Abstract
A pre-column derivatization method for the simple, sensitive determination of biogenic amines using 10-ethyl-acridine-3-sulfonyl chloride (EASC) as labeling reagent with fluorescence detection and mass spectrometry (MS) identification has been developed. After pre-column derivatization, the labeled biogenic amines were separated on a Hypersil BDS-C18 column by gradient elution. The derivatives showed an intense protonated molecular ion corresponding m/z [M + H]+ in positive-ion mode. The collision-induced dissociation of protonated molecular ion formed specific fragment ions at m/z 196.5, m/z 222.7, m/z 224.4 and m/z 272.5, m/z 286.2. Satisfactory linear responses were observed at the concentration range of 0.02–10 μmol L−1 with coefficients of >0.9993. Detection limits obtained by the analysis of a derivatized standard containing 0.2 pmol of each biogenic amine, were from 20.22 to 109.2 fmol (at a signal-to-noise ratio of 3). The relative standard deviations of retention times and peak areas for each biogenic amine were <0.96 and 3.22%, respectively. Recoveries except for PUT were in the range of 96.7–103.6% for chicken sausage and 95.8–104.6% for pork sausage The established method for the determination of biogenic amines except for PUT from real samples was satisfactory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biogenic amines are nitrogenous and low molecular weight organic compounds of aliphatic, aromatic or heterocyclic structures that are mainly formed by microbial decarboxylation of the corresponding amino acid, with the exception of physiological polyamine [1]. They can be found in many beverages, fermented and spoiled foods, such as cheese, soy bean products, wine, beer, fish products and aged meat [2]. Many biogenic amines have powerful physiological effects (e.g., histamine, tyramine) and an important biological activity [3]. Moreover, secondary amines such as putrescine and cadaverine play an important role in food poisoning as they can potentiate the toxicity of histamine [4]. Low levels of biogenic amines in food are not considered as a serious risk. However, their presence in excessive amounts may induce several health disorders such as nausea, respiratory discomfort, hot flushes, cold sweat, palpitations, headaches, red rash, hyper/hypo tension, etc. [5]. The determination of biogenic amines in foods is of great interest not only due to their toxicity, but also because they can be a good indication of the freshness of food. For these reasons, it is important to monitor biogenic amine levels in foods.
Biogenic amines are usually determined by thin layer chromatography (TLC) [6, 7], capillary electrophoresis (CE) [8–10], gas chromatography (GC) [11, 12] and liquid chromatography (LC) with ultra-violet (UV) or fluorescence detection [13–23, 25]. Among these methods, LC is the most popular and frequently used for the separation and quantification of biogenic amines. However, only few positive confirmations using mass spectrometry after LC have been reported.
Biogenic amines exhibit low absorption at the visible or ultraviolet wavelengths, and no fluorescence, pre-column or post-column chemical derivatization is usually performed to increase the sensitivity and selectivity in determining biogenic amines. There are many derivatization reagents usually applied for biogenic amines analysis such as bis-3,5-dinitrobenzoyl chloride (DNBZ-Cl) [13], dabsyl chloride (Dbs-Cl) [14], benzoyl chloride [15], 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) [16], [2-ethylhexyl] sulphosuccinate (AOT) [17], dansyl chloride (Dns-Cl) [18], 9-fluorenylmethyl chloroformate (FMOC) [19], o-phthalaldehyde (OPA) [20], N-hydroxysuccinimidyl fluorescein-o-acetate (SIFA) [21], 1,2-naphthoquinone-4-sulfonate (NOS) [22] and 4-chloro-3,5-dinitrobenzotrifluoride (CNBF) [23]. In spite of the popularity of these pre-column methods, there have also been many reports describing various shortcomings in their applications, such as short detection wavelengths, poor reproducibility and stability, redundant hydrolysates, incomplete reactions and serious interference for the determination of real samples.
In this study, a simple and sensitive method for the determination of biogenic amines in food samples by pre-column labeling with 10-ethyl-acridine-3-sulfonyl chloride was described. This reagent showed many advantages including (1) reaction with primary and secondary amino groups and aromatic primary amino groups in the presence of sodium borate buffer (pH 9.0) can be carried out at mild condition without pretreatment steps to remove excess of reagent; (2) EASC was stable both in crystal state and in solution in the dark, and the derivatives were stable at room temperature in acidic conditions (pH 6–7); (3) EASC contains nitrogen and carbonyl oxygen atoms in contraposition, resulting in intramolecular isomerization easily and making an improvement to the ionizable efficiency of EASC biogenic amine derivatives with ESI–MS in positive-ion detection mode [24]. To the best of our knowledge, this is the first time that EASC fluorescent probe was applied to determine biogenic amines. In this study, the optimal derivatization conditions such as buffer pH, reaction time and temperature were investigated. Linearity, detection limits and precision of the proposed method were also evaluated. At the same time, the method reported here has been successfully applied for the determination of biogenic amines from two food samples.
Experimental
Instrumentation
Experiments were performed using 1100 Series LC/MSD-Trap-SL liquid chromatograph-mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). The chromatographic systems consisted of an Agilent 1100 Series LC instrument equipped with a vacuum degasser (model G1322A), a quaternary pump (model G1311A), an autosampler (model G1329A), a thermostated column compartment (model G1316A), a fluorescence detector (model G1321A) and a diode array detector (DAD) (model G1315A). Derivatives were separated on a Hypersil BDS-C18 column (200 mm × 4.6 mm, 5 μm, Dalian Yilite, China). The LC system was controlled by HP Chemstation software. Mass spectrometer was equipped with an electrospray ionization (ESI) source; dry temperature, 350 °C; nebulizer, 35.00 psi; dry gas, 9.0 L min−1. The mass spectrometer system was controlled by Esquire-LC NT software, version 4.1. Fluorescence excitation and emission wavelength were obtained at F-7000 fluorescence spectrophotometer (Hitachi). The mobile phase was filtered through a 0.2 μm nylon membrane filter (Alltech, Deerfiled, IL, USA).
Chemicals
Putrescine (PUT), histamine (HIS), 2-phenylethylamine (2-PHE), cadaverine (CAD), 1,6-hexamethylenediamine (HEX), tyramine (TYR), spermidine (SPD) and spermine (SPM) were from Sigma (St. Louis, MO, USA). LC grade acetonitrile (ACN) was from Yucheng Chemical Reagent (Shandong Province, China). N,N-Dimethylformamide (DMF) and acetic acid was analytical grade from Shanghai Chemical Reagent (Shanghai, China). Water was purified on a Milli-Q system (Millipore, Bedford, MA). EASC was synthesized in our laboratory [24]. Other chemicals used were analytical grade from Jining Chemical Reagent (Shandong, China).
Preparation of Standard Solution
Individual stock solution of biogenic amines was prepared in ACN and proper DMF. The standard biogenic amines for LC analysis at an individual concentration of 1.0 × 10−3 mol L−1 were prepared by diluting the corresponding stock solution (1.0 × 10−2 mol L−1) of each biogenic amine with ACN. When not in use, all standards were stored at 4 °C. The derivatizing reagent solution (5 × 10−3 mol L−1) was prepared by dissolving 16 mg EASC in 10 mL ACN.
Preparation of Sample Solution
To extract biogenic amines, 8 mL of 5% trichloroacetic acid (TCA) was added to 5 g of each sample, and the mixture was homogenized for 40 min in an ultrasonic bath. The mixture was then centrifuged for 20 min at 3,000 rpm, the supernatant was collected, and the residue was extracted again with 8 mL of 5% TCA. Both supernatants were combined and the final volume was adjusted to 20 mL with 5% TCA. The extract was filtered and then 0.2 mL of each extract was used for derivatization with EASC followed by LC analysis.
Derivatization Procedure
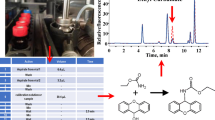
To 10 μL biogenic amines (or 100 μL tested sample supernatants) in a 2 mL vial were successively added 280 μL derivatization reagent (5 × 10−3 mol L−1), and 100 μL of sodium borate buffer (0.1 mol L−1, pH adjusted to 9.0 with NaOH). The vial was then sealed and heated at 50 °C for 15 min in a thermostatic water-bath. After the reaction was completed, the mixture was cooled to room temperature in a water-bath and 10 μL of 50% acetic acid and 200 μL 50% ACN were added. A 10 μL volume of the crude reaction mixture was diluted to 100 μL with acetonitrile. The diluted solution (10 μL) was injected directly onto the LC system. The derivatization process of putrescine is shown in Fig. 1.
Liquid Chromatography
Derivatives were separated on a Hypersil BDS-C18 column (200 mm × 4.6 mm, 5 μm, Dalian Yilite, China) by a gradient elution. Eluent A was 20% of ACN (including 20 mmol L−1 ammonium/formic acid buffer); B was ACN (100%). Gradient conditions were selected at 20–25% (B) from 0 to 10 min; 25–27% (B) from 10 to 22 min; 25–35% (B) from 22 to 30 min; 35–100% (B) from 30 to 40 min. Before injection of the next sample, the column was equilibrated with mobile phase A for 10 min. The flow rate was constant at 1.0 mL min−1 and the column temperature was set at 30 °C. The fluorescence excitation and emission wavelengths were set at λex 270 and λem 430 nm, respectively. The detection and identification of EASC-biogenic amine derivatives were performed by online post-column fluorescence detection and ESI–MS identification.
Results and Discussions
Optimization of Derivatization
The Effects of EASC Concentrations on the Derivatization
EASC, a highly sensitive fluorescence labeling reagent, had good sensitive and selectivity for amino compounds. They can react with amines at low concentration to form stable derivatives under base conditions, and the excessive reagents were hydrolyzed to corresponding sulfonic acid. The hydrolysis compound can be written as 10-ethyl-acridine-3-sulfonic acid (EASC-OH).
Derivatization of biogenic amines (1 × 10−3 mol L−1) with EASC can be achieved within 15 min at 50 °C in the presence of sodium borate buffer (0.1 mol L−1, pH adjusted to 9.0 with NaOH). The effects of EASC concentrations on the derivatization yields were investigated for biogenic amine derivatives. The concentration of EASC was critical for the labelling reaction, the fluorescence intensity of EASC-amine derivatives increased with the increasing amounts of EASC. Maximal fluorescence intensity was achieved with the addition of eightfold molar reagent excess to total molar biogenic amines, further increasing the excess of reagent beyond this level resulted in a slightly decrease in yields. With less than threefold molar excess of derivatization reagent to the total molar amines, the derivatization of biogenic amines was incomplete. To an unknown sample concentration complete derivatization was guaranteed by using a large excess of EASC for constant peak intensity of detector responses. Therefore, all derivatization reactions were performed eightfold molar excess of derivatization reagent to the total molar amines.
The Effect of pH on Derivatization
The derivatization reaction of biogenic amines with EASC is usually accelerated in the presence of base catalyst. To obtain high derivatization yields, several types of basic catalysts were evaluated in this study, including NaOH, Na2CO3, NaHCO3 and Na2B4O7. The results showed that Na2B4O7 was found to be the best choice. The optimum reaction pH was determined by derivatizing each of the eight biogenic amines at pH values ranging from 8.0 to 10.5. The results showed that pH 9.0 may result in maximal sensitivity and high pH value may result in serious hydrolysis. Therefore, we chose pH 9.0 sodium borate buffer as optimal buffer.
There was about a 10–20% loss of the derivatives over a 24 h period, mainly due to the high pH condition resulting in the hydrolysis of EASC-biogenic amine derivatives. However, the derivatized biogenic amines were found to be stable for more than 54 h at room temperature when the derivatization solution was neutralized with 50% acetic acid solution. Therefore, we added 50% acetic acid to neutralize the derivatization solution after cooling to room temperature.
The Effect of Temperature and Time on Derivatization
The effect of temperature on derivatization was also studied. The results indicated that detection responses remarkably increased with the increasing of temperature from 25 to 50 °C except for tyramine, spermidine and spermine. With further higher temperature >50 °C, a slight decrease in detection response was observed. This is probably due to the fact that high derivatization temperature may result in some side-reactions. Taking low side-reaction into consideration, derivatization temperature was set at 50 °C. The reaction time was a critical factor for the derivatization reaction. The effect of reaction time on derivatization yield was studied over the period of 10–40 min, while keeping all the other parameters constant. It was clear that peak areas of all the derivatives of biogenic amines requiring 15 min reached an optimum value. Therefore, reaction temperature of 50 °C and reaction time of 15 min were chosen.
LC Separation for Derivatized Biogenic Amines
For the separation of derivatized biogenic amines, several mobile phase compositions were investigated. They included methanol and acetonitrile in aqueous mixtures. For the simultaneous separation of eight biogenic amine derivatives, an Hypersil BDS-C18 column (200 × 4.6 mm, 5 μm, Dalian Yilite, China) was selected and eluted with (A) 20% of ACN containing 20 mmol L−1 ammonium/formic acid buffer and (B) ACN (100%). The gradient elution (see Experimental section) was used to give the best resolution with shortest retention times, and reduce the interfererence with EASC-amino acid in real samples. The separation for derivatized biogenic amine standards is shown in Fig. 2. As can be seen from Fig. 2, the presence of by-products did not interfere with the separation of standard biogenic amine derivatives under the proposed conditions.
Chromatogram for 25 pmol biogenic amine standards. The column temperature was 30 °C; the excitation and emission wavelengths were λex 270 nm and λem 430 nm; PUT putrescine, HIS histamine, 2-PHE 2-phenylethylamine, CAD cadaverine, HEX 1,6-hexamethylenediamine, TYR tyramine, SPD spermidine, SPM spermine, EASC-OH 10-ethyl-acridine-3-sulfonic acid
Identification with ESI–MS
The ionization and fragmentation of the isolated derivatized biogenic amines using EASC as labeling reagent was studied by mass spectrometry with electrospray ionization source detection in positive-ion detection mode. As expected, the derivatized biogenic amines exhibited excellent ionizable efficiency. Derivatives show intense protonated molecular ion corresponding m/z [M + H]+ in positive-ion mode. All molecular ions [M + H]+ and corresponding fragment ions for eight biogenic amine derivatives are shown in Table 1. The MS and MS–MS spectra of representative putrescine derivative are shown in Fig. 3a, b. For example, the EASC-putrescine derivative produced an intense molecular ion peak at m/z 659.5. The collision induced dissociation of the molecular ion forms corresponding characteristic fragment ions at m/z 222.7 corresponding to the 10-ethyl-acridine core structure moiety. In addition, the collision induced dissociation of the molecular ion at m/z [M + H]+ for the EASC-putrescine derivative formed corresponding fragment ions at m/z 356.6, m/z 272.5, m/z 224.4, m/z 263.5, m/z 196.6 and m/z 436.2 (Fig. 3b). The cleavage mode is also shown in Fig. 3c. The pattern of fragmentation of EASC-derivatives was similar to other amino compounds and phenolic derivatives as observed in our laboratory (data not shown). Using ion-trap ESI–MS, a modified McLafferty rearrangement mechanism give rise to the specific fragment ion at m/z 222.7, this fragment ion from the cleavage of the C–S bond, followed by the rearrangement of one hydrogen atom toward the 10-ethyl-acridine nucleus. This is a significant improvement to the fragmentation efficiency of EASC amine derivatives. The selected reaction monitoring, based on the m/z [M + H]+ → m/z 222.7 transition, was specific for biogenic amine derivatives. Although other endogenous phenolic and amino compounds present in food samples are presumably co-extracted and derivatized by EASC, no interferences are observed due to using specific parent m/z and the corresponding characteristic fragment ion in the m/z [M + H]+ 222.7 transition.
Methods Validity
Detection Limits and Linearity
Detection limits were an important consideration when the components of biological matrices were analyzed, particularly when they were present at low or trace concentrations. Based on the injection of 0.2 pmol (0.02 μmol L−1) of each biogenic amine derivatives, the calculated detection limits (at a signal-to-noise ratio = 3:1) for each derivatized biogenic amine were from 20 to 109 fmol (2.02–10.92 nmol L−1) (Table 2). To establish calibration curves, we prepared six concentration levels of the biogenic amine standards in the concentration range from 0.02 to 10 μmol L−1. A linear calibration curve was constructed using the regression of the peak area versus concentration. All of the biogenic amines were found to give satisfactory linear responses over this range, with correlation coefficients of >0.9993 (Table 2).
Repeatability, Precision and Accuracy
The repeatability of the method was established under the optimized conditions with fluorescence detection. A standard containing 25 pmol EASC biogenic amine derivatives was prepared to examine the method repeatability by injecting six times on the same day (intra-day) and over successive 6 days (inter-day). The relative standard deviations of retention times and peak areas for each biogenic amine were <0.96% and 3.22%, respectively (Table 2). The precisions were examined by using three chicken sausage samples, which were respectively spiked with 0.05, 0.1, and 1 μmol L−1 of biogenic amine. The intra- and inter-day precision expressed as RSD, proved to be equal to or lower than 5.68%. The accuracy was evaluated with recoveries of biogenic amines from chicken sausage and pork sausage samples. In the two sample extracts, a known amount of biogenic amines except for PUT was added, respectively (Table 3). Then the extract was derivatized with EASC as described in text and the analyses were carried out (n = 5). Table 3 shows satisfactory results for biogenic amines except for PUT, with recoveries ranging from 96.7 to 103.6% for chicken sausage and from 95.8 to 104.6% for pork sausage. Therefore, all these biogenic amines except for PUT can be quantified with the proposed method under the selected conditions (here, PUT is not determined due to a large disturbance peak introduced by the real sample).
Comparison of Detection Limits of the Reagents Reported for Biogenic Amines
The EASC core structure exhibits a rigid plane, which contains nitrogen and carbonyl oxygen atoms. It is the fact that nitrogen and carbonyl are in contraposition, resulting in a large n–π conjugation system and giving rise to a strong fluorescence response. The comparison of EASC with recently developed labeling reagents with respect to the sensitivity for the derivatized biogenic amines is summarized in Table 4. As can be seen from Table 4, EASC provided high sensitivity for the labeled biogenic amines with the detection limits in the range of 20.22–109.2 fmol. This is at an equivalent level with the reported excellent labeling reagents, such as CEOC-Cl, and much lower than OPA [20], AQC [16], FMOC-Cl [19] and Dns-Cl [18] which were pmol level. Moreover, OPA can only react with the primary amines, and the derivatives are not very stable. AQC method is rapid and convenient and gives stable derivatives. However, only 10% of the fluorescent intensity in aqueous solution relative to that in pure acetonitrile solution is observed for its derivatives. Thus, the detection limits for the early eluted amine derivatives are usually higher than the latter. Dns-Cl forms derivatives not only with primary but also secondary amines, furthermore, the products are more stable than those formed by using OPA. EASC has the same function group reacting with amines as Dns-Cl, so it can easily react with primary, secondary amines and aromatic primary amino groups to form stable derivatives. The EASC derivatization for quantitative analysis of biogenic amines can also be carried out at mild condition without pretreatment steps to remove excess of reagent, and derivatization can be completed as rapidly as that of chloroformates reagent. Moreover, chloroformate should be carefully stored to avoid humidity of the air, while EASC was stable both in crystal state and in solution in the dark. Comparing to CEOC-Cl core structure, EASC has a carbonyl in 9-position, which would result in a large n–π conjugation system and give rise to a strong fluorescence response. Therefore, EASC is of prospective significance as a pre-column derivatizing reagent for biogenic amines in terms of sensitivity and stability.
Applications
In the analysis of five food samples, peak identification was based on the comparison between the retention times of standard compounds and confirmed by online ESI–MS. Quantification was based on the external standard method using calibration curves fitted by linear regression analysis. Figure 4 showed the application of this method for the determination of biogenic amines present in a chicken sausage sample. As can be seen from Fig. 4a, the content of the relevant biogenic amines in the chicken sausage sample exhibited significant difference, the major biogenic amines in chicken sausage sample were tryptamine (0.15 ± 0.01 ng g−1), spermidine (0.20 ± 0.03 ng g−1), spermine (0.08 ± 0.02 ng g−1). The content of biogenic amines in pork sausage were cadaverine (0.17 ± 0.01 ng g−1), tryptamine (0.17 ± 0.02 ng g−1), spermidine (2.94 ± 0.02 ng g−1), spermine (1.49 ± 0.01 ng g−1) (Fig. 4b), The results indicate that the main contents of biogenic amines in the tested samples were cadaverine, tryptamine, spermidine and spermine.
LC–FL chromatogram of biogenic amine extracted from chicken sausage (a) and pork sausage (b) (Chromatographic conditions and peaks as Fig. 2)
The established method was suitable for the determination of the biogenic amine composition from foods with satisfactory results. Except for biogenic amines, there are some amine acids existing in the analyzed samples. To exclude the interference of amino acids in samples, the retention times of familiar amino acid derivatives were investigated. It was found that under the selected chromatographic condition, the peaks of amino acid derivatives and those of EASC by-products overlapped. That is, retention times of biogenic amines were much longer than those of amino acids. Therefore, amino acids do not interfere the analysis. The facile EASC-Cl derivatization coupled with mass spectrometry identification allowed the development of a highly sensitive and specific method for the quantitative analysis of trace levels of biogenic amines from food or natural environmental samples.
Conclusions
The study described here introduces a highly sensitive and selective method for biogenic amines determination using EASC as labeling reagent with superior properties including convenient derivatization procedure and excellent sensitivity. Complete derivatization in the presence of sodium borate buffer at 50 °C took not longer than 15 min. The improved performance of the reagent EASC for the quantitative analysis of amines has been demonstrated in details. The biogenic amines derivatives were stable under room temperature in acetonitrile–water samples. The LC separation for the derivatized biogenic amines showed good repeatability. Detection limits were in the femtomole range. Current studies should further explore the derivatization of different amino containing compounds such as aromatic amines and amino acids.
References
Santos MHS (1996) J Food Microbiol 29:213–231. doi:10.1016/0168-1605(95)00032-1
Önal A (2007) J Food Chem 103:1475–1486. doi:10.1016/j.foodchem.2006.08.028
Shalaby AR (1996) J Food Res Int 29:675–690. doi:10.1016/S0963-9969(96)00066-X
Bjeldanes LF, Schutz DE, Morris MM (1978) J Food Cosmet Toxicol 16:157–159. doi:10.1016/S0015-6264(78)80196-5
Pérez-Serradilla JA, Luque de Castro MD (2008) J Food Chem 111:447–456. doi:10.1021/ac0708801
Lapa-Guimarães J, Pickova J (2004) J Chromatogr A 1045:223–232. doi:10.1016/j.chroma.2004.06.014
Latorre-Moratalla ML, Bover-Cid S, Veciana-Nogués T, Vidal-Carou MC (2009) J Chromatogr A 1216:4128–4132. doi:10.1016/j.chroma.2009.02.045
Jayarajah CN, Skelley AM, Fortner AD, Mathies RA (2007) Anal Chem 79:8162–8169. doi:10.1021/ac071306s
Simó C, Moreno-Arribas MV, Cifuentes A (2008) J Chromatogr A 1195:150–156. doi:10.1016/j.chroma.2008.05.004
Zhang N, Wang H, Zhang ZX, Deng YH, Zhang HS (2008) Talanta 76:791–797. doi:10.1016/j.talanta.2008.04.027
Macfarlane RG, Midgley JM, Watson DG, Evans PD (1990) Insect Biochem 20:305–311. doi:10.1016/0020-1790(90)90048-Y
Awan MA, Fleet I, Thomas CLP (2008) Anal Chim Acta 611:226–232. doi:10.1016/j.aca.2008.01.083
Kirschbaum J, Rebscher K, Brückner H (2000) J Chromatogr A 881:517–530. doi:10.1016/S0021-9673(00)00257-0
Castillo MA, Castells RC (2001) J Chromatogr A 921:121–133. doi:10.1016/S0021-9673(01)00867-6
Paleologos EK, Chytiri SD, Savvaidis IN, Kontominas MG (2003) J Chromatogr A 1010:217–224. doi:10.1016/S0021-9673(03)01068-9
Busto O, Guaschn J, Borrull F (1996) J Chromatogr A 737:205–213. doi:10.1016/0021-9673(96)00022-2
Romero L, Keunchkarian S, Reta M (2006) Anal Chim Acta 565:136–144. doi:10.1016/j.aca.2006.02.054
Loukou Z, Zotou A (2003) J Chromatogr A 996:103–113. doi:10.1016/S0021-9673(03)00558-2
Lozanov V, Benkova B, Mateva L, Petrov S, Popov E, Slavov C, Mitev V (2007) J Chromatogr B 860:92–97. doi:10.1016/j.jchromb.2007.10.020
Lehtoneh P, Saarinen M, Vesanto Riekkola M-L (1992) Z Lebensm Unters Forsch A 1431–4630:434–437. doi:10.1007/BF01197724
Deng YH, Zhang HS, Du XL, Wang H (2008) J Sep Sci 31:990–998. doi:10.1002/jssc.200700399
Garcıa-Villar N, Saurina J, Hern S (2006) Anal Chim Acta 575:97–105. doi:10.1016/j.aca.2006.05.074
Tang T, Shi TY, Qian K, Li PL, Li JQ, Cao YS (2009) J Chromatogr B 877:507–512. doi:10.1016/j.jchromb.2008.12.064
You JM, Zhao HX, Sun ZW, Xia L, Yan T, Suo YR, Li YL (2009) J Sep Sci 32:1351–1362. doi:10.1002/jssc.200800724
You JM, Zhang YK (2002) Chromatographia 56:43–50. doi:10.1007/BF02490245
Acknowledgment
This work was supported by the 100 Talents Programme of The Chinese Academy of Sciences (No. 328).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, L., You, J., Sun, Z. et al. LC Determination of Trace Biogenic Amines in Foods Samples with Fluorescence Detection and MS Identification. Chromatographia 73, 43–50 (2011). https://doi.org/10.1007/s10337-010-1826-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-010-1826-0