Abstract
It is crucial to study the presence of transformation products (TPs) of emerging contaminants that can be potentially found in the environment after biological or chemical degradation. This review focuses on the potential and shortcomings of high-resolution mass spectrometry (HRMS) to identify these TPs, with emphasis on recent developments in mass analyzers, data evaluation, and compound identification workflows and applications. Advances in HRMS technologies, including direct introduction or in-line chromatographic separation modes, ionization techniques, mass analyzers, and detection methods, have led to powerful tools to assess the molecular changes and the opening of new horizons to identify unknown molecules. Advances in HRMS pertaining to the generation of analytical data for the main methods to identify TPs, including nontargeted and targeted approaches as they are applied to elucidate the structure of TPs, are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the growing presence of emerging contaminants, such as pharmaceuticals (both human and veterinary), personal-care products, illicit drugs, pesticides, and perfluorinated compounds detected in the water cycle, has been referred to as one of the most imperative environmental concerns [1–3]. After human and/or animal use, these compounds are excreted or release unchanged and as free and/or conjugate metabolites. Transformation products (TPs) of emerging contaminants can be found in wastewater treatment plants (WWTPs) or in the environment as a result of a multitude of abiotic and biotic processes (such as hydrolysis, photolysis, oxidation, and microbial metabolism) acting on the parent compounds or the metabolites [4–7]. TPs are of environmental concern particularly if they are biologically active or resistant to biodegradation [8–10]. However, there is only limited information in the literature on the fate of these TPs, and many of them remain undiscovered [11]. The importance of further investigation of the subject is highlighted by the fact that some TPs are equally active as or even more active than the precursor compounds on aquatic ecosystems or, in the last instance, on humans [10, 12–15].
There are already some reviews that address the experience of several research groups in the analysis of emerging contaminants, including their TPs, with particular emphasis on liquid chromatography (LC)–mass spectrometry (MS)-based techniques, describing the state of the art and highlighting gaps and future needs [9, 16–19]. Recently, some reviews have been published that included current information regarding the background of (biotic and abiotic) transformations of emerging contaminants [7, 9, 14, 15, 20–22], including their fate, occurrence, and distribution. Different reviews [1, 23–26] have also presented and discussed the main analytical techniques used for the determination of the emerging contaminants and their TPs.
Historically, identification of TPs has been conducted using radiolabeled compounds, thin-layer chromatography, and gas chromatography or LC with unspecific detectors [4]. However, a serious weakness of all these methods is that they lack sufficient specificity for identification of the TPs. Thus, MS is a key element. Because of the polar nature of TPs, the most commonly used separation technique is high-performance LC (HPLC) or simply LC [17, 27]. TPs can be identified using nominal mass. However, quadrupoles are not sensitive in full-scan mode (required to identify unknowns), and much of this work has been done with standards or pure water solutions at relatively high concentrations [24, 28]. Conventional LC–MS interfaces involve soft ionization techniques that produce little fragmentation but provide information on the molecule. The fragmentation of the analyte required for the structural elucidation is most commonly produced by collision-induced dissociation, which can be done in a specialized collision cell or in the intermediate-pressure part of the mass spectrometer (so-called in-source collision-induced dissociation). The fragmentation pattern of the molecule helps, after the application of long and complicated processes and the use of a number of additional confirmatory analyses, to establish the structure of unknown TPs [21, 22]. An additional obstacle to identify these TPs is that there are no extensive standard libraries that would help to identify the chemical composition of the molecule and its fragments (unlike in gas chromatography). Another limitation of the nominal-mass LC–MS methods to determine traces of these TPs is the need to use target methods, i.e., they detect only what they are designed to look for. Ultratrace target methods, based on low-resolution MS, are designed to preserve only a small amount of information about the composition of the sample; the rest of this analytical information is lost [29–31].
High-resolution MS (HRMS) transcends the major limitations of nontarget LC–MS and targeted MS/MS analysis. HRMS instruments [e.g., time-of-flight (TOF) and Orbitrap instruments] provide high-quality information by combining sensitive full-spectrum data with high mass resolution and mass accuracy [32, 33]. In theory, the presence of an unlimited number of compounds can be investigated at the proper sensitivity, without requiring the preselection of analytes or even without having reference standards available. In HRMS, ions are measured at a high resolving power, i.e., ions with slightly different m/z ratios can be distinguished from one another [34]. The most important feature of HRMS is the capacity to determine the molecular formulas of analytes from accurate-mass measurements. An HRMS instrument can be coupled to quadrupoles and ion traps to add MS/MS and/or MSn capabilities that allow to obtain mass spectra of multiple product ions with accurate-mass measurements [1, 35]. For this reason, HRMS is a promising technique that has opened new horizons in screening and rapid identification of a wide range of compounds and unknowns. The trends and emerging applications of HRMS in the analysis of micropollutants, but without pinpointing the problem of TPs, have also been reviewed by several authors [23, 28, 36, 37].
This review compiles information from publications that have appeared in the last 5 years (2011–2015) related to the study of TPs in the environment to clearly demonstrate the potential of HRMS for the identification of the myriad of TPs that are still unknown. The advantages and disadvantages of this technique are also examined, and strategies are proposed in order to improve current analytical performance and streamline data analysis.
Approaches to mimic degradation processes and to identify TPs
There are two different approaches to identify TPs: laboratory studies and environmental screening, as schematized in Fig. 1. These approaches are complementary and mutually enriching rather than the opposite of each other. Commonly, laboratory studies offer the advantage of simulating transformation processes under well-defined conditions with appropriate control that facilitates the establishment of differences in the samples that contain the compounds. However, the identification in the environment of at least a few of these compounds is the next necessary step. Tables 1 and 2 show the most recent applications in laboratory and environmental monitoring studies, respectively.
TPs are mostly generated during water treatment processes, including oxidation and biotransformation by activated sludges [87]. A large number of chemicals used in industry and households, including pharmaceuticals, biocides, and personal-care products, are released into aquatic environments because of their incomplete removal in WWTPs [3, 88]. These plants are optimized for nutrient removal and biological degradation of natural organic compounds, but more recalcitrant chemicals are only partially degraded during the treatment process [16, 22, 32, 34, 36]. As a consequence, their TPs are formed and emitted via WWTP effluents into the aquatic environment. Once released into the environment, emerging contaminants are also subject to processes (e.g., biodegradation in soil, sediment, and biota, as well as hydrolysis and photochemical degradation in surface water and groundwater) that contribute to their elimination.
The laboratory studies are focused on the different types of degradation mechanisms—biotic and abiotic. They can be at different scales. For batch processes, bench-scale testing is typically conducted on samples of 1–20 L or less, whereas pilot-scale testing is performed with samples of 20–100 L. Demonstration scale essentially involves operating the equipment at full commercial feed rates over extended time periods to prove operational stability.
Biodegradability is mostly evaluated under different treatment conditions in activated sludge batch experiments [38–43, 50]. The sludge is aerated to suspend the microorganisms and is used as the biodegradation medium or autoclaved for use as a sterile control to eliminate the influence of abiotic processes. Batches spiked and not spiked with the analyte are also run in parallel to avoid interferences. These experiments can be performed under different operational conditions: aerobic, anaerobic, and anoxic. The pH, dissolved oxygen content, temperature, and content of total suspended solids are monitored and adjusted to allow direct comparison with environmental conditions [39, 44, 51, 52]. The sorption behaviors of compounds in soils or sewage sludges can be determined by adjusting experimentally these parameters according to several OECD guidelines, among them 304A and 314A [46, 51]. Several recent studies have identified micropollutant TPs in laboratory studies with activated sludge and field investigations in WWTPs, pointing to oxidative and hydrolytic reaction pathways [38–52]. Evaluation of the importance of different reaction pathways, however, is very difficult owing to problems related to the identification of TPs (e.g., the lack of protocols and standardized methods, spectral libraries, or analytical standards to confirm the identity) and their quantification (e.g., again lack of analytical standards). Batch experiments have also been performed to establish biodegradation in soil and sediments, where microorganisms play an important role in degradation [51, 52]. Li et al. [52] used a test system with artificial river water and sediment collected from two rivers to identify microbial TPs from nine pharmaceuticals in a water/sediment test, with special focus on TPs that are not easily degraded and thus accumulate during the incubation period. The biotransformation in soil of two representative perfluorooctanesulfonic acid precursors—N-ethyl perfluorooctane sulfonamide and N-ethyl perfluorooctane sulfonamide ethanol—has also been studied using a similar approach [51].
Most of the pilot-scale experiments are performed using alternative water treatment technologies such as membrane bioreactors that better simulate the conditions in the full-scale system. These membrane bioreactors are commonly fed with synthetic wastewater and inoculated with activated sludge from the full-scale WWTP. The TPs are commonly searched for in the membrane bioreactor effluent [53]. Batch and pilot-scale experiments need to be correlated with the detection of the TPs identified in the real WWTPs or in the environment. Those studies that report searching for the identified TPs in real matrices demonstrated that at least some of them are present in wastewaters and surface waters [41, 44, 47], pointing out the interest in small-scale simulation to increase knowledge of TPs [46, 51].
The physicochemical degradation occurs concurrently with engineered processes such as oxidation reactions with chlorine, chlorine dioxide, or ozone and transformations by ultraviolet light. There have also been a number of batch and pilot-scale studies [55–71]. Among the various advanced oxidation processes, heterogeneous photocatalysis using titanium dioxide as a catalyst is a technology that appears to be a promising tool for water and wastewater treatment as it has been successfully applied for the removal of pharmaceuticals and other micropollutants from water in laboratory-scale reactors [58]. Another rapidly developing field in advanced oxidation processes for applications in environmental remediation is the use of ultrasound irradiation to destroy or accelerate the destruction of liquid-phase contaminants. In the case of reverse osmosis concentrates, a subsequent electrochemical treatment using boron-doped diamond electrodes offers potential removal of contaminants, since electrolysis itself profits from enhanced electrical conductivity owing to the high salinity and the capability of boron-doped diamond to generate hydroxyl radicals [69]. In situ chemical oxidation is an emerging technology for groundwater remediation because of its applicability to a wide range of contaminants, relatively fast treatment, potentially enhanced postoxidation microbial activity, and cost-effectiveness [67]. Chlorination is the most commonly used chemical process for disinfecting swimming pools and drinking water. Formation of halogenated disinfection by-products in chlorinated water is unavoidable, particularly for substances containing phenolic and/or amino groups [70]. Biotic and abiotic degradations occur together to transform emerging contaminants during the biological wastewater treatment. An abiotic transformation process of recent interest is the nitration of phenol moieties and the formation of nitrophenolic TPs during biological wastewater treatment [72].
Environmental studies have been performed in WWTP effluents and in surface water matrices. Nonetheless, in the field of environmental chemistry, the detection of trace levels creates an extra difficulty in the analytical development required as well as in its performance [81]. These studies as outlined in Table 2 are much scarcer. A number of studies have focused on effluent wastewaters because the concentrations of TPs are higher than in surface waters [74–76]. Extraction procedures able to isolate and preconcentrate the analytes are extremely important, and conventional solid-phase extraction (SPE) is commonly used in the case of water. These procedures are sometimes also used in laboratory studies. The extraction procedures are not sophisticated, but are generic and robust since the physicochemical characteristics of the TPs are sometimes unknown. Hydrophilic-lipophilic balance reversed-phase sorbents are the most popular [74–76] owing to their ability to retain quite polar compounds.
The long-term contamination of sediments by emerging contaminants such as pharmaceuticals, personal-care products, household chemicals, or pesticides as well as their possible degradation products has not been well explored [82–84]. Pressurized liquid extraction followed by quick, easy, cheap, effective, rugged, and safe (QuEChERS)-like cleanup has been used to reduce solvent consumption and to avoid the use of time-consuming cleanup techniques that can increase the loss of polar compounds.
The use of marine organisms as a tool for the monitoring of a pharmaceutical and its TPs in marine environments was evaluated in some studies [85, 86]. The extraction method applied to these matrices is the QuEChERS extraction that involves miniaturized extraction with acetonitrile by salting-out with sodium chloride and magnesium sulfate and a cleanup step, which is done by mixing the acetonitrile extract with several sorbents (dispersive SPE). Successful determination of TPs of venlafaxine and carbamazepine has been reported.
The features of LC separation prior to HRMS
A question that arises when one is collecting information about how to determine the TPs of different emerging contaminants is whether it is necessary to use a separation technique prior to the determination by HRMS, taking into account its specificity.
Analysis of the literature summarized in Tables 1 and 2 shows that HRMS is always used in combination with LC, which has been used indistinctly as either classical HPLC or the recently developed ultra-high-performance LC [38–41]. The major advantages of ultra-high-performance LC (1.7-μm particle size in the stationary phase) over classical HPLC (particle size between 3 and 5 μm) include improved resolution within a shorter retention time and higher analytical sensitivity.
The columns used are mostly reversed-phase LC columns and, in a few particular cases, hydrophilic interaction LC (HILIC) columns [62]. The attractiveness of the latter variant arises from the fact that the mobile phase composition is fully compatible with electrospray ionization, with an elevated percentage of organic solvent in the mobile phase enhancing evaporation of the solvent and altering ion suppression and thus improving detection sensitivity [62]. HILIC columns play an interesting role in achieving chromatographic separation of very polar parent compounds or even their more polar TPs. Figure 2 illustrates the chromatographic separation on a HILIC column achieved for zanamivir and its TPs. The reported advantages of the HILIC column in comparison with a C18 column were that it substantially improves the retention of zanamivir (an antiviral) and that it separates an isobaric TP, TP332, that was co-eluted with the parent compound.
Extracted ion chromatograms corresponding to separation by ultra-high-performance liquid chromatography (UHPLC) of zanamivir (ZAN) and its photoproducts on a hydrophobic interaction liquid chromatography column (acquired with an LTQ Orbitrap mass spectrometer) present in a irradiated high-performance liquid chromatography water sample (initial zanamivir concentration of 40 mg L−1). The figure depicts the ion traces of the molecular ions of a zanamivir (m/z 333) and TP332 (m/z 333), b TP322 (m/z 323), c TP274 (m/z 275), and d TP111 (m/z 112). (Reproduced from [62] with permission of Elsevier)
Chromatographic separation is very important for the identification of the TPs because firstly it helps to properly identify the mass spectra corresponding to the TPs. The parent compound and the TPs normally have part of the molecule in common, and the mass spectra have ions in common; therefore, the separation is important. In addition, this separation, which is usually based on the polarity, gives additional information about whether the assigned structure matches the expected polarity.
The role of HRMS in identification and structural elucidation of compounds
The high-resolution mass analyzers commonly employed are TOF, Orbitrap, and hybrid systems (e.g., quadrupole TOF [27, 51–54, 56, 57, 59, 61, 64–66, 70–76, 78–82], linear ion trap Orbitrap [38–41, 43–45, 47, 49, 50, 60, 62, 63, 69, 77], or quadrupole Orbitrap [42, 46, 48, 55, 67, 83–86]). As can be seen in Tables 1 and 2, despite the fact that HRMS can provide accurate mass, with which the empirical formula of the molecule can be calculated, and thus make possible elucidation of the molecular structure, it is hardly used alone, and all applications confirm the identification by an MS/MS study. The occurrence and variations in patterns of multi-isotopic elements often make the identification of TPs easy. Many emerging contaminants contain chlorine or bromine; these produce a distinctive mass spectral isotopic pattern corresponding to their natural isotopic abundance. By searching for compounds that show the same isotopic pattern as the parent compound, one can quickly associate TPs with the parent drug. The isotopic pattern capabilities have been demonstrated for several compounds, such as diazepam [27, 65].
The MS/MS spectrum can be recorder dependent or independent of the precursor ion data. In the data-dependent acquisition (DDA) mode, a precursor ion is selected for MS/MS, which is inherently biased owing to the existence of many compounds in the sample. For the precursor, a non-overlapping m/z window is selected, and all the precursor ions within the window are co-fragmented, yielding more fragment ions per sample, which increases the analyte identification score.
The commonest way to work with hybrid mass spectrometers has been with DDA or information-dependent acquisition. Both refer to the same way of working, but the nomenclature depends on the instrument. In this mode, the high-resolution MS/MS instrument first performs a survey scan, from which certain ions chosen “a priori” by the analyst or “not chosen” but meeting some pre-defined criteria (e.g. the intensity above a predefined threshold value) are selected, isolated, fragmented, and sequenced by product ion scanning in the instruments. Additional selection criteria, such as dynamic exclusion, background subtraction, and charge state selection, are also used to prevent redundant acquisition of the most abundant ions, or to avoid acquiring product ion spectra of the interferences. With use of this strategy, co-eluted matrix compounds or noisy peaks can be easily excluded, facilitating the identification and quantification of known or novel analytes in a single run. Figure 3 shows the fragmentation pattern of macrolide TPs obtained in a QqTOF instrument after analyst selection of the precursor ions. Figure 4 illustrates data-dependent MS/MS acquisition of the five most intense ions detected in a full-scan spectrum with dynamic exclusion enabled. The intensity threshold for triggering the MS/MS events was set to 80,000 counts in the full-scan event. Parent ions were fragmented by high-energy C-trap dissociation (HCD). With use of DDA, MS/MS data were obtained in a single run for all compounds investigated.
Time-of-flight (TOF) tandem mass spectrometry (MSMS) spectra of the transformation products of azithromycin (AZI) and roxithromycin (ROX) identified in membrane bioreactor effluent obtained in positive polarity mode. CE collision energy, CLA cladinose, ES electrospray, DESP phosphorylated desosamine, PI positive ionization. (Reproduced from [53] with permission of Springer)
The positive electrospray ionization (ESI) Orbitrap data-dependent tandem mass spectrometry spectra of methotrexate (a) and its biotransformation products: DAMPA (b), TP325 (c), TP312 (d), TP207 (e), and TP342 (f). (Reproduced from [53] with permission of Elsevier)
Data-independent acquisition (DIA) experiments are conducted using a hybrid system, with full-scan data being recorded in a TOF, Orbitrap, or other high-resolution mass analyzer. A major difference in these methods lies in the width (or the absence) of the precursor windows in the first stage of ion isolation. This, in turn, determines the range of ions being transmitted, fragmented, and sequenced. A consequence of having a wide precursor isolation window in DIA approaches is that it produces a very complex data structure and requires coherent and intricate data processing.
MSE is the commonest DIA approach. It acquires two full-scan mass spectra in an unbiased and parallel manner. As such, it increases both the number of compounds detected and the reproducibility. During data acquisition, the energy of the gas-filled traveling-wave collision cell is dynamically switched between a low-energy and a high-energy state. This produces alternating composite mass spectra of all intact molecular ions, followed by hypothetical fragmented mass spectra of all precursors. MSE has been a popular choice, with a number of reported applications to identify TPs [52, 74, 75, 78, 82]. Figure 5 shows the detection and identification of clopidogrel carboxylic acid in an effluent wastewater sample [75]. The accurate mass of the protonated molecule of the suspected clopidogrel carboxylic acid (retention time 5.64 min) was m/z 308.0524 from the low-energy MS spectrum (Fig. 5, panel B, bottom). The combined spectrum for this chromatographic peak showed a typical monochlorinated isotopic pattern, in accordance with the elemental composition of the protonated molecule. The high-energy TOF-MS spectrum was also investigated (Fig. 5, panel B, top). Up to seven fragment ions, all showing the chlorine isotopic pattern, were obtained. A possible concern of the MSE approach is that co-eluted compounds might “contaminate” the high-energy spectrum, which might also contain ions unrelated to the analyte, complicating the interpretation of the spectrum. As Fig. 5, panel C shows, all extracted ion chromatograms, for the protonated molecule and for the seven main fragment ions, led to a chromatographic peak at the same retention time (5.63 min).
Detection and identification of clopidogrel carboxylic acid in effluent wastewater by UHPLC–quadrupole time-of-flight (QqTOF) mass spectrometry (MS) (MSE approach). Chromatograms (A) and spectra (B) of the sample. C extracted ion chromatograms with a 20-mDa mass window for different ions observed in high-energy (HE) mode. ES electrospray, LE low energy. (Reproduced from [75] with permission of Elsevier)
All-ion fragmentation (AIF) is the DIA system developed for the Orbitrap. AIF, despite being named differently, is an acquisition mode similar to MSE in which all precursor ions are fragmented without a preselection by the quadrupole. Fragmentation, however, is obtained with the HCD cell, located at the far side of the C-trap. During filling of the HCD collision cells, the energy can be set to step between values at specified percent values around the chosen middle energy regardless of the ion’s characteristics. Figure 6 illustrates the AIF MS/MS spectrum of carbamazepine TPs, which was obtained in an additional experiment using a 10-eV. In HCD operation mode, ions were passed from the C-trap into a multipole collision cell, where they were fragmented and stored. The HCD cell voltages were then applied, and ions were transferred back into the C-trap and injected into the Orbitrap mass analyzer for detection.
Product ion mass spectra obtained using a quadrupole Orbitrap instrument by all-ion fragmentation for epoxycarbamazepine (epoxy) and trans-carbamazepine (TRANS) at 10 eV, and proposed fragmentation pathway for trans-carbamazepine. MS/MS tandem mass spectrometry, RDBE ring and double bond equivalents, R.T. retention time. (Reproduced from [86] with permission of Elsevier)
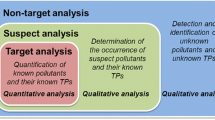
LC–HRMS enables one to determine many target polar contaminants and detect their TPs because of its sensitivity and selectivity across a wide range of m/z values. In addition to target compounds [77], LC–HRMS detects other contaminants not considered a priori in the analysis but present in the samples (screening of suspects or unexpected compounds against a library or database), even after measurement (post-target screening), and identifies the chemical nature of unknown or unidentifiable signals (Fig. 7).
Most of the wide-target and suspect screening is done using an algorithm that extracts m/z values from a database, either homemade or commercially provided by the instrument manufacturer. These databases automatically extract peaks of an accurate mass by comparing them with those compiled in a database of known compounds. In addition to the theoretical accurate mass (calculated according to the empirical formula), if available, other characteristics of the molecule, such as the isotopic pattern, double bounds (double bond equivalents), and MS/MS data, help to better match the possible chemicals [22, 45, 77]. The database can be automatically extended in the laboratory by injecting analytical standards, or if there are no analytical standards available, the empirical formula found in the literature can be added [44, 77].
The prediction of possible TPs to add their empirical formula to the library can be done using in silico prediction tools [20]. Commercially available or freely accessible programs have been applied in the prediction step, but more so in biochemical studies than in environmental ones.
The identification of unknown signals is commonly very difficult because there is no information available on the empirical formula corresponding to the analytes. Then, peaks are detected using automated peak detection and spectral deconvolution algorithms able to find thousands of peaks in an individual water sample [11, 15, 77]. Subsequently, the most probable empirical formula can be established according to the accurate mass and isotopic pattern of the mass spectrum corresponding to the peak. However, each molecular formula can be related to a quite large number of candidate structures, which have to be ranked or filtered to obtain a useful list of possible compounds for confirmation against the reference standards. In these cases, high-resolution instruments generate huge quantities of data that are difficult to export to the appropriate software and that are complex to evaluate. There is a range of free or commercial software able to predict MS properties of candidates, such as MSn fragmentation energies, product ion spectra, retention times, and ion mobility drift times, to facilitate the task by comparing predicted behavior with experimental data [28, 11, 77].
For this reason, postacquisition data-processing tools are necessary; computer-aided techniques provide rapid, accurate, and efficient data mining. There are quite a large number of software options for nontarget screening depending on the instrument vendor. The main problem with such software is the high price and the lack of compatibility between instruments. There are some free options, but they are often not user-friendly, as is required for analysts not specialized in bioinformatics [1, 74].
The use of mass spectral libraries for the confirmation of compounds is still limited for LC–(HR)MS data, as the libraries are small and the comparability of spectra is limited among different instruments. The compounds are identified by comparison with literature data, from retention times and MS and MS/MS analysis. The identity is confirmed on the basis of the mass accuracy of the molecular ion (5 ppm or less), the appropriate number of rings and double bonds, and the isotopic profile confirmed by simulation and the coincidence of the characteristic MS/MS data. The elucidation of the possible structure is done using chemical databases such as ChemSpider, and a search in the scientific literature related to the compound.
The ultimate confirmation of the identity always implies the use of analytical standards and the comparison of the behavior of the suspected substance identified with the behavior of the standards. This is time-consuming and very expensive.
Conclusions and future trends
The ability to perform identification of unknowns on the basis of accurate-mass measurements is one of the most powerful applications of HRMS to identify TPs of emerging contaminants. The adoption of “omics” techniques such as in the environmental sciences has opened up new horizons in the study of transformation processes. Unknown identification is further enhanced with MS/MS or multistage MS (MSn) since fragmentation of precursor ions reveals additional structural information that helps eliminate potential matches. In HRMS methods, the detection is performed over a wide m/z range (e.g., 50–1,000 m/z). Therefore, the possibility to reanalyze acquisition files to screen samples for the presence of “new” TPs is an attractive feature that is not possible using target analysis with quadrupole instruments.
It is important to stress that now that technological advancements have put such powerful instruments in the hands of scientists, emphasis should be given to the correct method. Key steps before HRMS analysis such as sample preconcentration and purification, ionization, and separation are thus essential to this technique. Sample preconcentration can be improved by using SPE phases with different chemistries such as reversed-phase columns and ion exchangers in order to maximize analyte retention. Finally, LC remains one of the most important aspects of method development since proper separation facilitates identification and can reduce matrix interferences. Recent techniques such as HILIC offer an interesting alternative to established techniques such as reversed-phase LC for the separation of polar compounds.
The applications outlined in this review for HRMS in environmental analysis of emerging contaminant TPs show its capacity to help solve some of the problems faced by scientists in the field because of the complexity of environmental samples. HRMS, coupled wit the use of proper sample preparation techniques, databases, and data-processing algorithms, has shown potential both for the screening of thousands of compounds simultaneously in a given sample and to identify new substances generated during wastewater treatment or in the environment. HRMS has the potential to become one of the most important analytical techniques for elucidation of TPs.
References
Hernández F, Ibañez M, Bade R, Bijlsma L, Sancho JV (2014) Investigation of pharmaceuticals and illicit drugs in waters by liquid chromatography-high-resolution mass spectrometry. Trends Anal Chem 63:140–157
Kosma CI, Lambropoulou DA, Albanis TA (2014) Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ 466:421–438
Carmona E, Andreu V, Picó Y (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci Total Environ 484:53–63
Devier MH, Mazellier P, Ait-Aissa S, Budzinski H (2011) New challenges in environmental analytical chemistry: identification of toxic compounds in complex mixtures. C R Chim 14:766–779
Andrés-Costa MJ, Rubio-López N, Morales Suárez-Varela M, Picó Y (2014) Occurrence and removal of drugs of abuse in wastewater treatment plants of Valencia (Spain). Environ Pollut 194:152–162
Van Doorslaer X, Dewulf J, Van Langenhove H, Demeestere K (2014) Fluoroquinolone antibiotics: an emerging class of environmental micropollutants. Sci Total Environ 500:250–269
Postigo C, Richardson SD (2014) Transformation of pharmaceuticals during oxidation/disinfection processes in drinking water treatment. J Hazard Mater 279:461–475
Liu J, Avendano SM (2013) Microbial degradation of polyfluoroalkyl chemicals in the environment: a review. Environ Int 61:98–114
Hübner U, von Gunten U, Jekel M (2015) Evaluation of the persistence of transformation products from ozonation of trace organic compounds – a critical review. Water Res 68:150–170
Toolaram AP, Kümmerer K, Schneider M (2014) Environmental risk assessment of anti-cancer drugs and their transformation products: a focus on their genotoxicity characterization-state of knowledge and short comings. Mutat Res 760:18–35
Petsas A, Vagi M, Nikolaou A, Kostopoulou M (2013) Trends in the analysis of pollutant transformation products in the marine environment. In: Proceedings of the 13th international conference on environmental science and technology, 474, CEST, Athens, Greece. http://cest2013.gnest.org/. Accessed 11 May 2015
Fatta-Kassinos D, Kalavrouziotis IK, Koukoulakis PN, Vasquez MI (2011) The risks associated with wastewater reuse and xenobiotics in the agroecological environment. Sci Total Environ 409:3555–3563
Mitrano DM, Motellier S, Clavaguera S, Nowack B (2015) Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int 77:132–147
Evgenidou EN, Konstantinou IK, Lambropoulou DA (2015) Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: a review. Sci Total Environ 505:905–926
Haddad T, Baginska E, Kümmerer K (2015) Transformation products of antibiotic and cytostatic drugs in the aquatic cycle that result from effluent treatment and abiotic/biotic reactions in the environment: an increasing challenge calling for higher emphasis on measures at the beginning of the pipe. Water Res 72:75–126
Agüera A (2012) Photodegradation pathways of emerging contaminants in water. In: Belgiorno V, Rizo L (eds) Emerging contaminants into the environment: contamination pathways and control, ASTER, Fisiciano, Italy, pp 27–44
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303
Postigo C, Barceló D (2015) Synthetic organic compounds and their transformation products in groundwater: occurrence, fate and mitigation. Sci Total Environ 503:32–47
Horvat AJM, Petrovic M, Babic S, Pavlovic DM, Asperger D, Pelko S, Mance AD, Kastelan-Macan M (2012) Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. Trends Anal Chem 31:61–84
Bletsou AA, Jeon J, Hollender J, Archontaki E, Thomaidis NS (2015) Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. Trends Anal Chem 66:32–44
Rosi-Marshall EJ, Royer TV (2012) Pharmaceutical compounds and ecosystem function: an emerging research challenge for aquatic ecologists. Ecosystems 15:867–880
Stasinakis AS (2012) Review on the fate of emerging contaminants during sludge anaerobic digestion. Bioresour Technol 121:432–440
Farré M, Kantiani L, Petrovic M, Pérez S, Barceló D (2012) Achievements and future trends in the analysis of emerging organic contaminants in environmental samples by mass spectrometry and bioanalytical techniques. J Chromatogr A 1259:86–99
Fischer K, Fries E, Korner W, Schmalz C, Zwiener C (2012) New developments in the trace analysis of organic water pollutants. Appl Microbiol Biotechnol 94:11–28
Jakimska A, Kot-Wasik A, Namiesnik J (2014) The current state-of-the-art in the determination of pharmaceutical residues in environmental matrices using hyphenated techniques. Crit Rev Anal Chem 44:277–298
Zonja B, Aceña J, Pérez S, Barceló D (2013) methods for elucidation of transformation pathways: identification of intermediate products, chiral, and isotope-ratio mass spectrometry analysis. In: Petrovic M, Barceló D, Pérez S (eds) Comprehensive analytical chemistry. Elsevier, Amsterdam, pp 593–610
Kosjek T, Perko S, Zupanc M, Hren MZ, Dragicevic TL, Zigon D, Kompare B, Heath E (2012) Environmental occurrence, fate and transformation of benzodiazepines in water treatment. Water Res 46:355–368
Hernández F, Sancho JV, Ibáñez M, Abad E, Portoles T, Mattioli L (2012) Current use of high-resolution mass spectrometry in the environmental sciences. Anal Bioanal Chem 403:1251–1264
Guillen D, Ginebreda A, Farré M, Darbra RM, Petrovic M, Gros M, Barceló D (2012) Prioritization of chemicals in the aquatic environment based on risk assessment: analytical, modeling and regulatory perspective. Sci Total Environ 440:236–252
Delgado LF, Charles P, Glucina K, Morlay C (2012) The removal of endocrine disrupting compounds, pharmaceutically activated compounds and cyanobacterial toxins during drinking water preparation using activated carbon—a review. Sci Total Environ 435:509–525
Delgado LF, Charles P, Glucina K, Morlay C (2012) QSAR-like models: a potential tool for the selection of PhACs and EDCs for monitoring purposes in drinking water treatment systems - a review. Water Res 46:6196–6209
Agüera A, Bueno MJM, Fernández-Alba AR (2013) New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ Sci Pollut Res 20:3496–3515
Zenker A, Cicero MR, Prestinaci F, Bottoni P, Carere M (2014) Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J Environ Manag 133:378–387
Clarke RM, Cummins E (2014) Evaluation of "classic" and emerging contaminants resulting from the application of biosolids to agricultural lands: a review. Hum Ecol Risk Assess 21:492–513
Lange FT, Scheurer M, Brauch HJ (2012) Artificial sweeteners—a recently recognized class of emerging environmental contaminants: a review. Anal Bioanal Chem 403:2503–2518
Zedda M, Zwiener C (2012) Is nontarget screening of emerging contaminants by LC-HRMS successful? A plea for compound libraries and computer tools. Anal Bioanal Chem 403:2493–2502
Nurmi J, Pellinen J, Rantalainen AL (2012) Critical evaluation of screening techniques for emerging environmental contaminants based on accurate mass measurements with time-of-flight mass spectrometry. J Mass Spectrom 47:303–312
Prasse C, Wagner M, Schulz R, Ternes TA (2011) Biotransformation of the antiviral drugs acyclovir and penciclovir in activated sludge treatment. Environ Sci Technol 45:2761–2769
Wick A, Wagner M, Ternes TA (2011) Elucidation of the transformation pathway of the opium alkaloid codeine in biological wastewater treatment. Environ Sci Technol 45:3374–3385
Wang N, Buck RC, Szostek B, Sulecki LM, Wolstenholme BW (2012) 5:3 polyfluorinated acid aerobic biotransformation in activated sludge via novel "one-carbon removal pathways". Chemosphere 87:527–534
Rubirola A, Llorca M, Rodriguez-Mozaz S, Casas N, Rodriguez-Roda I, Barceló D, Buttiglieri G (2014) Characterization of metoprolol biodegradation and its transformation products generated in activated sludge batch experiments and in full scale WWTPs. Water Res 63:21–32
Kosjek T, Negreira N, López de Alda M, Barceló D (2015) Aerobic activated sludge transformation of methotrexate: identification of biotransformation products. Chemosphere 119:S42–S50
Beel R, Eversloh CL, Ternes TA (2013) Biotransformation of the UV-filter sulisobenzone: challenges for the identification of transformation products. Environ Sci Technol 47:6819–6828
Luft A, Wagner M, Ternes TA (2014) Transformation of biocides Irgarol and terbutryn in the biological wastewater treatment. Environ Sci Technol 48:244–254
Chen X, Casas ME, Nielsen JL, Wimmer R, Bester K (2015) Identification of triclosan-O-sulfate and other transformation products of triclosan formed by activated sludge. Sci Total Environ 505:39–46
Mardal M, Meyer MR (2014) Studies on the microbial biotransformation of the novel psychoactive substance methylenedioxypyrovalerone (MDPV) in wastewater by means of liquid chromatography-high resolution mass spectrometry/mass spectrometry. Sci Total Environ 493:588–595
Huntscha S, Hofstetter TB, Schymanski EL, Spahr S, Hollender J (2014) Biotransformation of benzotriazoles: insights from transformation product identification and compound-specific isotope analysis. Environ Sci Technol 48:4435–4443
Gulde R, Helbling DE, Scheidegger A, Fenner K (2014) pH-dependent biotransformation of ionizable organic micropollutants in activated sludge. Environ Sci Technol 48:13760–13768
Tseng N, Wang N, Szostek B, Mahendra S (2014) Biotransformation of 6:2 fluorotelomer alcohol (6:2 FTOH) by a wood-rotting fungus. Environ Sci Technol 48:4012–4020
Llorca M, Rodríguez-Mozaz S, Couillerot O, Panigoni K, de Gunzburg J, Bayer S, Czaja R, Barceló D (2015) Identification of new transformation products during enzymatic treatment of tetracycline and erythromycin antibiotics at laboratory scale by an on-line turbulent flow liquid-chromatography coupled to a high resolution mass spectrometer LTQ-Orbitrap. Chemosphere 119:90–98
Mejia Avendano S, Liu J (2015) Production of PFOS from aerobic soil biotransformation of two perfluoroalkyl sulfonamide derivatives. Chemosphere 119:1084–1090
Li Z, Maier MP, Radke M (2014) Screening for pharmaceutical transformation products formed in river sediment by combining ultrahigh performance liquid chromatography/high resolution mass spectrometry with a rapid data-processing method. Anal Chim Acta 810:61–70
Terzic S, Senta I, Matosic M, Ahel M (2011) Identification of biotransformation products of macrolide and fluoroquinolone antimicrobials in membrane bioreactor treatment by ultrahigh-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Anal Bioanal Chem 401:353–363
Pérez-Parada A, Agüera A, Del Mar Gomez-Ramos M, Garcia-Reyes JF, Heinzen H, Fernández-Alba AR (2011) Behavior of amoxicillin in wastewater and river water: identification of its main transformation products by liquid chromatography/electrospray quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 25:731–742
Aceña J, Pérez S, Gardinali P, Abad JL, Eichhorn P, Heuett N, Barceló D (2014) Structure elucidation of phototransformation products of unapproved analogs of the erectile dysfunction drug sildenafil in artificial freshwater with UPLC-Q Exactive-MS. J Mass Spectrom 49:1279–1289
Diez-Mato E, Cortezon-Tamarit FC, Bogialli S, García-Fresnadillo D, Marazuela MD (2014) Phototransformation of model micropollutants in water samples by photocatalytic singlet oxygen production in heterogeneous medium. Appl Catal B 160:445–455
Eichhorn P, Pérez S, Aceña J, Gardinali P, Abad JL, Barceló D (2012) Identification of phototransformation products of sildenafil (Viagra) and its N-demethylated human metabolite under simulated sunlight. J Mass Spectrom 47:701–711
Jelic A, Michael I, Achilleos A, Hapeshi E, Lambropoulou D, Pérez S, Petrovic M, Fatta-Kassinos D, Barceló D (2013) Transformation products and reaction pathways of carbamazepine during photocatalytic and sonophotocatalytic treatment. J Hazard Mater 263:177–186
Souissi Y, Bouchonnet S, Bourcier S, Kusk KO, Sablier M, Andersen HR (2013) Identification and ecotoxicity of degradation products of chloroacetamide herbicides from UV-treatment of water. Sci Total Environ 458:527–534
Haddad T, Kümmerer K (2014) Characterization of photo-transformation products of the antibiotic drug ciprofloxacin with liquid chromatography–tandem mass spectrometry in combination with accurate mass determination using an LTQ-Orbitrap. Chemosphere 115:40–46
Michael I, Achilleos A, Lambropoulou D, Torrens VO, Pérez S, Petrovic M, Barceló D, Fatta-Kassinos D (2014) Proposed transformation pathway and evolution profile of diclofenac and ibuprofen transformation products during (sono)photocatalysis. Appl Catal B 147:1015–1027
Zonja B, Goncalves C, Pérez S, Delgado A, Petrovic M, Alpendurada MF, Barceló D (2014) Evaluation of the phototransformation of the antiviral zanamivir in surface waters through identification of transformation products. J Hazard Mater 265:296–304
Segura PA, Kaplan P, Yargeau V (2013) Identification and structural elucidation of ozonation transformation products of estrone. Chem Cent J 7:74
Tay KS, Rahman NA, Bin Abas MR (2013) Ozonation of metoprolol in aqueous solution: ozonation by-products and mechanisms of degradation. Environ Sci Pollut Res 20:3115–3121
Bautitz IR, Velosa AC, Nogueira RFP (2012) Zero valent iron mediated degradation of the pharmaceutical diazepam. Chemosphere 88:688–692
Sirtori C, Agüera A, Carra I, Pérez JAS (2014) Identification and monitoring of thiabendazole transformation products in water during Fenton degradation by LC-QTOF-MS. Anal Bioanal Chem 406:5323–5337
Ji YF, Ferronato C, Salvador A, Yang X, Chovelon JM (2014) Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: implications for remediation of groundwater contaminated by antibiotics. Sci Total Environ 472:800–808
Michael I, Hapeshi E, Aceña J, Pérez S, Petrovic M, Zapata A, Barceló D, Malato S, Fatta-Kassinos D (2013) Light-induced catalytic transformation of ofloxacin by solar Fenton in various water matrices at a pilot plant: mineralization and characterization of major intermediate products. Sci Total Environ 461:39–48
Eversloh CL, Henning N, Schulz M, Ternes TA (2014) Electrochemical treatment of iopromide under conditions of reverse osmosis concentrates - elucidation of the degradation pathway. Water Res 48:237–246
Grbovic G, Trebse P, Dolenc D, Lebedev AT, Sarakha M (2013) LC/MS study of the UV filter hexyl 2-[4-(diethylamino)-2-hydroxybenzoyl]-benzoate (DHHB) aquatic chlorination with sodium hypochlorite. J Mass Spectrom 48:1232–1240
Boix C, Ibáñez M, Bijlsma L, Sancho JV, Hernández F (2014) Investigation of cannabis biomarkers and transformation products in waters by liquid chromatography coupled to time of flight and triple quadrupole mass spectrometry. Chemosphere 99:64–71
Jewell KS, Wick A, Ternes TA (2014) Comparisons between abiotic nitration and biotransformation reactions of phenolic micropollutants in activated sludge. Water Res 48:478–489
Laurence C, Rivard M, Martens T, Morin C, Buisson D, Bourcier S, Sablier M, Oturan MA (2014) Anticipating the fate and impact of organic environmental contaminants: a new approach applied to the pharmaceutical furosemide. Chemosphere 113:193–199
Ibáñez M, Gracia-Lor E, Sancho JV, Hernández F (2012) Importance of MS selectivity and chromatographic separation in LC-MS/MS-based methods when investigating pharmaceutical metabolites in water. Dipyrone as a case of study. J Mass Spectrom 47:1040–1046
Hernández F, Ibáñez M, Gracia-Lor E, Sancho JV (2011) Retrospective LC-QTOF-MS analysis searching for pharmaceutical metabolites in urban wastewater. J Sep Sci 34:3517–3526
Gómez-Ramos MD, Pérez-Parada A, Garcia-Reyes JF, Fernandez-Alba AR, Aguera A (2011) Use of an accurate-mass database for the systematic identification of transformation products of organic contaminants in wastewater effluents. J Chromatogr A 1218:8002–8012
Hug C, Ulrich N, Schulze T, Brack W, Krauss M (2014) Identification of novel micropollutants in wastewater by a combination of suspect and nontarget screening. Environ Pollut 184:25–32
Masiá A, Ibáñez M, Blasco C, Sancho JV, Picó Y, Hernández F (2013) Combined use of liquid chromatography triple quadrupole mass spectrometry and liquid chromatography quadrupole time-of-flight mass spectrometry in systematic screening of pesticides and other contaminants in water samples. Anal Chim Acta 761:117–127
Masiá A, Campo J, Blasco C, Picó Y (2014) Ultra-high performance liquid chromatography–quadrupole time-of-flight mass spectrometry to identify contaminants in water: an insight on environmental forensics. J Chromatogr A 1345:86–97
Muller A, Schulz W, Ruck WKL, Weber WH (2011) A new approach to data evaluation in the non-target screening of organic trace substances in water analysis. Chemosphere 85:1211–1219
López SH, Ulaszewska MM, Hernando MD, Bueno MJM, Gómez MJ, Fernández-Alba AR (2014) Post-acquisition data processing for the screening of transformation products of different organic contaminants. Two-year monitoring of river water using LC-ESI-QTOF-MS and GCxGC-EI-TOF-MS. Environ Sci Pollut Res 21:12583–12604
Hernández F, Portoles T, Ibáñez M, Bustos-Lopez MC, Diaz R, Botero-Coy AM, Fuentes CL, Peñuela G (2012) Use of time-of-flight mass spectrometry for large screening of organic pollutants in surface waters and soils from a rice production area in Colombia. Sci Total Environ 439:249–259
Souchier M, Benali-Raclot D, Benanou D, Boireau V, Gomez E, Casellas C, Chiron S (2015) Screening triclocarban and its transformation products in river sediment using liquid chromatography and high resolution mass spectrometry. Sci Total Environ 502:199–205
Chiaia-Hernández AC, Krauss M, Hollender J (2013) Screening of lake sediments for emerging contaminants by liquid chromatography atmospheric pressure photoionization and electrospray ionization coupled to high resolution mass spectrometry. Environ Sci Technol 47:976–986
Bueno MJM, Boillot C, Munaron D, Fenet H, Casellas C, Gomez E (2014) Occurrence of venlafaxine residues and its metabolites in marine mussels at trace levels: development of analytical method and a monitoring program. Anal Bioanal Chem 406:601–610
Bueno MJM, Boillot C, Fenet H, Chiron S, Casellas C, Gomez E (2013) Fast and easy extraction combined with high resolution-mass spectrometry for residue analysis of two anticonvulsants and their transformation products in marine mussels. J Chromatogr A 1305:27–34
Vona A, di Martino F, Garcia-Ivars J, Picó Y, Mendoza-Roca JA, Iborra-Clar MI (2015) Comparison of different removal techniques for selected pharmaceuticals. J Water Process Eng 5:48–57
Pascual-Aguilar J, Andreu V, Gimeno-García E, Picó Y (2015) Current anthropogenic pressures on agro-ecological protected coastal wetlands. Sci Total Environ 503–504:90–199
Acknowledgments
This work was supported by the Spanish Ministry of Economy and Competitiveness through the projects “Assessing and Predicting Effects on Water Quantity and Quality in Iberian Rivers Caused by Global Change (SCARCE)” (no. CSD2009-00065; http://www.scarceconsolider.es) and “Evaluation of Emerging Contaminants in the Turia River Basins: From Basic Research to the Application of Environmental Forensics (EMERFOR)” (GCL2011-29703-C02-02; http://mefturia.es).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection High-Resolution Mass Spectrometry in Food and Environmental Analysis with guest editor Aldo Laganà.
Rights and permissions
About this article
Cite this article
Picó, Y., Barceló, D. Transformation products of emerging contaminants in the environment and high-resolution mass spectrometry: a new horizon. Anal Bioanal Chem 407, 6257–6273 (2015). https://doi.org/10.1007/s00216-015-8739-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8739-6