with high performance liquid chromatography for the analysis of trace polycyclic aromatic hydrocarbons in aqueous samples. Analyst. 2012;137:2716–22.
Wang W, Ma R, Wu Q, Wang C, Wang Z. Magnetic microsphere-confined graphene for the extraction of polycyclic aromatic hydrocarbons from environmental water samples coupled with high performance liquid chromatography–fluorescence analysis. J Chromatogr A. 2013;1293:20–7.
Bunkoed O, Kanatharana P. Extraction of polycyclic aromatic hydrocarbons with a magnetic sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim Acta. 2015;182:1519–26.
Morales-Cid G, Fekete A, Simonet BM, Lehmann R, Cárdenas S, Zhang X, et al. In situ synthesis of magnetic multiwalled carbon nanotube composites for the clean-up of (fluoro)quinolones from human plasma prior to ultrahigh pressure liquid chromatography analysis. Anal Chem. 2010;82:2743–52.
Moreno V, Zougagh M, Ríos Á. Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nanoC18SiO2 composites for solid phase extraction of mycotoxins prior to their determination by LC-MS. Microchim Acta. 2016;183:871–80.
Yang F, Long Y, Shen R, Chen C, Pan D, Zhang Q, et al. Ultrasonication extraction coupled with magnetic solid-phase clean-up for the determination of polycyclic aromatic hydrocarbons in soils by high-performance liquid chromatography. J Sep Sci. 2011;34(6):716–23.
Acknowledgements
The authors thank Ministerio de Economía y Competitividad (Spain) for financial support through project CTQ2016-78793-P and Junta de Comunidades de Castilla – La Mancha (Spain) through project PEIC-2014-001-P. The support given through an “INCRECYT” research contract to M. Zougagh is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Corps Ricardo, A.I., Guzmán Bernardo, F.J., Zougagh, M. et al. Magnetic nanoparticles—carbon nanotubes hybrid composites for selective solid-phase extraction of polycyclic aromatic hydrocarbons and determination by ultra-high performance liquid chromatography. Anal Bioanal Chem 409, 5125–5132 (2017). https://doi.org/10.1007/s00216-017-0459-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0459-7