Abstract
The preparation of magnetic nanoparticles (MNPs) coated with a layer of octadecyl group-modified silica containing multiwalled carbon nanotubes (MWCNTs) is described. Synthesis was accomplished by in-situ high temperature decomposition of the precursors ferric ion, octadecyltrichlorosilane (C18) and MWCNTs in ethylene glycol solvent. Transmission electron microscopy confirmed that the MNPs are encapsulated in the composite silica coating. These MNPs were used for clean-up of maize samples and the preconcentration of zearalenone and its secondary metabolites, namely β-zearalenol, β-zearalanol, α-zearalenol, α-zearalanol, and zearalanone at trace levels, prior to their determination by liquid chromatography-mass spectrometry. A comparative study of analyte adsorption and desorption was conducted with MNPs prepared with and without C18 and showed both solid phases to adsorb the analytes to some extent, but higher recoveries were obtained by using the C18-modified MNPs which therefore was selected for extraction of the mycotoxins. Under the optimum conditions, the MNPs can be re-used 6 times at least without loss of their extraction efficiency. Preconcentration factors are ~25, detection limits range from 0.6 to 1.0 μg mL−1, recoveries from spiked samples from 91.6 to 98.3 %, and relative standard deviations are <3.9 %.

Mycotoxins were selectively extracted on magnetic multiwalled carbon nanotube nanoC18SiO composites, and sensitively determined by liquid chromatography-mass spectrometry (LC- MS). The method meets the requirements established by the European Commission Directive 856/2005/EC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zearalenone (ZON) and its metabolites α-zearalenol (α-ZOL), β-zearalenol (β-ZOL), α-zearalanol (α-ZAL), β-zearalanol (β-ZAL) and zearalanone (ZAN) (Fig. 1) are highly toxic secondary metabolites. They are produced by fungal species, which belong to the group of macrocyclic lactone mycotoxins (MLM) [1]. They are chemical compounds of low molecular weight produced by fungi, which have pathological effects in humans and in animals. Mycotoxins are found in the top of the list of most common natural contaminants in food worldwide. As a result, it has begun to attach importance now. Recently, there are many withdrawals of animal feed in which these substances have been detected. Thousands of mycotoxins exit in food and feed, but only a few present significant food safety challenges. The natural fungal flora associated with food is dominated by three genera like Aspergillus, Fusarium and Penicillum. They grow in a wide range of agricultural goods before, during and after harvest [2]. Mycotoxin intake through food causes harmful effects on human and animal health and, because of this strict limits have been established around the world in various food products. These analytes are also used illegally as feed additives to increase animal growth, due to their ability to increase the efficiency [3]. They have been considered very chronic dietary risk factor and, many of them, are classified as cytotoxic, carcinogenic or mutagenic, among others. Nuts, fruits and cereals, especially the latter, are among the mayor food commodities affected by mycotoxin contamination, involving a serious risk for public and livestock health [4]. Rice and maize are the most consumed cereals and their contamination is dependent on environmental factors like temperature, humidity, insect damage, drought and inadequate moderate climates, so their consumers also may be controlled in food and feed [5–8]. To protect consumers, the European Union has set out the maximum levels of Fusarium toxins to be adopted in July 2006 [9]. Maximum levels of 200 and 100 μg kg−1 have been fixed for ZON in unprocessed corn and unprocessed cereals other than corn, respectively.
For the determination of ZON and its metabolites, many techniques have been reported: thin-layer chromatography (TLC) [10], gas chromatography–mass spectrometry (GC-MS) [11], liquid chromatography using UV diode array [12], fluorescence [13], electrochemical detection [1] and mass spectrometric detection [14]. To perform the extraction of samples with these methods cleaning procedures, are needed in order to remove most of the interfering and to pre-concentrate the analytes of interest. These procedures include liquid-liquid extraction (LLE) [15], solid phase extraction (SPE) [16–18], pressurized liquid extraction (PLE) [19] and supercritical fluid extraction (SFE) [20].
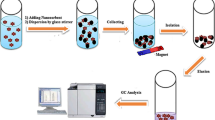
Octadecyltrichlorosilane silica gel (C18) and carbon nanotubes (CNTs) are good adsorbents for ZON and its metabolites, because of their hydrophobicity, large specific surface area, and excellent chemical stability [1, 21]. These sorbent materials were used in cartridge mode, which often results in a tedious column packing procedure, high backpressure and low flow rate. In order to avoid these disadvantages, the incorporation of CNTs and C18 in magnetic solid phase extraction (MSPE) is an excellent alternative. The experimental procedure is simple [22]. The phase separation can be simply carried out by applying an external magnetic field and, furthermore, it is possible to recycle the magnetic NPs.
One way to prepare magnetic C18 nanoparticles is by the reaction between magnetite and octadecyltrichlorosilane (C18) [23]. The difference between C18 and C18 nanoparticles is that the latter were prepared from SiO2 NPs, so that its particle size is smaller. This material is not stable because the magnetite is easy oxidized in the air. Liu et al. [24] prepared monodisperse and homogeneous magnetic C18 microspheres based on three-step reactions: solvothermal reduction, silanization and alkylation. In fact, this procedure involves complicated, time-consuming operations, and it is frequently difficult to achieve a controlled coupling between MNPs and C18. An alternative method, involving the in situ synthesis of MNPs onto the surface of C18, can be more advantageous for a large-scale synthesis.
CNTs are important polymorphs carbon structures. In general, the properties of CNTs mainly depend on the following factors: the number of concentric layers, the way they are arranged, and the pipe diameter. They present several interesting properties, such as high electrical conductivity, mechanical strength and chemical stability. Additionally, CNTs possess a high surface area and a pore structure in layers, which is ideal for storing various items and chemicals. Multiwalled carbon nanotubes are selective for the adsorption of mycotoxins because they possess their unique tubular structures and extremely high surface area and a porous structure capable of storing various elements and chemicals [21]. During the past decade, the synthesis of nanosized magnetic materials has been the subject of intensive research, owing to their biocompatibility and potential applications. When Fe3O4 particles are encapsulated, they are magnetically susceptible. Thus, they can be easily isolated from the sample by applying an external magnetic field, without the need for complicated centrifugation steps or filtration [25]. On the contrary, as disadvantages, when using these materials in SPE without MNPs it is necessary to pack the sorbent material in a cartridge or a minicolumn. This process is time consuming and requires more time to perform the procedures of packing of the adsorbent material, and to carry out the corresponding filtration or centrifugation steps. There are several methods used to modify the surface of the CNTs [26–31]. One method to prepare magnetic CNTs is based on the use of MNPs anchored to the surface of iron(III) oxide particles by chemical deposition of Fe3O4 MNPs onto CNTs [30] or by electrostatic self-assembling [26, 27]. Other alternative is caging Fe3O4 MNPs and MWCNTs into calcium alginate beads [31].
Gao et al. [28] and Morales-Cid et al. [29] used in situ high magnetic decomposition temperature of the precursor [iron(III)] and MWCNTs, in ethylene glycol. Although a high temperature is required for an efficient Fe3O4 decoration CNTs with NPs, the reaction is relatively selective and sensitive. In addition, CNTs did not require any modification, and the size and density of the MNPs coverage can be easily adjusted through the precursor MWCNT/magnetite ratio, the temperature, and the synthesis time.
Taken into account these precedents, we exploited the advantages of the solvothermal approach for decorating C18 and MWCNTs with MNPs to prepare magnetic hybrid nanoparticles. Additionally, these nanoparticles showed a super operational stability and retained excellent adsorption. Furthermore, magnetic properties even after a 6-cycle run for the adsorption and desorption of ZON and its metabolites. The synthetized material was characterized by transmission electron microscopy analysis. The present work is focused on developing a straightforward decoration method, in order to obtain composites for the use in the targeted analysis of ZON and its metabolites in maize samples. These types of samples commonly contain the higher amounts of mycotoxins. In addition, mycotoxins are gaining increasing global attention for the significant economic losses they represent and the risks associated with animal and human health. Finally, no research on the application of the highly stable magnetic MWCNTs-C18 composites for the extraction of trace ZON and its metabolites as an SPE format in food analysis has been reported.
Experimental
Chemicals, materials and samples
ZON, α-ZOL, β-ZOL, α-ZAL, β-ZAL and ZAN standards were purchased from Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com). Stock standard solutions were prepared in acetonitrile and stored in the dark at −20 °C until use. The concentration of the stock standard solutions was in the range from 2.5 to 3 mg mL−1, depending on the particular mycotoxin. Working standard solutions were made by appropriate dilution of the stock standard solutions with the mobile phase consisting of water/methanol/ acetonitrile (H2O: MeOH: ACN = 35:55:10, v/v, 15 mM ammonium acetate, pH 7.4). Acetonitrile, methanol (HPLC grade) and ammonium acetate were supplied by Panreac (Barcelona, Spain, www.panreac.es). Water was purified with a Milli-Q system (Millipore). All solutions prepared for HPLC were passed through a 0.45 μm nylon filter before use. Multi-walled carbon nanotubes (30 ± 15 nm diameter) with 95 % purity were obtained from NanoLab (Brighton, MA, www.nano-lab.com). MWCNTs were used with no pretreatment. Silica nanopowder (12 nm) at 99.8 % purity, sodium acetate, ethylene glycol, iron(III) chloride hexahydrate, methanesulfonic acid and octadecyltrichlorosilane were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Characterization measurements
TEM images were obtained with Jeol JEM 2011 operating at 200 kV and equipped with an Orius Digital Camera (2 × 2 MPi) from Gatan. The samples were prepared by deposition of a drop of the synthesized material suspension onto a lacey carbon/format-coated copper grid. The digital analysis of the HRTEM micrographs was done using Digital Micrograph TM 1.80.70 for GMS 1.8.0 Gatan.
Chromatographic analysis
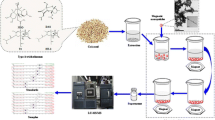
A LC pump (Agilent series 1100, Waldbronn, Germany) was used for the chromatographic system. For the chromatographic separation of the analytes a C18 reverse phase Ascentis Express (150 × 4.6 mm, 2.7 m) from Supelco (Bellefonte, PA, USA) was used, including an injection valve with an inner loop of 40 μL for direct injection of the analytes into the chromatographic column. Detection was carried out with a UV–Vis diode array detector (Agilent, 1200 series) equipped with a 2 μL flow cell coupled in series with a MS detector (Agilent, 6140 series), equipped with an atmospheric pressure ionization source electrospray (API-ES). The wavelength was set at 236 nm. The optimal operating parameters for the MS detector in positive ion mode were: drying glass flow 13 mL min−1, drying gas temperature 350 °C, nebulizer pressure 60 psi and capillary voltage 3500 V. Single ion monitoring (SIM) was used to quantify the target analytes using external calibration. Previously, full scan mode was used to identify the analytes by matching the retention time and mass spectra with standards. The main mass spectra ions were 345.1 (α-ZAL and β-ZAL), 343.1 (α-ZOL and β-ZOL), 321.1 (ZAN) and 319.2 (ZON).
Chromatographic analyses were carried out using the following gradient: mobile phase water/methanol/ acetonitrile (H2O: MeOH: ACN = 35:55:10, v/v, 15 mM ammonium acetate, pH 7.4) in isocratic mode, running at a flow-rate of 0.4 mL min−1. Injection volume was 10 μL and the column was maintained at a temperature of 40 °C. Re-equilibration of the column was done in 35 min after each run. All solvents were filtered through a 0.45 μm nylon membranes (Teknokroma, www.teknokroma.es) before they were used and analyzed. It is very important to filter everything because otherwise the system may be damaged.
Preparation of nanoparticles of C18 sorbent (C18SiO2NPs)
Silica nanopowder is the common name for nanomaterials composed of silicon dioxide (SiO2) and occurs in crystalline and amorphous forms. Crystalline silica exists in multiple forms. Silica nanopowder (12 nm) used as the support material was activated in order to enhance the silanol groups on the silica surface in order to modify it. A 2 g of silica gel nanopowder was mixed with 15 mL of 33 % methanesulfonic acid and refluxed with stirring during 8 h. The solid product obtained was recovered by centrifugation, followed by wash with deionized water to a neutral pH and dried under vacuum at 70 °C for 8 h [32].
The preparation of the nanoparticles of C18 started by mixing of 0.5 g of [SiOx(OH)4-2x]n with 1.5 mL of anhydrous chloroform, vigorously stirring this solution. Then, 0.5 mL of octadecyltrichlorosilane was added to the solution. The mixture was stirred for 2 h. After reaction, the solid product was filtered, washed with chloroform, methanol and finally with dichloromethane. The final product obtained is left to dry for 8–10 h in oven at 100 °C, obtaining a clean material as white powder.
Preparation of hybrid nanoparticles
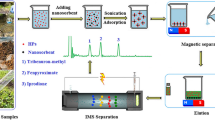
The hybrid nanoparticles were prepared by in situ high temperature decomposition of the magnetic precursor [iron(III)], MWCNTs and C18SiO2NPs, according to the previously described procedure [29], incorporating some modifications. This modified synthesis involves the addition of 14 mg of FeCl3.6H2O, 4 mg of MWCNTs and 2 mg of octadecyltrichlorosilane. This mixture was suspended in 0.75 mL of ethylene glycol in a glass vial. Then, 0.036 g of sodium acetate was added. The solution was allowed to stand at room temperature for 30 min, although previously was stirred and sonicated for a few minutes. Afterwards, the glass vial was heated in an oven at 200 °C for 24 h, in order to complete the reaction. After cooling to room temperature, the synthetic product was washed for 5 times with 1 mL of distillated water. The MNPs were then separated by applying a magnet. The nanoparticles thus obtained can be stored in Milli-Q-water (1 mL) or dried at 80 °C until needed. This experimental procedure was optimized in order to obtain as small nanoparticles as possible, in order to have a higher surface area and, hence, to increase the retention of the analytes onto the composite nanomaterial.
Sample preparation
The extraction process was performed by following a previously described protocol [33, 34]. Thus, 6 g of milled maize was homogenized and extracted in 24 mL of a mixture containing 75 % (v/v) acetonitrile and 25 % water. After centrifugation, the supernatant was filtered through a nylon filter and diluted to 25 mL. The procedure was as follows: 10 g of maize sample well homogenized were weighed onto aluminum foil and a microvolume of stock solutions of the analytes was slowly added dropwise. The solution being spread over the soil and the sample allowed standing for 30–60 min to allow acetonitrile to evaporate. The next step is to carried out the process of magnetic solid phase extraction with magnetic nanoparticles.
General procedure for preconcentration by magnetic nanomaterials
An amount of 5 mg of magnetic nanomaterials were put into a 50 mL vial. The first step was conditioning the samples with 3 mL of acetonitrile and deionized water in this order. After that, 25 mL of mycotoxins (standard or sample extract) was added to the vial. The mixture was mixed at room temperature for 5 min in order to form a homogenous dispersion solution. After the sample stand for 5 min, magnetic nanomaterials containing the adsorbed mycotoxins were rapidly removed from the solution under a strong external magnetic field. After discarding the supernatant solution, mycotoxins were eluted from the magnetic nanomaterials with 2 × 0.5 mL of acetonitrile. This solution (10 μL) was injected into the LC-MS system.
Results and discussion
Optimization of LC-MS
The following LC-MS parameters were optimized: (a) stationary phase, (b) mobile phase composition, (c) drying gas flow, (d) drying gas temperature, (e) nebulizer pressure, and (f) fragmenting voltage. Respective data are given in the electronic Supporting Information. The following experimental conditions were found to give best results: (a) reversed phase C18 column with a particle size of 2.7 μm of diameter, (b) water / methanol / acetonitrile (H2O: MeOH: ACN = 35:55:10, v / v, 15 mM ammonium acetate, pH 7.4) as mobile phase, (c) drying gas flow 13 mL min−1, (d) drying gas temperature 350 °C, (e) nebulizer pressure 60 psi, and (f) fragmenting voltage 3500 V. SIM was used to quantify the target analytes using external calibration.
Adsorption of macrocyclic lactone mycotoxins onto MNP-MWCNT- nanoC18SiO2 composite
The objective was to use the magnetic susceptibility of MNPs in order to simplify the sample treatment. For this purpose, CNTs material was combined with MNPs, as a hybrid composite material, and it must be capable to adsorb the mycotoxins. In this way, ZON and its metabolites were selectively extracted in the sample preparation step. MWCNTs were demonstrated to be more efficient than SWCNTs for the extraction of these compounds from the samples. The arguments were that MWCNTs have a higher adsorption capacity and they are less prone to aggregation. Tests were performed with six types of MWCNTs, purchased at purity greater than 95 % from NanoLab (Brighton, MA), and with different diameters and lengths. Table 1 shows the information about the types of MWCNTs used in the synthesis of the hybrid nanomaterial, and a comparison of recoveries obtained in the extraction of mycotoxins. As it can be seen in Table 1, the best recoveries were obtained by using MWCNTs with dimension of 30 ± 15 nm and diameter of 5–20 μm. Therefore, these MWCNTs were used for synthesis of MNP-MWCNTs. C18SiO2NPs was selected in order to improve the recoveries of mycotoxins. The hybrid nanoparticles were prepared using the same procedure as in the synthesis of MNP-MWCNTs except for the addition of 2 mg of C18SiO2NPs.
The nanomaterial thus obtained was rapidly isolated from the dispersion with the aid of an external magnetic field. No free MWCNTs or C18SiO2NPs remained in solution after the magnetic field was applied. The reusability of magnetic sorbent was evaluated in spiked maize samples spiked with 10 ng mL−1 of each mycotoxin. The recoveries of all extracted mycotoxin reminded constant after 6 uses of the magnetic sorbent without any treatment. After these uses it was observed a lost on the extraction efficiency, with recoveries decreasing down to 70 %. This result is especially interesting from an analytical point of view because the composite material can be easily dispersed and is stable when reused at list for six times. In fact, it remained in suspension throughout the preconcentration process. As can be seen in Fig. 2, using octadecyltrichlorosilane in the synthesis, the recoveries were increased from 70 to 89 % for ZON, 39 to 80 % for β-ZAL, 65 to 84 % for β-ZOL, 35 to 85 % for α-ZAL, 42 to 82 % for α-ZOL and 71 to 93 % for ZAN.
Characterization of MNP-MWCNT-nanoC18 composite
In order to confirm whether that product obtained was in fact the MNP-MWCNT-nanoC18 composite, the material was characterized by Transmission Electron Microscopy (TEM). Figure 3 shows TEM images for the three types of nanoparticles studied. Some differences can be immediately observed. Thus, the micrographs for the MNPs reveal the formation of nanosphere aggregates (Fig. 3a). Figure 3b shows a micrograph for the MNP-MWCNTs. The detailed views (20 nm scale) clearly reveal that the MNPs attach onto nanotubes surfaces and look like nodes growing from the tubes. Finally, Fig. 3c is a micrograph of the composite material (hybrid nanoparticles) that shows aggregates formed by C18 and MWCNTs attached to MNPs. Hybrid nanoparticles binding in the composite is strong enough to resist applied mechanical energy, such as that of manual shaking or sonication. The overall tubular structure of the MWCNTs remains intact after in situ decoration.
Analytical performance of the hybrid nanoparticles liquid chromatography-mass spectrometry method and application to the determination of macrocyclic lactone mycotoxins in maize samples
The performance of the hybrid nanoparticles LC-MS method was evaluated under the adsorption-elution conditions using spiked maize samples. External calibration curves using peak areas were obtained by injecting 6 standard solutions at the 4–40 ng mL−1 range. At least three replicates were performed at each concentration level. Each solution contained the 6 mycotoxin standards used in this work. The linear range, calibration equation and other figures of merit are summarized in Table 2. Repeatability values of peak areas and retention times were obtained by injecting a mixture containing 10 ng mL−1 of each standard (n = 11). Repeatability values ranging from 1.8 to 3.9 % and from 0.1 to 0.2 % were found for peak area and retention time, respectively. The reproducibility of the MNP-MWCNT-nanoC18SiO2 composite sorbent material was also investigated in terms of batch-to-batch series. Ten different batches of sorbent were used to extract a standard mixture containing 10 ng mL−1 of each mycotoxin. The relative standard deviations (RSD) ranged between 3.7 and 7.2 % were lower than 10 % in all cases, which was much better than the value recommended by the AOAC (32 %).
The limits of detection and limits of quantification were estimated for signal-to-noise ratios of 3 and 10, respectively. As observed in Table 2, the limits of detection and the limits of quantification were ranged from 0.6 to 1.0 ng mL−1 and from 1.9 to 3.3 ng mL−1, respectively. In all cases, the limits of quantification were below the values indicated in Amending Regulation (EC) N°466/2001 as regards Fusarium Toxins [12], when maize samples were treated. The applicability of the method was evaluated in the analysis of macrocyclic mycotoxins in maize samples, which were found to contain none of the six above mentioned mycotoxins. Thus, maize samples were spiked with the 6 mycotoxins at different concentration levels. The concentration of mycotoxins in the spiked samples was calculated from the calibration equations. Figure 4 shows three chromatograms of the standard solution (Fig. 4a), un-spiked and spiked maize samples (Fig. 4b and c) obtained by using the MNP-MWCNT-nanoC18SiO2 clean-up method in combination with LC-MS. The recovery results obtained are summarized in Table 3. As observed in this table, recoveries in the range of 91.6 to 98.3 %, depending on the analyte, were obtained.
Conclusions
Hybrid nanoparticles were successfully prepared and applied for the extraction of ZON and its metabolites from maize samples. The magnetic method for sample treatment presents several advantages when it is compared with the previously developed traditional SPE for mycotoxins [1]. The use of MNPs is one important trend topic in solid phase extraction techniques, because the application of magnetic separation technology simplifies sample treatment. The sorbent does not need to be packed into the cartridge (as it is usual in common SPE), the separation phase can be carried out easily by applying an external magnetic field, and the MNPs showed great stability, which can allow the reusability of the nanomaterials six times. In addition to the applied use, better precision, sensitivity and recoveries were obtained using this method. The limits of quantifications are below than the values reported in Directive 856/2005/EC [12] for these types of samples. Table 4 summarizes the advantages, as well as a comparison of this method with respect to others previously reported [1, 21, 35].
The limitation of this method is that the sample treatment and separation/detection are carried out off-line. A next step could be the on-line use of MNPs combined with more sensitive detectors, such as MS/MS, in order to improve sensitivity and thus expanding the applicability of the method to other samples, such as bread, pasties, snacks, cereal snacks and breakfast cereals. In these cases, the European Commission has established an acceptable level of ZON of 20–50 μg Kg−1 [36].
References
Fernando de Andrés F, Zougagh M, Castañeda G, Ríos A (2008) Determination of zearalenone and its metabolites in urine samples by liquid chromatography with electrochemical detection using a carbon nanotube-modified electrode. J Chromatogr A 1212:54–60
Krska R, Molinelli A (2007) Mycotoxin analysis: state-of-the-art and future trends. Anal Bioanal Chem 387:145–148
Lone KP (1997) Natural sex steroids and their xenobiotic analogs in animal production. Growth, carcass quality, pharmacokinetics, metabolism, mode of action, residues, methods and epidemiology. Crit Rev Food Sci Nutr 37:93–209
Cigić IK, Prosen H (2009) An overview of conventional and emerging analytical methods for the determination of mycotoxins. Int J Mol Sci 10:62–115
Hussein HS, Brasel JM (2001) Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicology 167:101–134
Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: occurrence, toxicology and exposure assessment. Food Chem Toxicol 60:2297–2303
Rodriguez-Carrasco Y, Ruiz MJ, Font G, Berrada H (2013) Exposure estimates to Fusarium mycotoxins through cereals intake. Chemosphere 93:218–237
Zain ME (2011) Impact of mycotoxins on humans and animals. J Saudí Chem Soc 15:129–144
Commission Regulation (EC) No 856/2005 amending Regulation (EC) No 466/2001 as regards Fusarium Toxins. European Union, Brussels
Josephs RD, Schuhmacher R, Krska R (2001) Determining mycotoxins and mycotoxigenic fungi in food and feed. Food Addit Contam 18:417–430
Tanaka T, Yoneda A, Inoue S, Sugiura Y, Ueno Y (2000) Simultaneous determination of trichothecene mycotoxins and zearalenone in cereals by gas chromatography–mass spectrometry. J Chromatogr A 882:23–28
Briones D, Gómez L, Cueva R (2007) Zearalenone contamination in corn for human consumption in the state of Tlaxcala. Mexico Food Chem 100:693–698
Mateo JJ, Mateo R, Hinojo MJ, Llorens A, Jiménez M (2002) Liquid chromatographic determination of toxigenic secondary metabolites produced by Fusarium strains. J Chromatogr A 955:245–256
Pallaroni L, Von Holst CH (2003) Comparison of alternative and conventional extraction techniques for the determination of zearalenone in corn. Anal Bioanal Chem 376:908–912
Cunnif P (1995) AOAC official methods of analysis, vol 49. AOAC International, Arlington, pp 45–46
Urraca JL, Marazuela MD, Merino ER, Orellana G, Moreno-Bondi MC (2006) Molecular imprinted polymers with a streamlined mimic for zearalenone analysis. J Chromatogr A 1116:127–134
Radova Z, Hajslova J, Kralova J, Papouskova L, Sykorova S (2001) Cereal Res Commun 29:435–442
Maragos CM, Appell M (2007) Capillary electrophoresis of the mycotoxin zearalenone using cyclodextrin-enhanced fluorescence. J Chromatogr A 1143:252–257
Urraca JL, Marazuela MD, Moreno-Bondi MC (2004) Analysis of zearalenone and α-zearalenol in cereals and swine feed using accelerated solvent extraction and liquid chromatography with fluorescence detection. Anal Chim Acta 524:175–183
Zougagh M, Ríos A (2008) Supercritical fluid extraction of macrocyclic lactone mycotoxins in maize flour samples for rapid amperometric screening and alternative liquid chromatographic method for confirmation. J Chromatogr A 1177:50–57
Ying YF, Wu YL, Wen Y, Yang T, Xu XQ, Wang YZ (2013) Simulteneous determination of six resorcyclic acid lactones in feed using liquid chromatography-tandem mass spectrometry and multiwalled carbon nanotubes as dispertive solid phase extraction sorbent. J Chromatogr A 1307:41–48
Bouri M, Gurau M, Salghi R, Cretescu I, Zougagh M, Ríos A (2012) Ionic liquids supported on magnetic nanoparticles as a sorbent preconcentration material for sulfonylurea herbicides prior to their determination by capillary liquid chromatography. Anal Bioanal Chem 404:1529–1538
Shen HY, Zhu Y, Wen XE, Zhuang YM (2007) Preparation of Fe3O4-C18 nano-magnetic composite materials and their cleanup properties for organophosphorous pesticides. Anal Bioanal Chem 387:2227–2237
Liu Y, Li H, Lin JM (2009) Magnetic solid-phase extraction based on octadecyl functionalization monodisperse magnetic ferrite microspheres for the determination of polycyclic aromatic hydrocarbons in aqueous samples coupled with gas chromatography–mass spectrometry. Talanta 77:1037–1042
Zhang Z, Yang X, Chen X, Zhang M, Luo L, Peng M, Yao S (2011) Novel magnetic bovine serum albumin imprinted polymers with a matrix of carbon nanotubes, and their application to protein separation. Anal Bioanal Chem 401:2855–2863
Gao C, Li WW, Morimoto H, Nagaoka Y, Maekawa TJ (2006) Magnetic carbon nanotubes: synthesis by electrostatic self-assembly approach and application in biomanipulations. J Phys Chem B 110:7213–7220
Correa-Duarte MA, Grzelczak M, Salgueirino-Maceira V, Giersig M, Liz-Marzán LM, Farle M, Sierazdki K, Diaz R (2005) Alignment of carbon nanotubes under low magnetic fields through attachment of magnetic nanoparticles. J Phys Chem B 109:19060–19063
Huiqun C, Meifang Z, Yaogang L (2006) Novel carbon nanotube iron oxide magnetic nanocomposites. J Magn Magn Mater 305:321–324
Morales-Cid G, Fekete A, Simonet BM, Lehmann R, Cardenas S, Zhang X, Valcárcel M, Schmitt-Kopplin P (2010) In situ synthesis of magnetic multiwalled carbon nanotube composites for the clean-up of (fluoro)quinolones from human plasma prior to ultrahigh pressure liquid chromatography analysis. Anal Chem 82:2743–2752
Gao L, Chen L (2013) Preparation of magnetic carbon nanotubes for separation of pyrethroids from tea samples. Microchim Acta 180:423–430
Bunkoed O, Kanatharana P (2015) Extraction of polycyclic aromatic hydrocarbons with a magnetic nanomaterials sorbent composed of alginate, magnetite nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182:1519–1526
Fang GZ, Tan J, Yan XP (2005) An ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique combined with a sol–gel process for selective solid-phase extraction of cadmium (II). Anal Chem 77:1734–1739
Mitterbauer R, Weindorfer H, Safaie N, Krska R, Lemmens M, Ruckenbauer P, Kuchler K, Adam G (2003) A sensitive and inexpensive yeast bioassay for the mycotoxin zearalenone and other compounds with estrogenic activity. Environ Microbiol 69:805–811
Schuhmacher R, Krska M, Grasserbauer W, Edinger W, Lew H (1998) Immuno-affinity columns versus conventional clean-up: a method comparison study for the determination of zearalenone in corn. Fresenius J Anal Chem 360:241–245
Wilcox J, Donnelly C, Leeman D, Markey E (2015) The use of immunoaffinity columns connected in tandem for selective and cost-effective mycotoxin clean-up prior to multi-mycotoxin liquid chromatographic-tandem mass spectrometric analysis in food matrices. J Chromatogr A 1400:91–97
European commission (EC) (2006) Commission regulation (EC) N° 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodtuffs. Off J Eur Union L364:5–31
Acknowledgments
The Spanish Ministry of Economy and Competitiveness (MINECO) and JJCC Castilla-La Mancha are gratefully acknowledged for funding this work with Grants CTQ2013-48411-P and JCCM PEIC- 2014-001-P, respectively. The support given through an “INCRECYT” research contract to M. Zougagh is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Moreno, V., Zougagh, M. & Ríos, Á. Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nanoC18SiO2 composites for solid phase extraction of mycotoxins prior to their determination by LC-MS. Microchim Acta 183, 871–880 (2016). https://doi.org/10.1007/s00604-015-1722-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1722-2