Abstract
Alkyl amines are surfactants used as quartz collectors in the reverse flotation of phosphate ores. It is important in both research and industrial practice to quantify amine concentration in the solution and/or wastewater. A simple and rapid method using high-performance liquid chromatography (HPLC) was developed to quantitatively determine the concentration of amine collectors (including dodecylamine, tetradecylamine, hexadecylamine, and octadecylamine) in a mineral flotation system. The method involved a sample derivatization procedure with 9-fluorenyl methoxycarbonyl chloride (FMOC-Cl) and separation/determination by HPLC coupled with a fluorescence detector. The calibration curves showed good linearity (R2 > 0.9956) in the range of 0.20–5.00 µg/mL, and the recoveries of dodecylamine, tetradecylamine, hexadecylamine, and octadecylamine were in the ranges of 84.93–97.64%, 89.93–101.92%, 85.29–98.37%, and 93.57–103.26%, respectively. The method was successfully used to quantify amines in wastewater from flotation experiments and artificial flotation wastewater (amine solution after activated carbon adsorption). The results from the analysis of four amines in the solution demonstrated that the proposed method is suitable for the simultaneous determination of amines in flotation pulp and wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphate ores contain variable amounts of carbonate and silicate gangue minerals. According to the major gangue mineral content, phosphate ore can be classified into siliceous phosphate ore, in which the main gangue mineral is silicate such as quartz [15], calcareous phosphate ore, which contains mainly carbonate gangue minerals (such as calcite and dolomite) [2, 8], and calcareous–siliceous phosphate ore [35, 44]. Flotation technology depends on the type of phosphate mineral and associated gangue minerals. For siliceous phosphate ore, silicate/quartz are the dominant gangue minerals; in general, silicate gangue will float using cationic amine-type collectors at weakly acidic or neutral pH, which also refers to reverse flotation processing [1, 9, 24].
It has been reported that alkyl amines have been widely used in reverse flotation to remove gangue minerals, i.e., quartz and silicate, from siliceous phosphate ore [3, 19, 29]. Unfortunately, residual amines in flotation wastewater will have a detrimental influence on flotation performance when recycled water is reused. Moreover, direct discharge of flotation wastewater without treatment will result in eutrophication of water bodies and is a waste of water resources [32]. Therefore, appropriate technologies are needed for the treatment and reuse of flotation wastewater. In addition, the adsorption behaviour of amines is important for understanding flotation behaviour [10, 21]. The function of flotation reagents is primarily to regulate the surface properties of valuable minerals and gangue minerals to improve the speed and selectivity of flotation separation [34, 37]. The interaction between mineral particles and reagents is studied indirectly by macroscopic methods such as adsorption density and adsorption heat calculation [40]. One common method to determine the adsorption of collectors is the concentration depletion method. For example, total organic carbon (TOC) analysis was used to determine the residue concentration in the solution and then calculate the adsorption of collectors on the surface of mineral particles [11, 16]. However, the amines commercially available for flotation commonly contain several alkyl amines with different carbon chain lengths (C8–C18). Furthermore, the combination of collectors is becoming an important research subject for flotation [30, 39]. Therefore, it would be helpful in both industry practice and research if the proportions of different amines in solution could be quantified. In this regard, a simple and accurate method for determining the concentration of alkyl amines in water and/or pulp is needed.
Diverse analytical methods have been developed for the analysis of aliphatic amines, including gas chromatography (GC) [46], liquid chromatography–mass spectrometry (LC–MS) [25], high-performance liquid chromatography (HPLC) [4, 13, 42], and ultraviolet spectroscopy (UV) [27]. Recently, HPLC has been the most commonly used technique due to its convenience, excellent reproducibility, and reliability. Fluorescence detection (FD) is a good technique for coupling with HPLC for aliphatic amine detection in complex samples due to its high sensitivity. However, the aliphatic amines under consideration here have no UV absorption spectra or fluorescent signal, and thus, chemical derivatization becomes a necessary procedure. Successful results have been recently reported with a variety of derivatization reagents. For the detection of aliphatic amines, a variety of derivatization reaction strategies have been used to produce highly sensitive and stable fluorophore-tagged aliphatic amines, and different reagents are being attempted to obtain a rapid, selective, stable and direct method, such as o-phthalaldehyde (OPA) [5], dansyl chloride (DNS-Cl) [25], naphthalene-2,3-dicarboxaldehyde (NDA) [38], and 4-chloro-7-nitrobenzofurazan (NBD-Cl) [12]. However, OPA does not react with secondary amines and cannot exhibit satisfactory reproducibility, stability, and detection sensitivity, nor is DNS-Cl an ideal derivative reagent, since it is not stable and the derivatized products have the fluorescence quenching phenomenon, thereby reducing the detection sensitivity. NDA needs to react with primary amines in the presence of cyanide (CN). The reagent NBD-Cl requires a high derivatization temperature and long reaction times. The fluorogenic reagent 9-fluorenylmethyl chloroformate, also known as 9-fluorenylmethyloxycarbonyl chloride (FMOC-Cl), has advantages of rapid reaction, high detection sensitivity, and ability to derive primary amine and secondary amine compounds [18, 20, 28, 36, 47]. The derivatives are formed in a reaction time of less than 1 min in a buffered aqueous solution at room temperature and yield stable derivatives. Thus, a sample can be immediately stabilized by derivatization.
There are few references dealing with the use of FMOC-Cl for chromatographic separation of aliphatic amines, although its application to amino acid separation has been well documented [6, 26]. Lopez et al. [23] described an FMOC-Cl derivatization method for dimethylamine separation in groundwater. Juan Xie [43] described a method based on ultrasound-assisted dispersive liquid–liquid microextraction after pre-column derivatization coupled with high-performance capillary electrophoresis for the determination of methylamine, ethylamine, dimethylamine and diethylamine in aquatic samples. However, FMOC-Cl has not been previously applied to the chromatographic determination of dodecylamine, tetradecylamine, hexadecylamine, and octadecylamine. This study intended to develop a simple and rapid HPLC method for the simultaneous determination of amines in flotation systems based on FMOC-Cl derivatization. The fluorogenic reagent 9-fluorenylmethyl chloroformate has been used, because alkyl amines react with this reagent very rapidly, producing highly fluorescent derivatives.
Materials and Methods
Materials and Reagents
Reagents and Solutions
All chemicals were of analytical or reagent grade or the highest purity available from several suppliers and were used as received. Standard amines (C12: dodecylamine; C14: tetradecylamine; C16: hexadecylamine; C18: octadecylamine) were purchased from Chengdu Aikeda Chemical Reagent Co. Ltd. (Chengdu, China), and 9-fluorenylmethyl chloroformate was purchased from GL Biochem (Shanghai) Ltd. (Shanghai, China). Supergradient HPLC grade methanol, acetonitrile and isopropanol were obtained from Aladdin Reagent (Shanghai) Co., Ltd. (Shanghai, China). Glacial acetic acid (GR) was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), whereas sodium tetraborate (AR) was obtained from Tianjin Yongda Chemical Reagent Co., Ltd. (Tianjin, China). Sulfuric acid was purchased from Chongqing Chuandong Chemical (Group) Co., Ltd. (Chongqing, China), while powdered activated carbon was obtained from Tianjin Guangfu Technology Development Co., Ltd. (Tianjin, China). Deionized water with a resistivity of over 18.2 MΩ·cm was used in the experiments.
The FMOC-Cl solutions were prepared daily by dissolving 20 mg of pure compound in 10 mL of acetonitrile to obtain a final concentration of 2 mg/mL. A 0.1 mol/L sodium tetraborate solution was prepared by dissolving 9.53 g of sodium tetraborate in 250 mL of DI water.
Standard stock solutions (1000 μg/mL) of four amines were prepared by separately dissolving 0.1 mg of each compound in 1 mL of glacial acetic acid and then diluting to 100 mL with DI water. The same volume of stock solution of four amines (dodecylamine, tetradecylamine, hexadecylamine, and octadecylamine) was taken and then mixed to obtain a 100 μg/mL working solution of mixed standards. Then, the standard working solution was diluted with DI water to six concentrations (0, 0.2, 0.5, 1.0, 2.0, and 5.0 μg/mL). All solutions were stored in a dark refrigerator at 4 °C.
Sample Preparation
Flotation wastewater: A flotation test was conducted on siliceous phosphate ore (quartz is the dominant gangue mineral). Microflotation tests were carried out in an XFGCII flotation machine (Jilin Exploration Machinery Plant, China) with a volume capacity of 40 mL. First, 2.0 g of mineral samples were placed in a plexiglass cell in which 40 mL of water was added. H2SO4 solution was added to adjust the pH of the slurry. The amines were added to the cell with a 3 min conditioning time, and then, flotation was conducted for another 4 min. Approximately 10 mL of pulp was taken from the flotation cell and filtered using filter paper to remove most of the particles. The filtrate was filtered again using 0.45 μm millipore filters to obtain a clear solution. The 10 times diluted solution with DI water was used as the sample for determination of different types of amines present in the solution.
Artificial flotation wastewater adsorbed by activated carbon [17]: A weighed amount (0.1 g) of powder activated carbon was added to amine solutions (50 µg/mL, 100 mL) and shaken continuously for 10 min in a Heheng TS-100C oscillator (Shanghai, China). Approximately 10 mL of pulp was taken from the carbon-amine solution as artificial flotation wastewater. The pulp was filtered using filter paper to remove most of the carbon particles. The filtrate was filtered again using 0.45 μm Millipore filters to obtain a clear solution for determination of the amine type and content in the solution.
Derivatization Procedure
For the solution derivatization method, 50 µL of the sample or the standard working solution, 50 µL of isopropanol, and 50 μL of 0.1 mol/L sodium tetraborate were placed in 2 mL glass vials, and then, 50 μL of the FMOC-Cl (2 mg/mL) solution was added with immediate mixing and allowed to react at 40 °C for 1 min. Then, 50 µL glacial acetic acid was added into the mixed solution to quench the reaction. Finally, a 5 µL aliquot of this mixture was injected into the HPLC for analysis.
Instrumentation and Analytical Conditions
Derivatization of the samples at temperatures up to 40 °C was conducted in 2 mL vials in a DLAB HB120-S (Beijing, China) thermostatic metal bath. Quantitation was performed using a reversed-phase HPLC system equipped with a fluorescence detector. Specifically, chromatography was optimized on an Agilent 1260 HPLC system comprising quaternary pumps (G7111B), an autosampler (G7129A), a thermostatic column compartment (G7116A), and a fluorescence detector (G7121A). Chromatograms and spectral data were collected and processed with Agilent OpenLAB CDS ChemStation software. Separation occurred on a ZORBAXEclipse XDB-C18 analytical column (250 × 4.6 mm, 5 μm) at 30 °C. The C18 column was equilibrated with the mobile phase for 30 min before analysis. Injections (5 µL) were made on the system equilibrated with 93% mobile phase A (methanol) and 7% mobile phase B (DI water) at a constant flow rate (1.0 mL/min). Gradient elution was used to separate the amine derivatives, and the gradient elution programme is listed in Table 1. FMOC-Cl-derivatized analytes were detected using excitation and emission wavelengths of 265–310 nm, respectively [7, 23, 36, 45].
Results and Discussion
Optimization of the HPLC Method
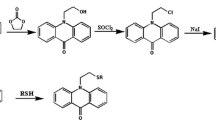
The derivatization reagent FMOC-C1, which reacts with alkyl amines and yields highly fluorescent and stable derivatives, has been used for the derivatization of alkyl amines. The chemical reaction of FMOC-Cl with alkyl amines is illustrated in Fig. 1 [22, 41].
The experimental chromatographic variables were optimized by utilizing the standard solution derivatized using the procedure mentioned before for the best separation in the shortest time with the highest sensitivity. Chromatography was performed in the mode of gradient elution ensuring that there was no overlap with additional peaks arising from the derivatization process. A chromatogram obtained for a standard solution containing each of the analytes at 1.0 mg/L is shown in Fig. 2. All tested derivatives were successfully eluted within 26 min, and satisfactory peaks were observed for the FMOC-Cl analyte derivatives. The peak shapes of the four amine derivatives were symmetrical without impurity interferences, suggesting that the mobile phase elution gradient met the requirement of simultaneously determining the four amine derivatives. Additional peaks were also observed at 6.8 min, 7.7 min, and 9.6 min. Since none of these peaks interfered with the analyte peaks, elimination of the excess reagent was found to be unnecessary.
Optimization of Derivatization Conditions
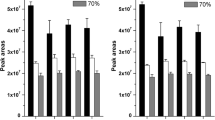
As described in the literature, FMOC-Cl derivatization reactions are typically carried out in an alkaline aqueous phase at ambient temperature for 2–30 min, [14, 31, 33]. The reaction conditions of derivatization were optimized with respect to reaction time and temperature, FMOC-Cl concentration, and pH (Fig. 3). The derivatization reactions were conducted at 25 °C, 40 °C, and 50 °C for 1–45 min. The detected derivative peak areas demonstrated that a mild difference was observed from 25 to 50 °C. However, the best results were obtained at 40 °C, which was selected for subsequent experiments. A reaction time of 1 min was sufficient for complete derivatization of the four amines. After heating for 1 min at 40 °C, maximal conversion to the derivative was achieved. However, extending the reaction time from 5 to 45 min did not obtain enhancement; in contrast, longer heating times reduced the peak area. The effect of pH on derivatization at 40 °C is shown in Fig. 3d. No significant changes in the derivative yield were observed when the reaction mixture was buffered at various pH values between 7.4 and 10.0 by different proportions of H3BO3–Na2B4O7 buffer. In this pH range, the reactions of the amino groups of the amines with FMOC were well developed, and all peaks were well resolved. However, when the pH value was lowered to 8.2, the derivative was unstable. The detected derivative peak areas decreased significantly over storage time. Therefore, a borate buffer with pH 9.18 was chosen for the analysis. The optimal values for pH, derivatization reagent concentration, reaction time, and temperature were 9.18, 2.0 mg/mL, 1 min, and 40 °C, respectively.
Reproducibility of the Proposed Method
FMOC-Cl was used as a derivatization reagent for different amines because of the stability of the derivatives and the mild conditions under which derivatization takes place. The reproducibility of the separation method was evaluated by replicate determinations with direct injection of amines after six derivatizations. Each derivative was evaluated in terms of the reproducibility of retention time and peak area. The results presented in Table 2 show that the relative standard deviations (RSDs) of retention time and peak area for individual amine derivatives were consistently below 0.60%. Thus, the reproducibility of the measurements for all analytes was acceptable.
Linearity, Detection Limit, and Quantification Limit
To verify the linearity of the response of different derivatives, standard solutions of 4 amines at concentrations from 0.2 µg/mL to 5.0 µg/mL were prepared and analysed in duplicate. The peak area was calculated to develop the standard curve for standard solutions with corresponding concentrations. The obtained standard curve regression equations and correlation coefficients are summarized in Table 3. Linearity was evaluated by determining the coefficient of the least square regression (R2). The coefficient of regression exceeded 0.9956 for all standard calibration curves with detection limits of 0.013 µg/mL and quantitation limits of 0.05 µg/mL, indicating the high sensitivity of our method and its competence for simultaneous determination. Method limits of detection (MLODs) and method limits of quantitation (MLOQs) were achieved by analysing the spiked blank samples at levels that provided signals at 3 and 10 times, respectively, above the background noises. The MLOQs were lower than those in previous studies [12] (MLOQs: dodecylamine, 0.46 µg/mL,tetradecylamine, 0.53 µg/mL; hexadecylamine, 0.60 µg/mL; and octadecylamine, 0.67 µg/mL), while the MLODs (MLODs: dodecylamine, 0.009 µg/mL; tetradecylamine, 0.010 µg/mL; hexadecylamine, 0.012 µg/mL; and octadecylamine, 0.013 µg/mL) were almost consistent. In this sense, relatively high analytical sensitivity could be obtained.
Application of the Method
Alkyl amines are surfactants used as quartz collectors in the reverse flotation of phosphate ore. It is important in both research and industrial practice to quantify amine concentration in the solution and/or wastewater. To evaluate the HPLC method described above for practical applications, flotation wastewater and artificial flotation wastewater adsorbed by activated carbon were used for analysis. The results are illustrated in Figs. 4 and 5.
HPLC chromatograms of a blank: (a) artificial flotation wastewater sample adsorbed by activated carbon (b), spiked with 1.0 µg/mL amines (c), spiked with 1.5 µg/mL amines (d), and spiked with 2.0 µg/mL amines (e). Peak number: (1) dodecyl amine, (2) tetradecylamine, (3) hexadecylamine, and (4) octadecylamine
QC (quality control) solutions (1.0 µg/mL, 1.5 µg/mL, and 2.0 µg/mL) were spiked into three batches of matrix blank samples to evaluate the matrix effect: matrix effect (%) = Sp/Ss × 100%, where Ss and Sp are the peak areas of the known QC solution and the matrix blank sample spiked with the QC solution, respectively. The results for the matrix effect showed a satisfactory range from 95.24% to 104.79% (Table 4). Spiked recovery is an important measure of method accuracy and is typically determined by spiking a known quantity of the analyte into a water sample. Recovery was calculated as recovery (%) = 100 × (C–Co)/Cs, where Co represents the mean measured value before spiking, C represents the mean measured value after spiking, and Cs represents the spiked amount (see Table 5). Based on the results for spiked samples, good recoveries were obtained for all amines, with mean recoveries for dodecylamine, tetradecylamine, hexadecylamine, and octadecylamine in the ranges of 84.93–97.64%, 89.93–101.92%, 85.29–98.37%, and 93.57–103.26%, respectively. The RSD values were lower than 3.38%. These data indicated that the proposed method can reliably quantitate the four amines in flotation water samples. The application of the developed method will be of great value in interpreting the results of amine flotation tests and guiding future work, as well as in flotation wastewater treatment.
Conclusion
In this study, a rapid and accurate method was developed to quantify alkyl primary amines (C12–C18) in flotation systems by derivatizing amine solution samples with 9-fluorenyl methoxycarbonyl chloride (FMOC-Cl), separating the derivatized amines by HPLC, and measuring the concentration of each derivatized amine by a fluorescence detector. The mobile phases methanol and DI water were chosen for separating the amines during HPLC analysis. The proposed method has various advantages, such as good correlation coefficients (R2 > 0.9956), low limits of detection (0.013 µg/mL) and quantification (0.05 µg/mL), and high recoveries (84.93–103.26%). The utility of the described approach was evaluated by analysing flotation wastewater and artificial flotation wastewater adsorbed by activated carbon. The proposed method is applicable to the determination of the concentration of the four amines in solutions from flotation pulp.
References
Abouzeid AZM (2008) Physical and thermal treatment of phosphate ores—An overview. Int J Miner Process 85:59–84
Abouzeid AZM, Negm AT, Elgillani DA (2009) Upgrading of calcareous phosphate ores by flotation: effect of ore characteristics. Int J Miner Process 90:81–89
Amirech A, Bouhenguel M, Kouachi S (2018) Two-stage reverse flotation process for removal of carbonates and silicates from phosphate ore using anionic and cationic collectors. Arab J Geosci 11:593
Breitbach ZS, Weatherly CA, Woods RM, Xu C, Vale G, Berthod A, Armstrong DW (2014) Development and evaluation of gas and liquid chromatographic methods for the analysis of fatty amines. J Sep Sci 37:558–565
Chang WY, Wang CY, Jan JL, Lo YS, Wu CH (2012) Vortex-assisted liquid–liquid microextraction coupled with derivatization for the fluorometric determination of aliphatic amines. J Chromatogr A 1248:41–47
Einarsson S (1985) Selective determination of secondary amino acids using precolumn derivatization with 9-fluorenylmethylchloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr A 348:213–220
Einarsson S, Josefsson B, Lagerkvist S (1983) Determination of amino acids with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. J Chromatogr A 282:609–618
El-Shall H, Zhang P, Abdel Khalek N, El-Mofty S (2004) Beneficiation technology of phosphates: challenges and solutions. Miner Metall Process 21:17–26
Fang G, Jun L (2011) Selective separation of silica from a siliceous–calcareous phosphate rock. Mining Sci Technol (China) 21:135–139
Fang J, Ge YY, Yu J (2019) Adsorption behavior and mechanism of an ether amine collector on collophane and quartz. Physicochem Prob Min Process 55:301–310
Han Y, Han S, Kim B, Yang J, Choi J, Kim K, You K, Kim H (2019) Flotation separation of quartz from apatite and surface forces in bubble–particle interactions: Role of pH and cationic amine collector contents. J Ind Eng Chem 70:107–115
Hao F, Lwin T, Bruckard WJ, Woodcock JT (2004) Determination of aliphatic amines in mineral flotation liquors and reagents by high-performance liquid chromatography after derivatization with 4-chloro-7-nitrobenzofurazan. J Chromatogr A 1055:77–85
Huang XF, Kao SJ, Lin J, Qin XF, Deng CR (2018) Development and validation of a HPLC/FLD method combined with online derivatization for the simple and simultaneous determination of trace amino acids and alkyl amines in continental and marine aerosols. PLoS ONE 13:e0206488
Kima J, Kim S, Lee HS, Kim I, Ahn MY, Ryu AKS (2003) Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J Chromatogr A 1002:93–99
Li G, Cao Y, Liu J, Wang D (2012) Cyclonic flotation column of siliceous phosphate ore. Int J Miner Process 110–111:6–11
Li XB, Zhang Q, Hou B, Ye JJ, Mao S, Li XH (2017) Flotation separation of quartz from collophane using an amine collector and its adsorption mechanisms. Powder Technol 318:224–229
Li XB, Ye JJ, Qiu YQ, Li LJ, Mao S, Liu ZH, Zhang Q (2017) Adsorption of residual amine collector HAY from aqueous solution by refined carbon from coal fly ash and activated carbon. J Cent South Univ 24:30–38
Lkhagva A, Shen C, Leung Y, Tai H (2020) Comparative study of five different amine-derivatization methods for metabolite analyses by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1610:460536
Medeirosa ARSD, Baltar CAM (2018) Importance of collector chain length in flotation of fine particles. Miner Eng 122:179–184
Moye HA, Boning AJ (1979) A versatile fluorogenic labelling reagent for primary and secondary amines: 9-fluorenylmethyl chloroformate. Anal Lett 12:25–35
Nunes APL, Peres AEC, Chaves AP, Ferreira WR (2019) Effect of alkyl chain length of amines on fluorapatite and aluminium phosphates floatabilities. J Mater Res Technol 8:3623–3634
Paul CH (2005) HPLC of amines as 9-fluorenyimethyl chloroformate derivatives. J Chromatogr Libr 70:471–501
Rodríguez López M, González Alvarez MJ, Miranda Ordieres AJ, Tuñón Blanco P (1996) Determination of dimethylamine in groundwater by liquid chromatography and precolumn derivatization with 9-fluorenylmethylchloroformate. J Chromatogr A 721:231–239
Ruan Y, He D, Chi R (2019) Review on beneficiation techniques and reagents used for phosphate ores. Minerals 9:253
Ruiz-Jiménez J, Hautala S, Parshintsev J, Laitinen T, Hartonen K, Petäjä T, Kulmala M, Riekkola M (2012) Aliphatic and aromatic amines in atmospheric aerosol particles: comparison of three ionization techniques in liquid chromatography-mass spectrometry and method development. Talanta 97:55–62
Schwarz EL, Roberts WL, Pasquali M (2005) Analysis of plasma amino acids by HPLC with photodiode array and fluorescence detection. Clin Chim Acta 354:83–90
Siddiqi Z, Pathania D (2003) Rapid, selective and direct spectrophotometeric determination of aliphatic amines with m-dinitrobenzene. Talanta 60:1197–1203
Smith LL, Francis KA, Johnson JT, Gaskill CL (2017) Quantitation of fumonisin B 1 and B 2 in feed using FMOC pre-column derivatization with HPLC and fluorescence detection. Food Chem 234:174–179
Snow R, Zhang P (2002) Surface modification for improved phosphate flotation. J Colloid Interface Sci 256:132–136
Suman SK, Kumar S (2020) Reverse flotation studies on iron ore slime by the synergistic effect of cationic collectors. Sep Sci Technol 55:1702–1714
Tandy S, Schulin R, Suter MJF, Nowack B (2005) Determination of [S, S’]-ethylenediamine disuccinic acid (EDDS) by high performance liquid chromatography after derivatization with FMOC. J Chromatogr A 1077:37–43
Thyabat AS, Zoubi AH (2012) Purification of phosphate beneficiation wastewater: Separation of phosphate from Eshydia Mine (Jordan) by column-DAF flotation process. Int J Miner Process 110–111:18–24
Titti E, Cecilia G (2005) Determination of polyamines in human tissues by precolumn derivatization with 9-fluorenylmethyl chloroformate and high-performance liquid chromatography. Anal Biochem 338:179–185
Tripathy SK, Angadi SI, Patra NK, Rao DS (2018) Comparative separation analysis of direct and reverse flotation of dolomite fines. Miner Process Extr Metall Rev 39:339–350
Tuo B, Yang J, Han L, Wang J, Yao Y (2016) Flotation experimental research of calcareous–siliceous phosphorite. Int J Miner Process 146:10–14
Verdú-Andrés J, Campíns-Falcó P, Herráez-Hernández R (2002) Liquid chromatographic determination of aliphatic amines in water using solid support assisted derivatization with 9-fluorenylmethyl chloroformate. Chromatographia 55:129–134
Vidyadhar A, Kumari N, Bhagat RP (2014) Adsorption mechanism of mixed cationic/anionic collectors in quartz-hematite flotation system. Miner Process Extr Metall Rev 35:117–125
Wang CY, Tung SY, Lo YS, Huang HL, Ko CH, Wu CH (2016) Sensitivity enhancement in the fluorometric determination of aliphatic amines using naphthalene-2,3-dicarboxaldehyde derivatization followed by vortex-assisted liquid–liquid microextraction. Talanta 152:475–481
Wang L, Sun W, Hu YH, Xu LH (2014) Adsorption mechanism of mixed anionic/cationic collectors in Muscovite – Quartz flotation system. Miner Eng 64:44–50
Wang XM, Lu Y (2018) Development of surface analytical techniques and their application in mineral engineering—atomic force microscopy. J Guizhou Univ (Nat Sci) 35:1–12
Westerholm R, Li H, Almén J (1993) Estimation of aliphatic amine emissions in automobile exhausts. Chemosphere 27:1381–1384
Xia LJ, Guo XF, Ji Y, Chen L, Wang H (2018) A long-wavelength fluorescent probe for amino compounds and its application in the determination of aliphatic amines. Anal Methods 10:3188–3196
Xie J, Li Y, Zhang J, Zeng L, Lu D, Liu Y, Yang Y, Sun C (2014) Simultaneous determination of four aliphatic amines in aquatic products by ultrasound-assisted dispersive liquid–liquid microextraction coupled with high performance capillary electrophoresis. Anal Methods 6:5140–5146
Ye J, Wang X, Li X, Mao S, Shen Z, Zhang Q (2018) Effect of dispersants on dispersion stability of collophane and quartz fines in aqueous suspensions. J Dispersion Sci Technol 39:1655–1663
Zhang L, Ping GC, Yu XM, Zhang LH, Zhang WB, Zhang YK (2004) Analysis of aliphatic amines by RP-HPLC with pre-column derivatization by 9-fluorenylmethylchloroformate. Mod Instr Med Treatment 14–16:18
Zhao YY, Cai LS, Jing ZZ, Wang H, Yu JX, Zhang HS (2003) Determination of aliphatic amines using N-succinimidyl benzoate as a new derivatization reagent in gas chromatography combined with solid-phase microextraction. J Chromatogr A 1021:175–218
Ziegler J, Abel S (2014) Analysis of amino acids by HPLC/electrospray negative ion tandem mass spectrometry using 9-fluorenylmethoxycarbonyl chloride (Fmoc-Cl) derivatization. Amino Acids 46:2799–2808
Acknowledgements
This work was financially supported by the National Key R&D Program of China (Grant No. 2018YFE0110300)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author(s) declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, X., Zhang, Q., Li, X. et al. An HPLC Method for the Determination of Amines in Flotation Pulp Based on Derivatization. Chromatographia 84, 463–471 (2021). https://doi.org/10.1007/s10337-021-04020-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04020-3