Abstract
A new analytical method was developed for simultaneous determination of 12 pharmaceuticals using ultrasound-assisted dispersive liquid–liquid microextraction (DLLME) followed by ultra-high performance liquid chromatography with tandem mass spectrometry (UHPLC-MS/MS). Six nonsteroidal anti-inflammatory drugs (NSAIDs, ketoprofen, mefenamic acid, tolfenamic acid, naproxen, sulindac, and piroxicam) and six antibiotics (tinidazole, cefuroxime axetil, ciprofloxacin, sulfamethoxazole, sulfadiazine, and chloramphenicol) were extracted by ultrasound-assisted DLLME using dichloromethane (800 μL) and methanol/acetonitrile (1:1, v/v, 1200 μL) as the extraction and dispersive solvents, respectively. The factors affecting the extraction efficiency, such as the type and volume of extraction and dispersive solvent, vortex and ultrasonic time, sample pH, and ionic strength, were optimized. The ultrasound-assisted process was applied to accelerate the formation of the fine cloudy solution by using a small volume of dispersive solvent, which increased the extraction efficiency and reduced the equilibrium time. Under the optimal conditions, the calibration curves showed good linearity in the range of 0.04–20 ng mL−1 (ciprofloxacin and sulfadiazine), 0.2–100 ng mL−1 (ketoprofen, tinidazole, cefuroxime axetil, naproxen, sulfamethoxazole, and sulindac), and 1–200 ng mL−1 (mefenamic acid, tolfenamic acid, piroxicam, and chloramphenicol). The LODs and LOQs of the method were in the range of 0.006–0.091 and 0.018–0.281 ng mL−1, respectively. The relative recoveries of the target analytes were in the range from 76.77 to 99.97 % with RSDs between 1.6 and 8.8 %. The developed method was successfully applied to the extraction and analysis of 12 pharmaceuticals in five kinds of water samples (drinking water, running water, river water, influent and effluent wastewater) with satisfactory results.

Twelve pharmaceuticals in water samples analyted by UHPLC-MS/MS using ultrasound-assisted DLLME
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals are bioactive chemicals mostly used for humans and animals for prevention or treatment of disease, etc. Because of the abuse and waste by human beings, those chemicals would be presented as environmental contaminants in environmental systems which might show potential toxicity to aquatic organism and plants and finally bring danger to the health of human beings [1–3]. Moreover, most of the pharmaceuticals are not eliminated by wastewater treatment plants due to insufficient technology for the removal of such contaminants. Because of this incomplete elimination, wastewater treatment plants are the cause of 70–80 % of pharmaceutical occurrence in ecosystems [4]: they have been detected in wastewater influent and effluent, surface water, drinking water, river water, and ground water [5–11]. Therefore, the problem of pharmaceuticals residues in environmental water samples has become a major concern to people [12, 13]. It is very difficulty to determine such trace levels of pharmaceuticals in wastewater using common analytical methods. Mostly recently, many novel pretreatment methods were investigated for the preconcentration of the trace chemicals which were determined via the liquid chromatography coupled to tandem mass spectrometry with the merits of sensitivity, versatility, and selectivity [14–16]. Among those commonly used methods, solid-phase extraction (SPE) was the most extensively used technique [17–19]. However, the shortcomings compared with microextraction techniques for SPE are time-consuming, strenuous, and waste of organic solvents [20]. In recent years, liquid-phase microextraction (LPME) technology has been developed with more and more interests [21, 22]. To be specially mentioned, this technology requires less amounts of solvent than LLE, and various configurations have recently been developed [23–26]. In 2006, Rezaee et al. [27] reported a novel LPME technique termed as dispersive liquid–liquid microextraction (DLLME) based on a ternary component solvent system after the extraction solvent and disperser being rapidly injected into an aqueous sample. The merits of DLLME method are included such as easy operation, rapidity, high recovery, high enrichment factor, and so on [28, 29]. Regueiro et al. [30] applied an ultrasound-assisted emulsification–microextraction method which achieved perfect results for the application of ultrasound making the organic solvent to dissipate into microdroplets resulting in a considerably large contact area of both the aqueous and organic phases [31]. Consequently, it is clearly shown that the strong reduction of reagents and solvents involved in miniaturization processes is welcome from the environmental point of view. Recently, several studies investigating the combined application of DLLME extraction and ultrasound-assisted extraction of the pharmaceuticals from water samples have been published [22, 32–34]. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ketoprofen, naproxen, mefenamic acid, tolfenamic acid, sulindac, and piroxicam, are commonly used in clinic for the treatment of inflammatory disorders and pain relief. Publication regarding NSAIDs in the environment confirms their toxicity to many animal species [35]. On the other hand, antibiotics are the most successful family of drugs developed for improving human health so far. The annual usage of antibiotics has been estimated to be between 100,000 and 200,000 t globally [36]. Recent researches indicated that antibiotics can exert adverse influence on ecology and human health even at the low concentrations [37]. For that reason, it is essential to analyze the content of these pharmaceuticals in environmental matrices. In previous work carried out by our group [38], we developed a SPE in combination with DLLME procedure for the preconcentration of 10 antibiotics from different water samples prior to their ultra-high performance liquid chromatography with tandem mass spectrometry (UHPLC-MS/MS) detection. The aim of this study was to develop a quick and selective analytical method to carry out the simultaneous determination of six pharmaceuticals from the group of NSAID (ketoprofen, mefenamic acid, tolfenamic acid, naproxen, sulindac, and piroxicam) and six antibiotics (tinidazole, cefuroxime axetil, ciprofloxacin, sulfamethoxazole, sulfadiazine, and chloramphenicol) at low concentrations. Their chemical structures are outlined and shown in Fig. 1. Furthermore, we developed a simple and effective ultrasound-assisted DLLME coupled to UHPLC-MS/MS method for the determination of 12 pharmaceuticals. The effect of various extraction parameters, such as the type and volume of extraction and dispersive solvent, vortex and ultrasonic time, sample pH, and ionic strength, was investigated. Moreover, the developed method was validated for the analysis of five different kinds of water samples (drinking water, running water, river water, influent and effluent wastewater). To the best of our knowledge, the procedure for the preconcentration and simultaneous determination of 12 pharmaceuticals selected has not been described in the literature. The use of UHPLC results in shorter analysis time and reduced labor costs, which is important for routine analysis.
Materials and methods
Reagent and standards
The ketoprofen, mefenamic acid, tolfenamic acid, naproxen, sulindac, piroxicam, tinidazole, cefuroxime axetil, ciprofloxacin, sulfamethoxazole, sulfadiazine, and chloramphenicol standards were purchased from National Institute for Food and Drug Control (Beijing, China) with purities higher than 99.7 %. Stock solutions were prepared by dissolving each substance in chromatographic-grade methanol at concentrations of 0.8–1.1 mg mL−1 and stored at 4 °C in darkness. The standard curve and spiking solutions were prepared from appropriate dilutions of the stock solutions with methanol. Working solutions were prepared immediately before use. All of the standard solutions and sample extract were filtered through 0.22 μm × 4 mm nylon 66 membrane syringe filters before injecting into the chromatographic system.
Acetonitrile and formic acid of chromatographic-grade were obtained from Fisher Scientific (Pittsburgh, PA, USA). Methanol of chromatographic-grade was purchased from Shandong Yuwang Chemical Co., Ltd. (Yucheng, Shandong, China). Chromatographic grade water was purified using a Milli-Q Reagent Water system (Millipore, Bedford, MA). The other chemicals and solvents in this experiments, such as dichloroethane, carbon tetrachloride, dichloromethane, chlorobenzene, ethanol, and acetone, were all of analytical grade.
Instruments
The UPLC-MS/MS method was carried out on a Waters AcquityTM UPLC-MS/MS System (Waters, Milford, MA, USA), which consisted of an autosampler, a quaternary pump, a column oven, and a XevoTM Triple Quadrupole MS/MS system equipped with an ESI source (Waters Corp., Milford, MA, USA). The system was controlled with UnifiTM software for data acquisition and analysis, which was supplied by Waters Technologies. The optimal UPLC-MS/MS parameters for the analysis are shown in Table 1. Chromatographic separation was carried out on a Waters ACQUITY UPLC® BEH Phenyl (50 mm × 2.1 mm, 1.7 μm) protected by a high-pressure column prefilter (2 μm) (Waters, USA). The mobile phase consisted of 0.1 % formic acid in water (A) and acetonitrile (B), with a gradient elution as follows: 35–65 % B at 0–4 min, 65 % B at 4–5 min, and 65–35 % B at 5–7 min. The analysis run time was 9 min. The injection volume was 5 μL. The column temperature was set at 35 °C, and the flow rate was set at 0.3 mL min−1. The optimal conditions were capillary voltage, 3.0 kV; desolvation temperature and source temperature, 350 and 120 °C, respectively; and desolvation gas flow, 700 L h−1. Argon was used as the collision gas in all cases and nitrogen as the auxiliary and sheath gas in the ESI source. Positive–negative ion fast switching techniques and multiple reaction monitoring (MRM) were used.
Samples
Influent and effluent wastewaters were collected at the urban wastewater treatment plant of Shenyang. River water samples were collected from the South Canal of Shenyang. The samples of running water were taken from the tap in the laboratory and drinking water from bottled water in Shenyang. All samples were collected in October 2015 and filtered by 0.45-μm nylon membrane and stored in amber glass bottles at −20 °C before analysis.
Ultrasound-assisted DLLME procedure
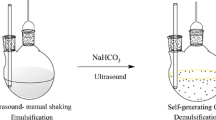
A 5-mL aliquot of the water sample (previously acidified using 0.1 mol L−1 HCl to pH 4) was placed in a 12-mL glass centrifuge tube with a conical bottom. Then, 1200 μL of methanol/acetonitrile (1:1, v/v; dispersive solvent) containing 800 μL of dichloromethane (extraction solvent) was rapidly injected into the sample solution by micropipette. The injection of the extraction mixture led to a cloudy sample solution which was vortexed for 60 s. And then, the tube was ultrasonicated for 10 min to enhance the extraction of the target analytes from the sample solution into the tiny droplets of dichloromethane. The phase separation was performed by a rapid centrifugation at 4000 rpm for 10 min. The supernatant was removed by a microsyringe. The remaining sedimented phase was evaporated with a gentle stream of nitrogen at 35 °C, and the residue was reconstituted with 100 μL acetonitrile/H2O (1:1, v/v). Finally, 5 μL of the filtered extract was injected into the UPLC-MS/MS system.
Method validation
Using the optimized conditions, the analytical characteristics of the proposed method were determined in terms of linearity, precision (intra-day precision and intra-day precision), accuracy, LOD, LOQ, and stability.
The linearity of the method was determined by analyzing standard solutions at nine concentrations. For each level, three replicate extractions were performed. All the experiments were carried out by a series of influent wastewater samples containing standards through a whole extraction procedure. Calibration curves were generated using linear regression analysis, and obtained linearity was assumed satisfactory when correlation coefficients (r 2) were higher than 0.99.
The precision of the method (expressed as relative standard deviation, RSD) was determined by the repeatability (intra-day precision) and reproducibility (inter-day precision) studies. Intra-day and inter-day precision data were obtained by analyzing the six replicates of the running water samples spiked at 10 ng mL−1, which were performed in 1 day and in three different days.
The recovery experiment was conducted to determine the accuracy of the method, which was evaluated by performing the determination of the influent wastewater samples spiked at three concentration levels (low, medium, and high). For each concentration level, three replicate experiments with the whole analysis process were performed. The data of recovery experiment was also used for the calculation of enrichment factor (EF). Calculations of recoveries were carried out according to the following equation: recovery (%) = [(C 1 − C 2) / C 3] × 100, where C 1 = concentration of the analyte in the final extract, C 2 = concentration of the analyte in the blank sample, and C 3 = concentration of the analyte added to the sample. The target analytes were extracted and purified according to the abovementioned procedure. Blanks (no-spiked samples) were analyzed to determine their concentration, which were afterwards subtracted to the spiked samples.
The LODs and LOQs were determined for influent wastewater samples spiked at serial dilution of working standard after extraction. The signal-to-noise ratios (S/N) 3:1 were used in calculating LOD and S/N 10:1 for calculating LOQ, respectively. The stability of the target analytes in the final extraction solution stored at −3∼−5 °C was tested by replicate assays of the solution at 0, 2, 4, 6, 8, 12, and 24 h.
Results and discussion
Optimization of the ultrasound-assisted DLLME conditions
Effect of the pH of sample solution
The pH of sample solution is a significant parameter for DLLME. Only when the sample solution was adjusted to a desired pH where the analytes were uncharged, it could be extracted effectively with organic extractants. In order to deionize the analytes, the sample solution should be adjusted to acidic with their pKa values between 3.3 and 9.6. Therefore, the pH of the solution was adjusted from 2 to 6 by using 0.1 mol L−1 HCl solution. As shown in Fig. 2, pH 4 provided satisfactory recoveries for most of the analytes, so it was selected for the subsequent experiment.
Type of the extraction solvent and its volume
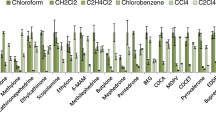
In DLLME, the type and volume of the extraction solvent pay important influence on the recoveries of the analytes. The suitable extraction solvent should have higher density than water, low solubility in water, and high extraction capability for the analytes. Different chlorinated solvents (dichloroethane, chlorobenzene, chloroform, dichloromethane, and carbon tetrachloride) with density higher than water and different polarities were tested to obtain a good extraction efficiency. As can be seen in Fig. 3, dichloromethane and chloroform showed similar extraction efficiency. The possibility is due to the greater polarity of dichloromethane and chloroform than the others, which leads to the higher solubility of the target analytes (containing polar groups such as carboxyl, hydroxyl, or amino groups) according to the principle of “like dissolve like” and hence higher extraction efficiency. Therefore, dichloromethane was selected as the extraction solvent in this study due to its lower toxicity compared with chloroform. Then, the effect of the volume of dichloromethane on the extraction efficiency was also investigated. The solutions containing different volumes of dichloromethane (ranging from 500 to 1100 μL at intervals of 100 μL) were used. The results from Electronic Supplementary Material (ESM) Fig. S1 show the recoveries of the analytes increased from 500 to 800 μL and then decreased. The possible reasons might be that when the volume of dichloromethane was lower, the dichloromethane might not be dispersed effectively in the sample solution, while when the its volume increased to a certain volume, the extraction efficiency got a maximum profile. Considering these reasons, 800 μL of dichloromethane was finally selected as optimized volume of extraction solvent.
Type of the dispersive solvent and its volume
The most important factor affecting the selection of dispersive solvent is relative miscibility of the dispersive solvent with the dichloromethane and aqueous phase in this experiment. Methanol, ethanol, acetonitrile, and acetone exhibit adequate properties and were studied as dispersive solvents. Considering a previous report [38, 39], methanol/acetonitrile (1:1, v/v) was also investigated. According to ESM Fig. S2, the mixture of methanol and acetonitrile gave a higher extraction efficiency for most of the analytes. This behavior might result from an increase in the dispersion of the dichloromethane in the aqueous phase and resulted in an excellent cloudy solution. And the solubility of the analytes in dichloromethane was increased, leading to an increase recoveries of the analytes. Therefore, methanol/acetonitrile (1:1, v/v) was selected as the dispersive solvent in the following experiments.
The volumes of dispersive solvent (ranging from 800 to 1400 μL at intervals of 100 μL) used in the extraction were also studied. The results (see ESM Fig. S3) indicated that 1200 μL could achieve higher recovery than others. Therefore, 1200 μL was selected as the volume of dispersive solvent.
Effect of vortex time
In 2010, Yiantzi et al. [40] developed LLME assisted by vortex to analyze alkylphenols. In this study, the vortex time was investigated in the ranges of 0–120 s (data not shown). The extraction efficiency of analytes had no remarkable effect upon increasing the vortex time. It was obvious that the surface area between extraction solvent and sample solution was very large after the sample solution becoming cloudy. Therefore, the transfer of the analytes from sample solution to extraction solvent was fast, and the equilibrium state was quickly achieved. Consequently, 60 s of vortex time was chosen in the following experiments.
Effect of the ultrasonic time
The effect of ultrasonic time on the extraction efficiency was examined within the range from 0 to 20 min. As shown in ESM Fig. S4, ultrasonic time of 10 min was the optimal condition and longer ultrasonic time could not afford higher extraction efficiency. It is probable that when the ultrasonic time was too short, the extraction solvent would not reach enough contact with the sample solution. However, if too longer, the volatilization of analytes and extraction solvent caused by consequent thermal effect would lead to the loss of analytes. Therefore, 10 min was chosen as the optimum ultrasonic time.
Effect of ionic strength
Generally, the addition of salts can reduce the solubility of the analytes and improve their partitioning into the organic phase for LPME [41]. In order to investigate the effect of ionic strength, different concentrations of NaCl from 0 to 20 % (m/v) was optimized (data not shown). The results indicated that salt concentration showed nonsignificant effect on the recoveries of the analytes. Hence, NaCl was not added in the current method.
Application of ultrasound-assisted DLLME in water samples
Matrix effect
Matrix effect (ME) was originally discussed by Kebarle and Tang [42] in the early 1990s, which should be necessary to be investigated for DLLME combined with UPLC-MS/MS.
ME, recovery of sample preparation procedure (R), and overall process efficiency (PE) were established according to Niessen [43]. ME, recovery of extraction procedure (RE), and overall PE were calculated by the following equation:
A refers to the responds of each real samples (drinking water, running water, river water, influent and effluent wastewaters), B refers to the standard solution, C refers to pre-extraction spiked samples with concentration that was same as B, and D was the post-extraction.
EF were calculated using the equation:
The results of ME, overall PE, and their precision (RSD) in different water matrices spiked at the 10 ng mL−1 level were indicated in Table 2. The EF values were also showed in Table 2. These results proved that globally the proposed method did not suffer of matrix effects by virtue of the sample preparation.
Assay validation
All validation data are presented in Tables 3 and 4.
Linearity of the method was estimated in the working range of 0.04–20 ng mL−1 for ciprofloxacin and sulfadiazin, 0.2–100 ng mL−1 for ketoprofen, tinidazole, cefuroxime axetil, naproxen, sulfamethoxazole, and sulindac, and 1–200 ng mL−1 for mefenamic acid, tolfenamic acid, piroxicam, and chloramphenicol at nine concentration levels. The satisfactory correlation coefficients (0.9907–0.9996) were achieved. The inter-precision for all analytes was less than 8.9 %, the intra-precision was less than 3.9 %, and accuracy values were all more than 76.77 % with RSDs between 1.6 and 8.8 %. The LODs and LOQs were calculated for all pharmaceuticals in 100 μL extracts (without inclusion of preconcentration step). In extracts, LODs and LOQs ranged from 0.006 to 0.091 ng mL−1 and 0.018 to 0.271 ng mL−1, respectively. And the sample solution was found to be stable from until 24 h with their RSD values lower than 8.9 %, indicating a good stability of all target analytes.
Real sample analysis
The developed method was applied to the analysis of real water samples. As reported in Table 5, all analytes could be detected above the LOD in influent and effluent wastewater. In common, concentrations of the analytes were lower in effluents than those in influent. What is more, the concentrations of naproxen and tinidazole were higher in effluents than influents, which might be because that the samples from both influent and effluent were taken at the same time, indicating that they did not originate from the same portion of wastewater [44]. Generally, pharmaceuticals have been detected in environmental water samples mainly due to their widely consumption and incomplete treatment.
Comparison of ultrasound-assisted DLLME with other methods
To highlight the robust application of the presented ultrasound-assisted DLLME combined with UHPLC-MS/MS method, it was compared to several published methods for the determination of pharmaceuticals such as ultrasound-assisted DLLME followed by UHPLC-PDA, SPE-DLLME combined with UPLC-MS/MS, SPE combined with GC-MS/MS, etc. [38, 45–48]. As listed in Table 6, better sensitivity is achieved as the LODs were 0.006–0.091 ng mL−1, which were lower than those reported in our previous paper [38] and other papers [45–47]. However, LODs at a similar level can be found in the literature [48]. Nevertheless, a few-hour-long SPE procedure and the derivatization step had to be used to determination of these analytes.
Conclusion
In the present study, a new method was developed and applied to pretreat and detect 12 pharmaceuticals from environmental water samples. Ultrasound-assisted method was employed to promote the dispersion of the liquid droplets into the sample solution, which increased the extraction efficiency and reduced the equilibrium time. Moreover, the extraction material is cheap, easy-to-use, and consumes only minor amounts of organic solvent. Adequate repeatability, good linearity and recoveries, and the low quantification limits demonstrated that the method was sensitive and accurate for quantitative analysis of the 12 pharmaceuticals in real water samples (drinking water, running water, river water, influent and effluent wastewater). Accordingly, it shows great potential in the analysis of ultra-trace compounds in environmental water samples.
References
Fent K, Weston AA, Caminada D. Ecotoxicology of human pharmaceuticals. Aquat Toxicol. 2006;76:122–59.
Segura PA, Francois M, Gagnon C, Sauvé S. Review of the occurrence of anti-infectives in contaminated wastewaters and natural and drinking waters. Environ Health Perspect. 2009;117:675–84.
Kümmerer K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere. 2001;45:957–69.
Ternes TA, Joss A. Human pharmaceuticals, hormones and fragrances. The challenge of micropollutants in urban water management. London: IWA Publishing; 2006. p. 243–88.
Patrolecco L, Capri S, Ademollo N. Occurrence of selected pharmaceuticals in the principal sewage treatment plants in Rome (Italy) and in the receiving surface waters. Environ Sci Pollut Res. 2015;22:5864–76.
Yang GCC, Liou SH, Wang CL. The influences of storage and further purification on residual concentrations of pharmaceuticals and phthalate esters in drinking water. Water Air Soil Pollut. 2014;225:1–11.
Radović T, Grujić S, Petković A, Dimkić M, Laušević M. Determination of pharmaceuticals and pesticides in river sediments and corresponding surface and ground water in the Danube River and tributaries in Serbia. Environ Monit Assess. 2015;187:4092–109.
Fatta-Kassinos D, Meric S, Nicolaou A. Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Anal Bioanal Chem. 2011;399:251–75.
Meffe R, Bustamante I. Emerging organic contaminants in surface water and groundwater: a first overview of the situation in Italy. Sci Total Environ. 2014;481:280–95.
Gros M, Petrović M, Barceló D. Wastewaters treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro River basin (Northeast Spain). Environ Toxicol Chem. 2007;26:1553–62.
Halling-Sørensen B, Nielsen SN, Lanzky PF, Ingerslev F, Holten Lützhøft HC, Jørgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment: a review. Chemosphere. 1998;3:357–93.
Khan U, Nicell J. Human health relevance of pharmaceutically active compounds in drinking water. AAPS J. 2015;17:558–85.
Leung HW, Jin L, Wei S, Tsui MMP, Zhou BS, Jiao LP, et al. Pharmaceuticals in tap water: human health risk assessment and proposed monitoring framework in China. Environ Health Perspect. 2013;121:839–46.
Petrović M, Hernando MD, Díaz-Cruz MD, Barceló D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J Chromatogr A. 2005;1067:11–4.
Petrovic M, Farré M, Lopez de Alda M, Perez S, Postigo C, Köck M, et al. Recent trends in the liquid chromatography–mass spectrometry analysis of organic contaminants in environmental samples. J Chromatogr A. 2010;1217:4004–17.
Wille K, De Brabander HF, De Wulf E, Van Caeter P, Janssen CR, Vanhaecke L. Coupled chromatographic and mass-spectrometric techniques for the analysis of emerging pollutants in the aquatic environment. Trends Anal Chem. 2012;35:87–108.
Staniszewska M, Wolska L, Namieśnik J. New approach based on solid-phase extraction for the assessment of organic compound pollutions in so-called pharmaceutically pure water. Anal Bioanal Chem. 2008;391:1941–9.
Berthod L, Roberts G, Mills GA. A solid-phase extraction approach for the identification of pharmaceutical–sludge adsorption mechanisms. J Pharm. 2014;4:117–24.
David R, Kasprzyk-Hordernb BB. Critical evaluation of methodology commonly used in sample collection, storage and preparation for the analysis of pharmaceuticals and illicit drugs in surface water and wastewater by solid phase extraction and liquid chromatography–mass spectrometry. J Chromatogr A. 2011;1218:8036–59.
Padrón T, Esther M, Cristina AO, Zoraida SF, Santana-Rodríguez JJ. Microextraction techniques coupled to liquid chromatography with mass spectrometry for the determination of organic micropollutants in environmental water samples. Molecules. 2014;19:10320–49.
Farajzadeh MA, Sorouraddin SM, Mogaddam MRA. Liquid phase microextraction of pesticides: a review on current methods. Microchim Acta. 2014;181:829–51.
Hiroyuki K. New trends in sample preparation for analysis of plant-derived medicines. Curr Org Chem. 2010;14:1698–713.
Larsson N, Petersson E, Rylander E, Jönsson JA. Continuous flow hollow fiber liquid-phase microextraction and monitoring of NSAID pharmaceuticals in a sewage treatment plant effluent. Anal Methods. 2009;1:59–67.
Díaz-Álvarez M, Esther T, Martín-Esteban A. Hollow fibre liquid-phase microextraction of parabens from environmental waters. Int J Environ Anal Chem. 2013;93:727–38.
Sarafraz-yazdi A, Assadi H, Es’haghi Z, Danesh NM. Pre-concentration of non-steroidal anti-inflammatory drugs in water using dispersive liquid–liquid and single-drop microextraction with high-performance liquid chromatography. J Sep Sci. 2012;35:2476–83.
Zhang J, Lee HK. Application of dynamic liquid-phase microextraction and injection port derivatization combined with gas chromatography–mass spectrometry to the determination of acidic pharmaceutically active compounds in water samples. J Chromatogr A. 2009;1216:7527–32.
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A. 2006;1116:1–9.
Zgoła-Grześkowiak A, Grześkowiak T. Dispersive liquid-liquid microextraction. Trends Anal Chem. 2011;30:1382–99.
Mohammad R, Yadollah Y, Mohammad F. Evolution of dispersive liquid-liquid microextraction method. J Chromatogr A. 2010;1217:2342–57.
Regueiro J, Llompart M, Garcia-Jares C, Garcia-Monteagudo JC, Cela R. Ultrasound-assisted emulsification–microextraction of emergent contaminants and pesticides in environmental waters. J Chromatogr A. 2008;1190:27–38.
Albero B, Sánchez-Brunete C, García-Valcárcel AI, Rosa A, José P, Tadeo L. Ultrasound-assisted extraction of emerging contaminants from environmental samples. TrAC Trends Anal Chem. 2015;71:110–8.
Parrilla Vázquez MM, Parrilla Vázquez P, Martínez Galera M, Gil García MD, Uclés A. Ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction coupled with liquid chromatography-quadrupole-linear ion trap-mass spectrometry for simultaneous analysis of pharmaceuticals in wastewaters. J Chromatogr A. 2013;1291:19–26.
Yan H, Wang H, Qin X, Liu B, Du J. Ultrasound-assisted dispersive liquid–liquid microextraction for determination of fluoroquinolones in pharmaceutical wastewater. J Pharmaceut Biomed. 2011;54:53–7.
Wu H, Li G, Liu S, Liu D, Chen G, Hu N, et al. Simultaneous determination of six triterpenic acids in some Chinese medicinal herbs using ultrasound-assisted dispersive liquid–liquid microextraction and high-performance liquid chromatography with fluorescence detection. J Pharmaceut Biomed. 2015;107:98–107.
Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature. 2004;427:630–3.
Kümmerer K. Significance of antibiotics in the environment. J Antimicrob Chemother. 2003;52:5–7.
Liu X, Lee J, Ji K, Takeda S, Choi K. Potentials and mechanisms of genotoxicity of six pharmaceuticals frequently detected in freshwater environment. Toxicol Lett. 2012;211:70–8.
Liang N, Huang PT, Hou XH, Li Z, Tao L, Zhao LS. Solid-phase extraction in combination with dispersive liquid-liquid microextraction and ultra-high performance liquid chromatography-tandem mass spectrometry analysis: the ultra-trace determination of 10 antibiotics. Anal Bioanal Chem. 2016;408:1701–13.
Celano R, Piccinelli AL, Campone L, Rastrelli L. Ultra-preconcentration and determination of selected pharmaceutical and personal care products in different water matrices by solid-phase extraction combined with dispersive liquid-liquid microextraction prior to ultra high pressure liquid chromatography tandem mass spectrometry analysis. J Chromatogr A. 2014;1355:26–35.
Yiantzi E, Psillakis E, Tyrovola K, Kalogerakis N. Vortex-assisted liquid-liquid microextraction of octylphenol, nonylphenol and bisphenol-A. Talanta. 2010;80:2057–62.
Zhao RS, Wang X, Li FW, Wang S, Zhang LL, Cheng CG. Ionic liquid/ionic liquid dispersive liquid-liquid microextraction. J Sep Sci. 2011;34:830–6.
Kebarle P, Tang L. From ions in solution to ions in the gas phase. Anal Chem. 1993;65:972A–86.
Niessen WMA, Manini P, Andreoli R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2006;25:881–99.
Kotowska U, Kapelewska J, Sturgulewska J. Determination of phenols and pharmaceuticals in municipal wastewaters from Polish treatment plants by ultrasound-assisted emulsification-microextraction followed by GC–MS. Environ Sci Pollut Res. 2014;21:660–73.
Herrera-Herrera AV, Hernandez-Borges J, Borges-Mique TM, Rodriguez-Delgado MA. Dispersive liquid-liquid microextraction combined with ultra-high performance liquid chromatography for the simultaneous determination of 25 sulfonamide and quinolone antibiotics in water samples. J Pharmaceut Biomed. 2013;75:130–7.
Ferńandez P, Regenjo M, Ferńandez AM, Lorenzo RA, Carro AM. Optimization of ultrasound-assisted dispersive liquid–liquid microextraction for ultra performance liquid chromatography determination of benzodiazepines in urine and hospital wastewater. Anal Methods. 2014;6:8239–46.
Caldas SS, Rombaldi C, Arias JLDO, Marube LC, Primel EG. Multi-residue method for determination of 58 pesticides, pharmaceuticals and personal care products in water using solvent demulsification dispersive liquid–liquid microextraction combined with liquid chromatography-tandem mass spectrometry. Talanta. 2016;146:676–88.
Nishi I, Kawakami T, Onodera S. Monitoring the concentrations of nonsteroidal anti-inflammatory drugs and cyclooxygenase-inhibiting activities in the surface waters of the Tone Canal and Edo River Basin. J Environ Sci Health A. 2015;50:1108–15.
Acknowledgments
This work was sponsored by the National Natural Science Foundation of China (grant nos. 21477082 and 81503029), Liaoning Province Educational Department (L-2014160), Liaoning Province Natural Science Foundation of China (no. 2015020701), and by Large Instrument Sharing Service Construction Special of Shenyang Science and Technology Innovation Fund (no. F14-195-4-00).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 584 kb)
Rights and permissions
About this article
Cite this article
Guan, J., Zhang, C., Wang, Y. et al. Simultaneous determination of 12 pharmaceuticals in water samples by ultrasound-assisted dispersive liquid–liquid microextraction coupled with ultra-high performance liquid chromatography with tandem mass spectrometry. Anal Bioanal Chem 408, 8099–8109 (2016). https://doi.org/10.1007/s00216-016-9913-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9913-1