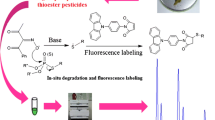
Abstract
A simple, sensitive, and selective high-performance liquid chromatography (HPLC) method using 9-(2-iodoethyl)acridone (IEA) as a novel fluorescence derivatizing agent for the simultaneous determination of six thiophenols has been developed. An efficient Pb2+-modified OASIS-MCX cartridge was used and could get good recoveries. IEA was successfully used to label thiophenols with high sensitivity and excellent selectivity. The effects of different solvents, pH, and surfactants on fluorescence properties of derivatives were investigated. To obtain the best labeling efficiency, derivatizing parameters including pH value, temperature, and concentration of IEA, as well as types of catalysts were also evaluated in detail. Under the optimal conditions, the separation could be achieved within 12 min with limits of detection (LODs) in the range of 0.6–5.8 μg L−1 and relative standard deviations (RSDs) < 3.9 %. This is the first time that IEA was applied to the analysis of thiophenols, and the established method has been successfully applied to the trace level detection of thiophenols in industrial wastewater samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiophenols, an important kind of raw materials which are commonly used for the production of pesticides, polymers, and pharmaceuticals [1, 2], have a significant impact on a variety of physiological and psychological parameters and attract people’s attention extensively. However, volatile thiophenols not only have an unbearable stench but also possess significant hazards, and aromatic thiols are more toxic than aliphatic thiols. It is reported that the median lethal dose (LC50) value is in a low range of 0.01–0.40 mM for fish [3]. In addition, they may lead to serious side effects on human health with long-term exposure to thiophenols environment, including central nervous system damage, increased respiration, muscle weakness, hind limb paralysis, coma, and even death [4]. So it is important to monitor the level of thiophenols in industrial wastewater samples as thiophenols may be dumped as industrial waste into the environment and thus contaminate water sources.
With the development of analytical technologies, a variety of methods for the determination of thiols compounds have been reported. The non-separation techniques, such as UV-spectrophotometry [5] and fluorescence (FL) detection, are widely used because of their simplicity and low cost. In particular, fluorescent sensor-based detection [6–8] is more and more frequently used in recent years due to its excellent sensitivity and great analytical performance. For example, Yao’s team accomplished the fluorescent dual detection of cysteine (Cys) and homocysteine (Hcy) in organisms at a trace level of 0.25 mM [9]. Moreover, Jun Li et al. completed the quantification of thiophenol and p-methoxy-thiophenol in water samples with extremely low limits of detection (LODs) of 30 and 18 nM, respectively [10]. Unfortunately, these methods only provide the total amount of thiols compounds without specific data for each single component. The applications of capillary electrophoresis (CE) [11, 12] and chromatographic methods including gas chromatography (GC) [13, 14] and high-performance liquid chromatography (HPLC) coupled with various detection techniques, such as UV [15, 16], fluorescence [17–19], and mass spectrometry [20, 21], can easily avoid this drawback mentioned above and greatly improve the sensitivity and selectivity. Among these methods, HPLC with fluorescence detection is widely used by the introduction of fluorescent chromophore to the thiols compounds [22–24]. For example, Xiao Feng Guo et al. developed a novel probe and applied to the quantitative analysis of six thiols in human plasma [22]. Seung K. Park et al. established a rapid HPLC method for the detection of glutathione by pre-column derivatization with o-phthalaldehyde (OPA) and successfully applied in grape juice and wine [23]. Up to now, there are a lot of studies about thiol compounds. However, most of studies mainly focused on the HPLC analysis of aliphatic thiols, especially thiol-containing peptides in biological sample [24–26]. To the best of our knowledge, there is no report on the quantitative determination of thiophenol compounds from industrial wastewater by pre-column derivatization with fluorescence detection (FLD).
Commonly used labeling reagents for sulfydryl include monobromobimane (mBrB) [27], o-phthaldialdehyde (OPA) [23, 28], 7-fluoro-2,1,3-benzoxadiazole-4-sulfonamide (ABDF) [29], 7-fluorobenzo-2-oxa-1,3-diazoloe-4-sulfonamide (SBDF) [27], N-(1-pyrenil)maleimide (NPM) [30], dansylaziridine [31], 5-iodoacetamidofluorescein (5-IAF) [32], 5-methyl-(2-(miodoacetylaminophenyl)benzoxazole (MIPBO) [25], and so on. However, it has been reported that these reagents have various shortcomings in their application, such as high derivatization temperature [29, 33], short wavelength of λex/λem [25, 27], long separation time [25, 28], and non-thiol-specific [28].
In previous studies [34–37], we described some applications for the sensitive determination of common amino, amino acid, and fatty acid compounds using some synthesized acridone derivatives. On the basis of the good fluorescence characteristics of acridone core moiety [35], we have synthesized a novel fluorescence reagent 9-(2-iodoethyl)acridone (IEA) and successfully used to label thiophenols. IEA has been found to be very stable in its crystal state and exhibits excellent fluorescence. In this study, the optimal derivatization conditions were investigated. Linearity, LODs, and precision of the procedure were also determined. To the best of our knowledge, this is the first time that IEA fluorescent probe and its applications for the determination of thiophenols have been reported. The suitability of the developed method for the analysis of thiophenols in industrial wastewater samples was satisfactory.
Experimental
Instrumentation
HPLC analysis was performed using an Agilent 1100 Series high-performance liquid chromatography. This LC apparatus was consisted of an on-line degasser (model G1322A), a quaternary pump (model G1311A), an autosampler (model G1329A), a thermostated column compartment (model G1316A), and a fluorescence detector (model G1321A). For identification of analytes, mass spectrometer (MSD Trap SL, model G2445D) from Bruker Daltonics (Bremen, Germany) was used. This mass spectrometer was equipped with atmospheric pressure chemical ionization (APCI) source (model G1947A), and ion source conditions were as follows: APCI in positive ion detection mode, nebulizer pressure 60 psi, dry gas temperature 350 °C, dry gas flow 5.0 L min−1, APCI Vap temperature 350 °C, corona current 4000 nA, and capillary voltage 3500 V. An F-7000 fluorescence spectrophotometer (Hitachi, Tokyo, Japan) was applied to record fluorescence excitation and emission spectra, and a FE-20 accurate acidimeter (Mettler-Toledo Instruments Co. Ltd., Shanghai) was used to adjust pH values.
Materials and reagents
Analytical standards of 3,4-dichlorothiophenol (Dic), 3,5-bis(trifluoromethyl)benzenethiol (Btf), 2-phenylthiophenol (Phe), thiophenol (Thi), p-toluenethiol (Pto), and 4-(trifluoromethyl)thiophenol (Tfm) were purchased from Aladdin Reagent Co. (Shanghai, China). HPLC-grade acetonitrile and methanol were purchased from Yucheng Chemical Reagent Co. (Shandong, China). Dimethylformamide (DMF), dimethylsulfoxide (DMSO), tetrahydrofuran (THF), acetone, dichloromethane, and chloroform were of analytical grade obtained from Shanghai Chemical Reagent Co. (Shanghai, China). Pure distilled water was supplied by Watsons (Guangzhou, China). All other reagents used were also of analytical grade. IEA was synthesized in our laboratory as described in the synthesis section (Fig. 1).
Synthesis of 9-(2-iodoethyl)acridone
Synthesis of 9-(2-hydroxyethyl)acridone
9-(2-Hydroxyethyl)acridone (HEA) was synthesized according to the method described in our previous studies with a little modification [36]. Acridone (10.0 g), ethylene carbonate (7.0 g), and trace amounts of KOH were dissolved together in 200 mL of DMF in a 500-mL flask. The contents of the flask were rapidly heated to reflux for 6 h with vigorous stirring. After cooling, the mixture was transferred into 400 mL water with stirring for 3 min. The precipitated solid was recovered by filtration, washed with water, and dried in an oven at 50 °C. The crude product was recrystallized twice from acetonitrile to afford a yellow crystal 7.4 g, yield 74 %.
Synthesis of 9-(2-chloroethyl)acridone
HEA (7.4 g) and SOCl2 (50 mL) were mixed in a 100-mL round-bottom flask and rapidly heated to 80 °C with vigorous stirring. After a 3–4 h reflux reaction, the mixture was transferred slowly into a solution containing 300 mL water and 20 g KOH with stirring for 5 min. After the solution was neutralized to pH 7.0, the mixture was filtrated and washed with water. The crude product was dried in an oven at 60 °C for 5 h and recrystallized twice from acetonitrile to afford a brownish red crystal 3.2 g, yield 43 %.
Synthesis of 9-(2-iodoethyl)acridone
To a solution containing 3.2 g 9-(2-chloroethyl)acridone (CEA) in 150 mL acetone in a 250-mL beaker, 12.0 g NaI was added. The contents of the beaker were slightly heated with stirring until completely dissolved. The mixture was then transferred into three 100-mL of Teflon-lined stainless steel high-pressure autoclave and heated to 100 °C for 8 h. After cooling, the mixture was poured into 300 mL of water with stirring for 3 min. The precipitated solid was recovered by filtration, washed with distilled water, and then dried at ambient temperature. The crude products were recrystallized twice from acetonitrile to give brownish red acicular crystals (1.8 g, yield 56 %). The characterization of the reagent is shown in the Electronic Supplementary Material (ESM).
Preparation of standard solutions
IEA solution (5.0 × 10−3 mol L−1) was prepared by dissolving 17.5 mg IEA in 10 mL of DMF. The corresponding low concentration of IEA solution (1.0 × 10−3 mol L−1) was obtained by diluting the stock solution with DMF. The mixed standard solution (1.0 × 10−4 mol L−1) containing six thiophenols was prepared by dilution of the corresponding stock solution (1.0 × 10−2 mol L−1) with acetonitrile. Borate buffer solution was prepared by dissolving 1.9 mg Na2B4O7·10 H2O in 100 mL water (0.05 M), and pH (12.2) was adjusted with NaOH (0.20 M) solution coupled with accurate acidimeter. All reagent solutions were stored at 4 °C in a refrigerator.
Chromatographic method
HPLC separation was carried out on a ZORBAX Eclipse XDB-C18 (250 × 4.6 mm, 5 μm) column with a binary gradient elution. Eluent A was 30 % acetonitrile in water and B was acetonitrile. The flow rate was constant at 1 mL min−1, and the gradient condition of mobile phase was as follows: 0–8 min, 70–82 % B; 8–10 min, 82–100 % B; 10–12 min, 100 % B. The column was pre-equilibrated with the mobile phase for 5 min before each analysis. The column temperature was set at 30 °C. The injection volume was 10 μL, and detection wavelength was set at λex/λem = 256/420 nm, respectively.
Samples
Water samples were collected from the highly polluted region in Shandong province of China. Sample in Tengzhou was industrial wastewater and collected from the dirty water that pesticide factory always discharged. Sample in Qufu was collected from the industrial wastewater that pharmaceutical factory discharged. The sample was collected by a 500-mL clean bottle with a tight-fitting lid during the process of discharge, and then ice packs were used to make it cool rapidly. All samples were stored at 4 °C in a refrigerator until analysis.
Enrichment preparation of thiophenols in industrial wastewater samples
Enrichment on metal-loaded solid-phase extraction (SPE) is based on the formation of coordinated bonds between sorbents and organic sulfur compounds. As the optimization results reported in [37], Pb2+ can achieve the best enrichment efficiency with great specificity. Therefore, Waters OASIS-MCX cartridges (30 mg) coupled with Pb2+ were used and the processing conditions were carried out according to literature [37] with a little modification. The cartridges were initially conditioned with methanol (1.0 mL) and distilled water (2.0 mL); the conditioned cartridges were then loaded with an excess of Pd(NO3)2 solution (1.5 mL, w/v = 0.1 %) and followed by 2 mL of distilled water. Wastewater samples were filtered twice through 0.45 μm cellulose acetate filters and 50 mL was forced to pass through the OASIS-MCX cartridges at a flow rate of approximately 1–2 mL min−1. The cartridges were then dried for 10 min under vacuum, and the analytes were eluted with 1 mL of acetonitrile without pressure.
Derivatization procedure
To 30 μL standard solution or 100 μL sample solution in a 2-mL vial, Na2B4O7–NaOH buffer solution (40 μL, pH 12.2) and IEA DMF solution (180 μL) were successively added. The mixture was sealed and allowed to react in a water bath at 40 °C for 50 min. After the derivatization was completed, 50 μL mixture was taken out and diluted with 600 μL acetonitrile. Finally, a portion of solution was syringe filtered using a 0.22 μm nylon filter and a 10-μL of solution was injected directly onto the chromatograph. The procedure of derivatization is shown in Fig. 1.
Method validation
The HPLC-FLD method was evaluated by several parameters, including linearity, LOD, limit of quantitation (LOQ), precision, and accuracy. Linearity was evaluated by the determination coefficients (R 2) of the analytical curves constructed with eight standard solutions containing six thiophenols compounds at different concentrations ranging from 0.02 to 6.68 μmol L−1 (prepared by environmental matrix solvent, n = 3). The parameters including slope, intercept, and R 2 were obtained from the calibration equation of each analyte. LOD and LOQ for each analyte were calculated at a signal-to-noise (S/N) ratio of 3 and 10, respectively. The instrument precision was determined by injection of a 10-μL sample with six replicates, and the method precision was carry out by repeating the whole procedure three times. Intraday and interday precision was carried out by repeating the analysis within 1 day and every other day, respectively. The accuracy of the analytical procedure was evaluated through recovery experiments by spiking wastewater samples with three different concentrations of standard solutions (1, 5, and 10 μg L−1). Independent triplicates of the fortified samples were analyzed, and recoveries were calculated based on the formula of (measured value − endogenous value)/added value × 100.
Results and discussion
Fluorescence excitation and emission of IEA–thiophenol
Acridone core moieties as the chromophoric group exhibited strong fluorescent properties [32, 34–40], and the synthesized IEA should also exhibit high fluorescence property. However, the labeled thiophenol derivatives are necessary to test the feasibility because some sulfur derivatives exhibited fluorescence quenching effect in different solvents. For this purpose, we measured the main factors that influenced fluorescence properties of derivatives including different solvents, pH, and surfactants (the effects of surfactants are shown in the ESM). The uncorrected excitation and emission spectra of the IEA–thiophenol in methanol and acetonitrile solution are investigated. The results indicate that the maximal excitation is 256 nm, and emission wavelengths are 420 and 440, respectively.
Effects of solvents on fluorescence intensity of IEA–thiophenol derivative
Solvents are classified into five groups: group 1, fatty alcohol series; group 2, fatty amine series; group 3, fatty acid series; group 4, dimethyl sulfoxide, tetrahydrofuran, acetonitrile, N,N-dimethylformamide (DMF), and acetone; group 5, acetonitrile/water mixture (0–100 % acetonitrile, v/v) and methanol/water mixture (0–100 % methanol, v/v). The effects of different solvents on IEA–thiophenol spectral properties are shown in ESM Table S1.
With group 1, the fluorescence emission intensity of IEA–thiophenol increases with increasing carbon number of alcohols. With C n > 5 (n: the number of carbon atoms of alcohols), thiophenol derivative showed a slight decrease in fluorescence intensity. This is probably due to the fact that the solubility of thiophenolic derivatives in high molecular weight alcohols is lower than that in low molecular weight alcohols. Besides, it is also probably due to the fact that the hydrogen bond forces between the derivative molecules and the alcoholic molecules decrease with the increase of the solvent viscosity. With group 2, the fluorescence intensity of IEA–thiophenol slightly increases with the increasing of carbon numbers of amine molecules, but it is worth noting that the fluorescence intensity in butylamine is close to four times higher than that in the other three kinds of alkyl amines. With group 3, the fluorescence intensity of IEA–thiophenol increases with increasing carbon numbers of acid molecules. This is probably due to the fact that IEA–thiophenol molecules are partially protonated in the relatively strong formic acid, resulting in corresponding weak fluorescence emission. The maximum intensity in butyric acid solvent is close to 3–4-fold higher than that in the other acid solvents. Besides, it is also found that the fluorescence intensity of IEA-derivative progressively decreases with further increasing of carbon numbers of acid molecules (C n > 4), and it should be attributed to the fact that the solubility of IEA–thiophenol molecule in relatively high molecular weight solvent is gradually decreased. With group 4, the different types of solvents were also tested for fluorescence spectra. The results indicated that the maximum excitation and emission wavelengths of IEA–thiophenol derivative in these solvents exhibited no obvious difference, and the maximum emission intensity was observed in acetone solvent.
With group 5 (Fig. 2), the maximum excitation and emission wavelengths of IEA–thiophenol derivative exhibited no obvious blue or red shift with increasing acetonitrile or methanol concentration from 0 to 100 %. The emission intensity of IEA–thiophenol in methanol was higher than that in acetonitrile (IMeOH/IACN = 1.09 %). Additionally, the increase of methanol and acetonitrile content from 0 to 100 % (v/v) resulted in a 25 and 20 % increase in the fluorescence intensity, respectively. This was probably due to the fact that the solubility of derivative increases with increasing content of methanol or acetonitrile.
Effect of pH on fluorescence spectra of IEA–thiophenol derivative
The pH value of the IEA–thiophenol solution is another key factor affecting fluorescence intensity; the fluorescence emission spectra of IEA–thiophenol derivative was evaluated in the pH range of 4–12. With pH 4–12, IEA–thiophenol derivative showed neither obvious fluorescence enhancement nor fluorescence quenching. At the same time, the fluorescence spectra exhibited no obvious blue or red shift with the further increasing of acidity or alkalinity.
Optimization of derivatization condition
Effects of co-solvents and basic catalysts on derivatization
Several types of solvents including DMF, DMSO, THF, acetonitrile, acetone, chloroform, and dichloromethane were evaluated for the optimal derivatization conditions (Fig. 3). The results indicated that DMF gave the highest detector response. In addition, DMF used as the derivatization co-solvent can obviously increase the solubility of polycyclic thiophenols derivatives and greatly avoid the problem of precipitation. Therefore, DMF was selected as reaction co-solvent. Several base catalysts, including K2CO3, Na2CO3, CsCO3, borax, sodium bicarbonate buffer, phosphate buffer, and borate buffer, were evaluated. As shown in Fig. 3, borate buffer was superior to the other catalysts. The effect of pH on the derivatization reaction was then evaluated with Na2B4O7–NaOH buffer (0.05 M) in the pH range of 9.5–12.2. The results indicated the relative peak area reached the maximum at pH 12.2. Therefore, 50 μL Na2B4O7–NaOH buffer solution (0.05 M) at pH 12.2 was used to adjust the pH.
Reagent concentration, temperature, and time effects for derivatization
In order to obtain the highest derivatization efficiency, the main parameters affecting the fluorescence labeling, including temperature, time, and reagent concentration were optimized by response surface methodology (RSM). Seventeen runs of experiments for interactive variables were listed in ESM Table S2. A regression equation that could predict the optimum point within the given range was obtained by applying multiple regression analysis on the experimental data. The second-order polynomial model was the following: Y = 30.18 − 5.60 X1 − 2.64 X2 + 0.46 X3 − 1.42 X1X2 − 1.08 X1X3 − 1.45 X2X3 − 4.59 X1 2 − 12.76 X2 2 − 9.26 X3 2, where Y is the predicted average peak areas and X1, X2, and X3 are the coded values of molar reagent excess, temperature, and time, respectively. The surface response plot was seen as a visual process of the predicted model equation to study the effects of parameters and their interactions on fluorescence labeling reaction. Response surface plots are shown in Fig. 4. The results of the analysis of variance (ANOVA) indicated all the linear parameters and quadratic parameters of the quadratic equation were significant at the level of p < 0.01. The value of R 2 was 0.9776, meaning that there was a satisfactory agreement between experimental and predicted values. The optimum conditions given by the model were as follows: 10-fold molar reagent excess, 40 °C reaction temperature, and 50 min labeling time.
Stabilities of reagent (IEA) and its derivatives
The stability of IEA reagent was evaluated by the analysis of the stored acetonitrile solution of IEA. When reagent was, respectively, stored at 4 °C for 1 month and 25 °C for 1 week, no significant changes of the peak area for the derivatized thiophenols were observed. The stability of corresponding thiophenol derivatives was also studied. The solution of IEA derivatives (1.0 × 10−5 mol L−1) was kept at 4 °C for at least 1 week and analyzed repeatedly by HPLC (n = 6); the relative standard deviation (RSD) of peak area for the labeled thiophenols were less than 6 %. It was indicated that the stability of IEA–thiophenols was sufficient enough for HPLC analysis.
HPLC separation and MS identification
To obtain the optimal separation for the target compounds with similar structures, three reversed-phase columns including ZORBAX SB-C18 (4.6 mm × 150 mm, 5 μm) column, Hypersil BDS C18 (4.6 mm × 200 mm, 5 μm) column, and ZORBAX Eclipse XDB-C18 (250 × 4.6 mm, 5 μm) column were tested. The results showed that the separation of six thiophenol derivatives could be achieved on a ZORBAX Eclipse XDB-C18 (250 × 4.6 mm, 5 μm) column with a good baseline resolution. Thus, ZORBAX Eclipse XDB-C18 (250 × 4.6 mm, 5 μm) column in conjunction with a gradient elution using acetonitrile and water as mobile phase was used in this study.
Thiophenol derivatives were dually identified by the retention time and on-line mass spectrometry. MS and MS/MS spectra of IEA–thiophenol are shown in ESM Fig. S1. As expected, thiophenol derivatives exhibited intense molecular ion peaks at m/z [M+H]+. The collision-induced dissociation spectra of m/z [M+H]+ usually contained intense fragment ions resulting from loss of IEA core moiety (195 Da). Taking thiophenol (Thi) as an example, Thi gave the molecular ion peak at m/z 331.9 [M+H]+, and MS2 spectrum of m/z 331.9 gave an abundant daughter ion at m/z 136.7. The ion was from the cleavage of C–N bond of the N-linked side chain and obtained by the neutral loss of 195 Da (IEA core structure). The product ions at m/z [M+H]+ and the corresponding neutral fragment loss at m/z [M+H-195]+ are the characteristic structural information of thiophenol derivatives. MS and MS/MS data for all thiophenol derivatives are listed in Table 1.
Method performance
The HPLC-FLD method was evaluated by the procedures described above, and the results were listed in Table 2. The correlation coefficients were of >0.9992, indicating excellent linearity of the analytes. The RSDs of the intraday and interday validation for the instrument precision were less than 0.68 and 2.46, respectively, while RSDs for method precision were less than 2.29 and 3.90, respectively. The LODs were in the range of 0.6–5.8 μg L−1 and LOQs were in the range of 1.8–17.4 μg L−1. The results for method accuracy are shown in Table 2, and recoveries for all tested compounds were in the range of 60–94 %. These results demonstrated that this method was suitable for the determination of trace thiophenols from industrial wastewater samples.
Matrix effect
Due to two reasons, the matrix effect was reduced to the minimum. Firstly, Pb2+-loaded SPE was highly specific and sensitive for organic sulfur compounds and other impurities were removed to a great extent in this procedure. Besides, a significant feature of the pre-column derivatization HPLC-FLD method is high selectivity, which means IEA only reacts with thiophenols under certain conditions, and acids, amines, and alcohols cannot interfere. For the foregoing reasons, the quantification of thiophenols in complex matrix can be practical and the analysis results are reliable.
Analysis of thiophenols in industrial wastewater
The proposed HPLC-FLD method was successfully applied for the analysis of six thiophenols in two industrial wastewater samples. The compounds were quantified by the corresponding peak area and analytical curves and verified by MS. The results indicated that only thiophenol was detected and the contents were, respectively, 5.21 and 3.78 μg L−1. Tfm, Btf, Pto, Dic, and Phe were not detected probably because their contents in samples were lower than detection limits. Figure 5 shows representative chromatograms (a: thiophenols standard; b and c: thiophenols derivatives in real sample).
The representative chromatograms for six thiophenols standards (a), industrial wastewater in Qufu (b), and industrial wastewater in Tengzhou (c). Column: ZORBAX Eclipse XDB-C18 (250 × 4.6 mm, 5 μm). Detection: fluorescence (λex/λem = 256/420 nm). Flow rate: 1 mL min−1. Injection volume: 10 μL. Standard thiophenols concentration: 300 μM for Btf; 100 μM for Tfm, Pto, and Dic; 50 μM for Thi and Phe
Comparisons of the proposed method with previously reported methods
As we can see from Table 3, several literatures reported the detection of thiophenols. Among them, water is widely used as sample and SPE is the most often used extraction method. The volume of sample is between 3 and 50 mL. The mainly used methods are GC [37], HPLC-UV [41], and fluorescence detection [42, 43]. As we can see from Table 3, the proposed method is distinguished by its high sensitivity. GC is a useful tool, but its sensitivity for thiophenols is not satisfying. HPLC-UV is an easier and cheaper analytical method, but interferences often occur and sensitivity is always keeping in a low level. Even though the sensitivity of few colorimetric fluorescence methods [43] was on the same level or a bit higher than the proposed method, they usually have limitations because they can only provide the total content of target compounds. This new method not only can provide the accurate content of each analyst but also has a low sensitivity of 0.0057–0.02 μM. More importantly, the synthesis of IEA needed only three steps with low cost, and the HPLC analysis could be separated within 12 min. This proposed method brings great help and much convenience and for HPLC users.
Conclusions
On the basis of the reaction between iodine and thiol group, a new fluorescent reagent of 9-(2-iodoethyl) acridone (IEA) was prepared for the simultaneous determination of six thiophenols in industrial wastewater samples by HPLC-FLD. The introduction of Pb2+-loaded solid phase extraction coupled with IEA derivatization greatly improves the detection sensitivity. Acids, amines, and alcohols did not interfere with derivatization reaction of thiophenols with IEA reagent. The proposed method offers a number of advantages: (1) Derivatization of thiols used IEA is relatively simple with little matrix interference; (2) derivatives are not only sensitive to fluorescence but also to MS ionization; (3) maximal yields close to 100 % are observed with a 10-fold molar reagent excess. In addition, the method exhibited high stability, good selectivity, low cost, and broad linear range with low detection limits (0.6–5.8 ng L−1 or 0.0057–0.02 μM). The established method was successfully applied to determine thiophenols in industrial wastewater samples with satisfied results. A possible disadvantage of this method is that the reaction of IEA with thiols in derivatization solution must be kept a small amount of water as far as possible.
References
Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev. 2005;105(4):1103–70.
Eychmüller A, Rogach AL. Chemistry and photophysics of thiol-stabilized II-VI semiconductor nanocrystals. Pure Appl Chem. 2000;72(1–2):179–88.
Hell TP, Lindsay RC. Toxicological properties of thio‐and alkylphenols causing flavor tainting in fish from the upper Wisconsin River. J Environ Sci Health B. 1989;24(4):349–60.
Amrolia P, Sullivan SG, Stern A, Munday R. Toxicity of aromatic thiols in the human red blood cell. J Appl Toxicol. 1989;9(2):113–8.
Santangelo F, Witko-Sarsat V, Drüeke T, Descamps-Latscha B. Restoring glutathione as a therapeutic strategy in chronic kidney disease. Nephrol Dial Transplant. 2004;19(8):1951–5.
Kand D, Mishra PK, Saha T, Lahiri M, Talukdar P. BODIPY based colorimetric fluorescent probe for selective thiophenol detection: theoretical and experimental studies. Analyst. 2012;137(17):3921–4.
Jung HS, Chen X, Kim JS, Yoon J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem Soc Rev. 2013;42(14):6019–31.
Sun W, Li W, Li J, Zhang J, Du L, Li M. Naphthalimide-based fluorescent off/on probes for the detection of thiols. Tetrahedron. 2012;68(27):5363–7.
Yao Z, Bai H, Li C, Shi G. Colorimetric and fluorescent dual probe based on a polythiophene derivative for the detection of cysteine and homocysteine. Chem Commun. 2011;47(26):7431–3.
Li J, Zhang C-F, Yang S-H, Yang W-C, Yang G-F. A coumarin-based fluorescent probe for selective and sensitive detection of thiophenols and its application. Anal Chem. 2014;86(6):3037–42.
Ševčíková P, Glatz Z. Specific determination of cysteine in human urine by capillary micellar electrokinetic chromatography. J Sep Sci. 2003;26(8):734–8.
Carlucci F, Tabucchi A. Capillary electrophoresis in the evaluation of aminothiols in body fluids. J Chromatogr B. 2009;877(28):3347–57.
Mestres M, Busto O, Guasch J. Chromatographic analysis of volatile sulphur compounds in wines using the static headspace technique with flame photometric detection. J Chromatogr A. 1997;773(1):261–9.
Valerio A, Baldo G, Tessari P. A rapid method to determine plasma homocysteine concentration and enrichment by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(4):561–7.
Amarnath K, Amarnath V, Amarnath K, Valentine HL, Valentine WM. A specific HPLC-UV method for the determination of cysteine and related aminothiols in biological samples. Talanta. 2003;60(6):1229–38.
Kuśmierek K, Głowacki R, Bald E. Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography. Anal Bioanal Chem. 2006;385(5):855–60.
Ferin R, Pavão ML, Baptista J. Methodology for a rapid and simultaneous determination of total cysteine, homocysteine, cysteinylglycine and glutathione in plasma by isocratic RP-HPLC. J Chromatogr B. 2012;911:15–20.
Janeš L, Lisjak K, Vanzo A. Determination of glutathione content in grape juice and wine by high-performance liquid chromatography with fluorescence detection. Anal Chim Acta. 2010;674(2):239–42.
Yue Y, Guo Y, Xu J, Shao S. A Bodipy-based derivative for selective fluorescence sensing of homocysteine and cysteine. New J Chem. 2011;35(1):61–4.
Ma J, Gu Y, Chen B, Yao S, Chen Z. High-performance liquid chromatography-electronspray ionization mass spectrometry for determination of tiopronin in human plasma. J Chromatogr A. 2006;1113(1):55–9.
Seiwert B, Karst U. Simultaneous LC/MS/MS determination of thiols and disulfides in urine samples based on differential labeling with ferrocene-based maleimides. Anal Chem. 2007;79(18):7131–8.
Guo X-F, Wang H, Guo Y-H, Zhang Z-X, Zhang H-S. Simultaneous analysis of plasma thiols by high-performance liquid chromatography with fluorescence detection using a new probe, 1, 3, 5, 7-tetramethyl-8-phenyl-(4-iodoacetamido) difluoroboradiaza-s-indacene. J Chromatogr A. 2009;1216(18):3874–80.
Park SK, Boulton RB, Noble AC. Automated HPLC analysis of glutathione and thiol-containing compounds in grape juice and wine using pre-column derivatization with fluorescence detection. Food Chem. 2000;68(4):475–80.
Wang H, Liang S-C, Zhang Z-M, Zhang H-S. 3-Iodoacetylaminobenzanthrone as a fluorescent derivatizing reagent for thiols in high-performance liquid chromatography. Anal Chim Acta. 2004;512(2):281–6.
Liang S-C, Wang H, Zhang Z-M, Zhang H-S. Determination of thiol by high-performance liquid chromatography and fluorescence detection with 5-methyl-(2-(m-iodoacetylaminophenyl) benzoxazole. Anal Bioanal Chem. 2005;381(5):1095–100.
Guo X-F, Zhao P-X, Wang H, Zhang H-S. Simple and rapid determination of thiol compounds by HPLC and fluorescence detection with 1, 3, 5, 7-tetramethyl-8-phenyl-(2-maleimide) difluoroboradiaza-s-indacene. J Chromatogr B. 2011;879(32):3932–6.
Ivanov A, Nazimov I, Baratova L. Determination of biologically active low-molecular-mass thiols in human blood: I. Fast qualitative and quantitative, gradient and isocratic reversed-phase high-performance liquid chromatography with photometric and fluorescence detection. J Chromatogr A. 2000;895(1):157–66.
Tcherkas Y, Denisenko A. Simultaneous determination of several amino acids, including homocysteine, cysteine and glutamic acid, in human plasma by isocratic reversed-phase high-performance liquid chromatography with fluorimetric detection. J Chromatogr A. 2001;913(1):309–13.
Ling BL, Dewaele C, Baeyens W. Micro liquid chromatography with fluorescence detection of thiols and disulphides. J Chromatogr A. 1991;553:433–9.
Higashi Y, Yamashiro M, Yamamoto R, Fujii Y. HPLC analysis of bucillamine by derivatization with N-(1-Pyrenyl) maleimide in human blood. J Liq Chromatogr Relat Technol. 2003;26(19):3265–75.
Bald E, Chwatko G, Glowacki R, Kusmierek K. Analysis of plasma thiols by high-performance liquid chromatography with ultraviolet detection. J Chromatogr A. 2004;1032(1):109–15.
Cocco L, Martelli AM, Billi AM, Matteucci A, Vitale M, Neri LM, et al. Changes in nucleosome structure and histone H3 accessibility: iodoacetamidofluorescein labelling after treatment with phosphatidylserine vesicles. Exp Cell Res. 1986;166(2):465–74.
Imai K, Toyo’oka T, Watanabe Y. A novel fluorogenic reagent for thiols: ammonium 7-fluorobenzo-2-oxa-1, 3-diazole-4-sulfonate. Anal Biochem. 1983;128(2):471–3.
Kang L, You J, Sun Z, Wang C, Ji Z, Gao Y, et al. LC Determination of trace biogenic amines in foods samples with fluorescence detection and MS identification. Chromatographia. 2011;73(1–2):43–50.
You J, Zhang W, Zhang Q, Zhang L, Yan C, Zhang Y. Development of a precolumn derivatization method for the determination of free amines in wastewater by high-performance liquid chromatography via fluorescent detection with 9-(2-hydroxyethyl) acridone. Anal Chem. 2002;74(1):261–9.
Xe Z, Wang H, Ding C, Suo Y, Sun J, Chen G, et al. Determination of free fatty acids from soil and bryophyte by HPLC with fluorescence detection and identification with mass spectrometry. Chin J Anal Chem. 2006;34(2):150–5.
Beiner K, Popp P, Wennrich R. Selective enrichment of sulfides, thiols and methylthiophosphates from water samples on metal-loaded cation-exchange materials for gas chromatographic analysis. J Chromatogr A. 2002;968(1):171–6.
You J, Lao W, You J, Wang G. Characterization and application of acridine-9-N-acetyl-N-hydroxysuccinimide as a pre-column derivatization agent for fluorimetric detection of amino acids in liquid chromatography. Analyst. 1999;124(12):1755–60.
You J, You J, Lao W, Wang G, Jia X. Fluorescence properties of carbazole-9-ylpropionic acid and its application to the determination of amines via HPLC with fluorescence detection. Analyst. 1999;124(3):281–8.
Fan X, You J, Kang J, Ou Q, Zhu Q. New reagents for determination of amino acids by liquid chromatography with pre-column fluorescence derivatization. Anal Chim Acta. 1998;367(1):81–91.
Wang T, Chamberlain E, Shi H, Adams CD, Ma Y. Identification of hydrolytic metabolites of dyfonate in alkaline aqueous solutions by using high performance liquid chromatography–UV detection and gas chromatography–mass spectrometry. Int J Environ Anal Chem. 2010;90(12):948–61.
Jiang W, Cao Y, Liu Y, Wang W. Rational design of a highly selective and sensitive fluorescent PET probe for discrimination of thiophenols and aliphatic thiols. Chem Commun. 2010;46(11):1944–6.
Wang Z, Han D-M, Jia W-P, Zhou Q-Z, Deng W-P. Reaction-based fluorescent probe for selective discrimination of thiophenols over aliphaticthiols and its application in water samples. Anal Chem. 2012;84(11):4915–20.
Acknowledgments
The research was supported by the National Natural Science Foundation of China (nos. 21475075 and 21275089).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 189 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Lv, Z., Sun, Z. et al. Determination of thiophenols with a novel fluorescence labelling reagent: analysis of industrial wastewater samples with SPE extraction coupled with HPLC. Anal Bioanal Chem 408, 3527–3536 (2016). https://doi.org/10.1007/s00216-016-9429-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9429-8