Abstract
A new, simple and efficient method, including dispersive liquid–liquid–solidified floating organic drop microextraction and then electrothermal atomic absorption spectrometry, has been developed for the preconcentration and determination of ultratrace amounts of indium. The method was applied to preconcentrate the indium–1-(2-pyridylazo)-2-naphthol complex in 25 μL 1-undecanol. The various factors affecting the extraction efficiency, such as pH, type and volume of extraction solvent, type and volume of disperser solvent, sample volume, ionic strength, and ligand concentration, were investigated and optimized. Under the optimum conditions, an enrichment factor of 62.5, precision of ±4.75%, a detection limit of 55.6 ng L−1, and for the calibration graph a linear range of 96.0-3360 ng L−1 were obtained. The method was used for the extraction and determination of indium in water and standard samples with satisfactory results.

Preconcentration of indium ions via liquid-liquid-solidified floating organic drop microextraction method and determination by ETAAS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Indium belongs to group 13 of the periodic table and is a crystalline, ductile, very soft, and malleable metal that can maintain its plastic properties at cryogenic temperatures. Also, it is a toxic element, and exposure to indium compounds can cause different types of cancer [1].
The estimated abundance of indium in Earth’s crust is very low (50 mg kg-1), and is similar to that of silver. Like other rare metals, indium can be recovered as a by-product of electrolytic refining of zinc [2].
Use of indium will increase significantly in the next few decades, particularly because of the production of new types of equipment, such as high-definition televisions, liquid crystal displays, semiconductors, different types of batteries, low-temperature solders, infrared photodetectors, and solar cells [3].
Most applications of indium are in the form of indium tin oxide (ITO). ITO is a sintered alloy containing a large portion of indium oxide and a small portion of tin oxide. It is a superior transparent and conductive material and is used extensively for the making of thin-film transistor liquid crystal displays for television screens, cell phone displays, and portable-computer screens [4, 5]. Also, ITO thin film is an optoelectronic material and has some important and unique properties, such as transparency to visible light, electrical conduction, and thermal reflection [6]. Because of the wide range of applications of this compound, more than 50% of the world’s yearly ITO production is used by industry [7].
In recent years, because of widespread applications of indium, its use has increased significantly. Although it is predicted that indium applications will increase in many of the future technology areas, minerals containing indium as a main constituent are unknown. Indium determination is difficult because of its low concentration in most real materials and the high concentration of interferences. The development of extraction and preconcentration methods for this rare metal is therefore very important [8]. Various techniques have been used for the separation and preconcentration of indium, such as cloud point extraction [9], coprecipitation [10], and electrochemical methods [11].
Liquid–liquid extraction is one of the commonest extraction procedures [12–14]. In recent decades, researchers have been interested in improving green analytical methods. The main goal of green analytical chemistry is the substitution or minimization of toxic solvents and reagents. Therefore, studies have focused on miniaturizing the traditional liquid–liquid extraction procedure by decreasing the volume ratio of the organic solvent to the aqueous phase. This has led to the development of microextraction techniques such as homogeneous liquid–liquid microextraction, single drop microextraction, dispersive liquid–liquid microextraction and solid-phase microextraction. The principal advantages of the techniques mentioned are the low volume of the solvents used and their capability to detect analytes at low concentrations [15].
In recent years, a new liquid–liquid microextraction method—namely, solidified floating organic drop microextraction (SFODME), which is a modified solvent extraction method—has been applied for the preconcentration and determination of different analytes. This method has benefits such as very low organic solvent consumption, low cost, simplicity, and a high enhancement factor [16–21].
To the best of our knowledge, there is no previous literature report on the use of dispersive liquid–liquid–SFODME (DLL-SFODME) and electrothermal atomic absorption spectrometry (ETAAS) for the preconcentration and determination of indium ions in real samples. In this procedure (DLL-SFODME), 1-(2-pyridylazo)-2-naphthol (PAN) was used as the complexing agent, 1-undecanol was used as the extraction solvent, and ethanol was used as the disperser solvent. The influence of some important parameters, such as pH, type and volume of extraction solvent, type and volume of disperser solvent, sample volume, ionic strength, and ligand concentration, was studied and optimized.
Experimental
Reagents and standards
Indium(III) stock solution (1000 mg L−1) was purchased from Merck (Darmstadt, Germany). Working standard solutions of indium were prepared freshly at different concentrations by dilution of the stock standard solution with deionized water. The complexing agent (0.05% w/v) solution was prepared by addition of 0.05 g PAN (Merck, Darmstadt, Germany) to a 100-mL volumetric flask and dilution with ethanol. 1-Undecanol, 2-undecanone, 1-hexadecanethiol, acetone, acetonitrile, Pd(NO3)2, hydrofluoric acid, hydrochloric acid, ethanol, and methanol were purchased from Merck (Darmstadt, Germany). A salt solution (10% w/v) was prepared by addition of 10.0 g sodium nitrate (Merck, Darmstadt, Germany) to a 100-mL volumetric flask and dilution to the mark with deionized water. All reagents used were of the highest purity, and deionized water was used throughout. The laboratory glassware and polyethylene tubes were kept in 10% HNO3 (Merck, Darmstadt, Germany) for 24 h, washed with deionized water, and dried.
Instrumentation
All indium measurements were performed with a Varian Spectra AA 220 atomic absorption spectrometer with a deuterium lamp for background correction equipped with a graphite furnace (GTA-110 series). The instrumental parameters for ETAAS were as follows: wavelength, 325.6 nm; spectral bandwidth, 0.5 nm; lamp current, 5 mA; signal measurement, peak height; sample volume, 20 μL; volume of Pd(NO3)2 as a modifier, 5 μL. The ETAAS thermal programs were as follows: drying in three steps (temperatures 85, 95, and 120 °C, times 5, 40, and 10 s, and argon flow rates 3.0 L min-1), ashing in two steps (first step: temperature 600 °C, time 6 s, and argon flow rate 3.0 L min-1; second step: temperature 600 °C, time 2 s, and argon gas off), and atomization (temperature 2700 °C, time 3.3 s, argon gas off and cleanup; temperature 2800 °C, time 2 s, and argon flow rate 3.0 L min-1).
A centrifuge (6000 rpm, model 88-2750, Sahand Teb Aria, Iran) was used for faster separation of the organic phase from the aqueous phase. The pH measurements were done with a Metrohm 827 pH meter.
Suggested procedure
For DLL-SFODME, a 2.0 mL solution containing 20.0 ng indium, 1.0 mL PAN solution (0.05% w/v), and 0.4 mL sodium nitrate solution (10% w/v) was poured into a polyethylene tube and its pH was adjusted to 7 (with 2.0 mL buffer solution). In the next step, 25.0 μL 1-undecanol (as the extraction solvent) was mixed with 500.0 μL ethanol (as the disperser solvent) and dispersed in the previously prepared sample. After the mixture had been centrifuged for 8 min at 3000 rpm, 1-undecanol droplets containing the indium complex floated on the top surface of the sample solution. The tube was inserted into an ice bath until solidification of 1-undecanol droplets occurred. Then, the solidified droplets were collected, transferred into a vial, and allowed to melt. Finally, to facilitate the injection, the volume of melted extraction solvent was increased to 400 μL with ethanol, and the resultant solution was injected.
Sample preparation
Water samples
Two water samples—tap water (Kerman drinking water, Kerman, Iran) and seawater (Caspian Sea, Gilan, Iran)—were chosen. These water samples were filtered to remove the suspended particles, placed in a refrigerator, and the method studied was applied for the determination of indium content.
Standard sample
A 0.1 g standard reference material (SRM 2710) was digested by application of 5 mL of a mixture containing hydrochloric acid, nitric acid, and hydrofluoric acid (4:4:2 v/v/v), transferred to a 100-mL measuring flask, and diluted to the mark with deionized water. An aliquot of this solution was taken and its indium content was measured by the suggested method.
Results and discussion
A combination of DLL-SFODME and ETAAS was used for the preconcentration and determination of ultratrace quantities of indium ions. To obtain the best recoveries, various parameters, such as pH, volume of PAN as the complexing agent, type and volume of disperser and extraction solvents, sample volume, and ionic strength, were studied and optimized. Eventually, the optimum method was used successfully for the determination of indium in some real and standard samples.
Effect of pH
The effect of pH was investigated between 3.0 to 10.0 and the results are reported in Fig. 1. As can be seen, indium was extracted quantitatively in the pH range from 3.8 to 8.5. Thus, further experiments were performed at pH 7 for convenience.
Type and volume of extraction solvent
A suitable solvent for indium extraction has properties such as density lower than that of water, high extraction capability for indium, low solubility in water, and melting point close to room temperature. To achieve impressive preconcentration, the choice of the extraction solvent and its volume have a significant effect in the recommended process. The organic solvents 1-hexadecanethiol, 2-undecanone, and 1-undecanol were studied, and the results show that 1-undecanol has the highest extraction efficiency (98.3%) in comparison with 2-undecanone (0%) and 1-hexadecanethiol (66.7%). So 1-undecanol was selected as the most appropriate extraction solvent.
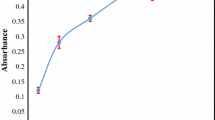
The effect of 1-undecanol volume on the extraction efficiency of indium ions was studied. For this purpose, different volumes of 1-undecanol (5–100 μL) were tested. Figure 2 shows that the minimum required amount of 1-undecanol for the quantitative extraction of indium is 25.0 μL. Therefore, to achieve the highest enrichment factor, 25.0 μL of 1-undecanol was used.
Effect of volume of the extractant solvent on the extraction efficiency of indium. The experimental conditions were the same as for Fig. 1. except for the 1-undecanol volume (pH 7)
Effect of PAN volume
PAN was chosen as a ligand because of its capability to form a strong complex with indium ions. PAN (0.05% w/v) volume was investigated in the range from 0.5 to 1.5 mL (4.4×10-4–1.1×10-3 mol L-1). The results obtained demonstrated that an enhancement in the extraction recovery of indium ions occurs with increasing PAN volume up to 1.0 mL (8.0×10-4 mol L-1) and remains constant for greater volumes. Thus, 1.0 mL PAN solution (0.05% w/v) (8.0×10-4 mol L-1) was used for subsequent experiments.
Type and volume of disperser solvent
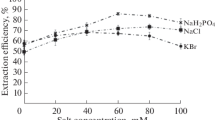
A suitable disperser solvent is miscible with both an aqueous solution and the extraction solvent. To identify the best disperser solvent, ethanol, acetone, methanol, and acetonitrile were tested. The results obtained (Fig. 3) show that the indium extraction efficiency with methanol and ethanol was quantitative, but because of the toxicity of methanol, ethanol was chosen as the disperser solvent.
Effect of disperser solvent type on the extraction efficiency of indium. The experimental conditions were the same as for Fig. 1. except for the disperser solvent (pH 7)
Furthermore, various volumes (50–1000 μL) of ethanol as the disperser agent were investigated. In the range from 100 to 450 μL, the extraction solvent was not sufficiently dispersed and the extraction recovery was not quantitative, but complete dispersal occurred in 500 μL and dispersal was less for greater ethanol volumes. So 500 μL was selected as the optimum volume of the disperser solvent.
Ionic strength
To study the ionic strength effect on the extraction recovery of indium, we performed various experiments by varying the NaNO3 (10% w/v) volume between 0.1 and 0.8 mL. The results show that the extraction recovery increases with increasing NaNO3 volume up to 0.4 mL and remains constant for greater volumes. Therefore, 0.4 mL NaNO3 (10% w/v) was selected as the optimum salt concentration.
Effect of sample volume
The preconcentration capability of the DLL-SFODME system was studied by application of various sample volumes (8–25 mL). The results demonstrated that quantitative preconcentration occurs for all volumes studied. Because of the restriction imposed by the size of the polyethylene tubes, the indium preconcentration procedure was not investigated for volumes larger than 25 mL. On the basis of the final prepared volume of 1-undecanol (400 μL) and the largest sample volume for which the extraction can be quantitative (25 mL), an enrichment factor of 62.5 was obtained.
Interference discussion
The applicability of the DLL-SFODME method for the preconcentration of indium ions in the presence of several anions and cations was studied. The endurance level was described as the maximum quantity of interference that can produce ±5% error in the determination of indium content. The endurance level of each interfering ion was studied (mole ratio of interfering ion to indium of 2000), and if interference was observed, the ratio was decreased until it stopped. Table 1 lists the species studied and their maximum tolerable amounts. As can be seen, various ions did not interfere even at high concentrations, and so this method is suitable for the determination of indium ions in different samples.
Performance characteristics
Under the optimized conditions, the performance characteristics of the recommended DLL-SFODME–ETAAS method were computed by use of indium standard solutions. The dynamic linear range for indium determination was from 96.0 to 3360 ng L−1 in the initial solution. The enrichment factor, detection limit, and quantification limit were 62.5, 55.6 ng L−1, and 185.3 ng L−1 respectively. The method’s relative standard deviation was calculated as ±4.75% by application of seven standard solutions containing indium at 800 ng L-1.
Method applications
To determine the validity of the method, the suggested procedure was used for the determination of indium ions in two water samples (tap water and seawater). The trustiness of the method was studied by the analysis of samples with the addition of known amounts of indium (spiking). As can be seen from Table 2, the recoveries of the spiked samples at the 95% confidence level are satisfactory.
To check the method’s accuracy, this procedure was used for indium determination in a standard reference material (SRM 2710); the results obtained are given in Table 3. As can be seen, the results are in good agreement with the reference values, and there is no considerable difference between the results and the accepted values. Thus, the suggested method is accurate for the determination of indium in different samples.
Comparison with previously reported methods
In Table 4, the performance characteristics of the recommended DLL-SFODME–ETAAS method are compared with those of other indium determination methods [9, 10, 22–30]. The suggested procedure has many advantages, such as high sensitivity, broad linear range, and high preconcentration factor. Also, it has the lowest detection limit, except for two previously reported methods [10, 25], and the best enrichment factor, except for three previously reported methods [10, 22, 24]. Furthermore, only 25.0 μL 1-undecanol was used as the extraction solvent, so this method can be considered as a green and environmentally friendly method.
Conclusion
In the present study, a new method including DLL-SFODME in combination with ETAAS was used for the extraction, preconcentration, and quantification of indium ions. Indium ions can form a strong and stable complex with PAN and preconcentrate in 25.0 μL 1-undecanol. In comparison with the traditional liquid–liquid extraction with high organic solvent consumption, this method uses only 25.0 μL of extraction solvent. Besides its high enrichment capability, some other advantages of the recommended technique are the enhancement of ETAAS sensitivity, simplicity, high speed, and environmental friendliness.
References
Tsuchiya T, Watanabe A, Niino H, Yabe A, Yamaguchi I, Manabe T, et al. Low temperature growth of metal oxide thin films by metallorganic laser photolysis. Appl Surf Sci. 2002;186:173–8.
Downs AJ. Chemistry of aluminum, gallium, indium and thallium. 1st ed. London: Blackie; 1993.
Schwarz-Schampera U, Herzig P. Technological applications and consumption of indium by industries. Indium. 2002;167–73.
Yang HF, Zhang YS, Qiu B, Lin ZY. The electrochemiluminescent behavior of nickel phthalocyanine (NiTSPc)/H2O2 system on a heated ITO electrode. Chin Chem Lett. 2012;23:711–4.
Yoon J, Cho T, Lim H, Kim J. Functionalization of indium tin oxide electrode with both of dendrimer-encapsulated Pt nanoparticles and chemically converted graphenes for enhanced electrochemiluminescence of luminol/H2O2. Anal Bioanal Chem. 2016;408:7165–72.
Alfantazi AM, Moskalyk RR. Processing of indium: a review. Miner Eng. 2003;16:687–94.
Latteyer F, Peisert H, Uihlein J, Basova T, Nagel P, Merz M, et al. Chloroaluminum phthalocyanine thin films: chemical reaction and molecular orientation. Anal Bioanal Chem. 2013;405:4895–904.
Adhikari BB, Gurung M, Kawakita H, Ohto K. Solid phase extraction, preconcentration and separation of indium with methylene crosslinked calix[4]- and calix[6]arene carboxylic acid resins. Chem Eng Sci. 2012;78:144–54.
Mortada WI, Kenawy IM, Hassanien MM. A cloud point extraction procedure for gallium, indium and thallium determination in liquid crystal display and sediment samples. Anal Methods. 2015;7:2114–20.
Minamisawa H, Murashima K, Minamisawa M, Arai N, Okutani T. Determination of indium by graphite furnace atomic absorption spectrometry after coprecipitation with chitosan. Anal Sci. 2003;19:401–4.
Medvecky L, Briancin J. Possibilities of simultaneous determination of indium and gallium in binary InGa alloys by anodic stripping voltammetry in acetate buffer. Chem Pap Slovak Acad Sci. 2004;58:93–100.
Guan J, Zhang C, Wang Y, Guo Y, Huang P, Zhao L. Simultaneous determination of 12 pharmaceuticals in water samples by ultrasound-assisted dispersive liquid–liquid microextraction coupled with ultra-high performance liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem. 2016;408:8099–109.
Fazelirad H, Taher MA, Nasiri-Majd M. GFAAS determination of gold with ionic liquid, ion pair based and ultrasound-assisted dispersive liquid-liquid microextraction. J Anal At Spectrom. 2014;29:2343–8.
Pérez RA, Albero B, Tadeo JL, Sánchez-Brunete C. Determination of endocrine-disrupting compounds in water samples by magnetic nanoparticle-assisted dispersive liquid–liquid microextraction combined with gas chromatography–tandem mass spectrometry. Anal Bioanal Chem. 2016;408:8013–23.
Viñas P, Campillo N, López-García I, Hernández-Córdoba M. Dispersive liquid–liquid microextraction in food analysis. A critical review. Anal Bioanal Chem. 2014;406:2067–99.
Li Y, Peng G, He Q, Zhu H, Al-Hamadani SMZF. Dispersive liquid–liquid microextraction based on the solidification of floating organic drop followed by ICP-MS for the simultaneous determination of heavy metals in wastewaters. Spectrochim Acta A. 2015;140:156–61.
Fazelirad H, Taher MA. Ligandless, ion pair-based and ultrasound assisted emulsification solidified floating organic drop microextraction for simultaneous preconcentration of ultra-trace amounts of gold and thallium and determination by GFAAS. Talanta. 2013;103:375–83.
Viñas P, Campillo N, Andruch V. Recent achievements in solidified floating organic drop microextraction. Trends Anal Chem. 2015;68:48–77.
Diao C-P, Wei C-H. Rapid determination of anilines in water samples by dispersive liquid–liquid microextraction based on solidification of floating organic drop prior to gas chromatography–mass spectrometry. Anal Bioanal Chem. 2012;403:877–84.
López-García I, Rivas RE, Hernández-Córdoba M. Liquid-phase microextraction with solidification of the organic floating drop for the preconcentration and determination of mercury traces by electrothermal atomic absorption spectrometry. Anal Bioanal Chem. 2010;396:3097–102.
Zhou Y, Han L, Cheng J, Guo F, Zhi X, Hu H, et al. Dispersive liquid–liquid microextraction based on the solidification of a floating organic droplet for simultaneous analysis of diethofencarb and pyrimethanil in apple pulp and peel. Anal Bioanal Chem. 2011;399:1901–6.
Hassanien MM, Kenawy IM, Mostafa MR, El-Dellay H. Extraction of gallium, indium and thallium from aquatic media using amino silica gel modified by gallic acid. Microchim Acta. 2011;172:137–45.
Uhrovčík J, Lesný J. Determination of indium in liquid crystal displays by flame atomic absorption spectrometry. J Ind Eng Chem. 2015;21:163–5.
Arslan Y, Kendüzler E, Ataman OY. Indium determination using slotted quartz tube-atom trap-flame atomic absorption spectrometry and interference studies. Talanta. 2011;85:1786–91.
Agnihotri NK, Ratnani S, Singh VK, Singh HB. Simultaneous determination of gallium and indium with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol in cationic micellar medium using derivative spectrophotometry. Anal Sci. 2003;19:1297–301.
Ghasemi J, Zolfonoun E. Ultrasound-assisted ionic liquid-based microextraction combined with least squares support vector machines regression for the simultaneous determination of aluminum, gallium, and indium in water and coal samples. Environ Monit Assess. 2012;184:3971–81.
Hang C, Hu B, Jiang Z, Zhang N. Simultaneous on-line preconcentration and determination of trace metals in environmental samples using a modified nanometer-sized alumina packed micro-column by flow injection combined with ICP-OES. Talanta. 2007;71:1239–45.
Liu H-M, Jiang J-K, Lin Y-H. Simultaneous determination of gallium(III) and indium(III) in urine and water samples with cloud point extraction and by inductively coupled plasma optical emission spectrometry. Anal Lett. 2012;45:2096–107.
Liu HM, Chang CY, Wu CC, Wei JM, Chen WY, Yeh CT. Determination of trace indium in urine after preconcentration with a chelating‐resin‐packed minicolumn. J Sep Sci. 2012;35:846–52.
Wei X-L, Gong Q, Ouyang K, Hong X. Polypropylene based anion exchange fiber for enrichment and determination of trace indium by GFAAS. Bull Chem Soc Ethiop. 2011;25:295–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ashrafzadeh Afshar, E., Taher, M.A., Fazelirad, H. et al. Application of dispersive liquid–liquid–solidified floating organic drop microextraction and ETAAS for the preconcentration and determination of indium. Anal Bioanal Chem 409, 1837–1843 (2017). https://doi.org/10.1007/s00216-016-0128-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0128-2