Abstract
A extraction medium based on chitosan-poly(m-phenylenediamine) (CS-PPD) @Fe3O4 nanocomposite was synthesized by chemical polymerization of m-phenylenediamine in the presence of chitosan coated magnetic nanocomposite, and for the first time, used as the sorbent for the magnetic solid-phase extraction (MSPE) of seven polychlorinated biphenyls (PCB28, PCB52, PCB101, PCB118, PCB138, PCB153, and PCB180) at trace levels in water samples. Gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) was used for PCBs quantification and detection. Several factors related to MSPE efficiencies, such as type and amount of sorbent, extraction time, sample pH, and desorption conditions were investigated. Under the optimized conditions, an excellent linearity was observed in the range of 1.0–200 ng L–1 for PCB180, 0.5–200 ng L–1 for the other six PCBs with the correlation coefficients ranging from 0.9954 to 0.9993. The good recoveries at spiked levels of 10.0, 20.0, and 50.0 ng L–1 were obtained in the range of 94 %–108 %, and the coefficients of variations were less than 6 %. The proposed method was feasible, rapid, and easy to operate for the trace analysis of the PCBs in local aquaculture water, livestock breeding water, and sewage water samples.

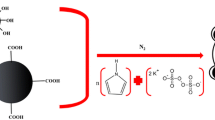
Fig. 1 Schematic diagram for the preparation of chitosan–poly(m-phenylenediamine) @Fe3O4 nanocomposite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polychlorinated biphenyls (PCBs) are one of the most widespread and persistent environmental pollutants since the middle of the last century [1, 2], and they are mainly used in transformers, capacitors, and paper and paint industry [3, 4]. Migration of chemicals from soil to water and vice versa may cause an accumulation of multiple residues in water and agricultural products designed for animal or human consumption [2]. Owing to their low water solubility, hydrophobic character, and resistance to metabolic degradation, PCBs pose a risk to environmental and human health. Although PCBs were banned many years ago, they have still occurred in the environment and food chains. Therefore, it is of great importance to develop an analytical method that is sensitive, rapid, and robust to quantify the trace levels of PCBs in the environment.
PCB residues are often at trace levels in the environment, so it is necessary to use a preconcentration step before their analysis. Liquid–liquid extraction (LLE) and solid phase extraction (SPE) are two classic and most widely used sample preparation methods for the analysis of PCBs [5–9]. However, LLE has several disadvantages; it is time-consuming, loss of target analytes, and large consumption of hazardous organic solvents. SPE often suffers from the plugging of cartridge, and the cartridge is expensive and not reusable. Therefore, many micro-extraction techniques involving no or small amount of solvents are developed over the past decades, such as solid-phase microextraction (SPME) [10–13] and liquid-phase microextraction (LPME) [14–17]. SPME is a practical solvent-free alternative for the extraction of PCBs from environmental samples. However, SPME is expensive, the fiber used is fragile and has a limited lifetime, and sample carryover is also a problem. LPME has several advantages with short extraction time, low cost, simplicity of operation, high enrichment factor, and can extract analytes into only a few microliters of organic solvent, but LPME is limited by the volume of the sample.
Magnetic solid-phase extraction (MSPE), which has drawn extensive attention in sample preparation in recent years, is a new mode of SPE based on the adoption of magnetic nanocomposite as sorbents. As for sample treatment procedures, the magnetic nanocomposite can be easily isolated from sample matrix with the aid of an external magnetic field without additional filtration or centrifugation procedure, which makes sample collection and separation easier and faster [18, 19]. Moreover, some magnetic adsorbents can be easily recycled after a simple washing operation. Finally, MSPE is suitable for direct analysis of samples containing particles or microorganisms, which may arouse blockage and lead to extraction failure on conventional SPE cartridges [20]. All of these merits mentioned above render MSPE as a promising technique for sample preparation. Recently, magnetic nanocomposite as adsorption materials have been employed for removal of trace PCBs from environment water samples [21–23]. The enrichment performances are determined by surface-modified material and structure of the used magnetic nanocomposite. Among different types of coating sorbents used for the extraction of organic analytes, composite of conductive polymers, because of their multifunctional properties, including hydrophobicity, acid-base character, π–π interaction, polar functional groups, ion exchange property, hydrogen bonding, and electroactivity, were quite prominent [24–26]. The different surface-modified material may result in different enrichment performance. Even if the same modified material was used, the different structure may also result in different enrichment performance [27].
In this work, chitosan-poly(m-phenylenediamine) (CS-PPD) @ Fe3O4 core-shell nanocomposite was synthesized for the MSPE of PCBs from water samples. The analyte concentration in the eluent was determined by gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) detection. Several factors related to MSPE efficiencies, such as type and amount of sorbent, extraction time, sample pH, and desorption conditions were investigated. The developed method was applied to the analysis of different aqueous samples for the determination of PCBs.
Experimental
Chemicals and standard solutions
Chitosan (CS), ammonium peroxydisulfate (APS), hydrochloric acid, sodium hydroxide, acetic acid, ethyl acetate, cyclohexane, acetone, FeCl3·6H2O, FeCl2·4H2O, and m-phenylenediamine (PD) were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). HPLC-grade n-hexane was purchased from Merck (Darmstadt, Germany). An N35-gradeNdFeB magnet (60 × 20 × 10 mm) was used for magnetic separation, which was purchased from Guanneng Magnetic (Yinzhou, Ningbo, China). 2,4,4′-Trichlorobiphenyl (PCB 28), 2,2′,5,5′-tetrachlorobiphenyl (PCB 52), 2,2′,4,5,5′-pentachlorobiphenyl (PCB 101), 2,3,4,4′,5-pentachlorobiphenyl (PCB 118), 2,2′,3,4,4′5-hexachlorobiphenyl (PCB 138), 2,2′,4,4′,5,5′-hexachlorobiphenyl (PCB 153), and 2,2,3,4,4′,5,5′-heptachlorobiphenyl (PCB 180) were selected as representative congeners of polychlorinated biphenyls (PCBs). A mixed standard solution with each concentration of 10 μg mL–1 for PCB28, PCB 52, PCB 101, PCB 118, PCB 138, PCB 153, PCB 180 were obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The working stock solution (0.1 μg mL–1) and the working solution were prepared by diluting the working stock solution with n-hexane to the corresponding concentrations, and stored at 4 °C until use. Ultrapure water was obtained from a Milli-Q system from Millipore (Milford, MA, USA). Twelve aquaculture water samples, 17 livestock breeding water samples, and six sewage water samples were collected from breeding base and sewage treatment plant in Jiangxi Province of China.
Synthesis of CS@ Fe3O4
First, 4.16 g FeCl3·6H2O and 1.6 g FeCl2·4H2O were dissolved into 200 mL acetic acid aqueous solution (0.25 % v/v) containing 2.5 g L−1 CS. After being stirred for 1 h at 40 °C under nitrogen atmosphere, sodium hydroxide solution (10 %) was added drop by drop into the solution under vigorous stirring for 1 h. The mixed hemimicelle of CS formed on the surface of Fe3O4 nanoparticles. Finally, the resulting brown precipitates were collected using a permanent magnet and washed consecutively with sodium hydroxide solution and doubly distilled water.
Preparation of chitosan-poly(m-phenylenediamine) (CS–PPD) @ Fe3O4 nanocomposite
The synthesis procedure for CS-PPD magnetic nanocomposite was performed according to self-assembly approach. CS @Fe3O4 had hydrophobic and hydrophilic moieties so they could facilitate the dissolution of m-phenylenediamine. CS-PPD magnetic nanocomposite was synthesized by addition of above-mentioned CS @Fe3O4 to 160 mL water containing different amounts of m-phenylenediamine monomers, stirring for 1 h at room temperature under the nitrogen atmosphere. Then saturated APS, as initiator, was added to the solution and stirred for 4 h at room temperature and CS-PPD magnetic nanocomposite was obtained (Fig. 1). The black CS-PPD@ Fe3O4 nanocomposite was collected using a permanent magnet and washed three times in double distilled water and methanol. The washing procedure was continued until the filtrate became colorless.
The size and morphology of the magnetic nanocomposite were investigated by using a FEI Quanta 200 scanning electron microscope (SEM) (Philips-FEI, The Netherlands). Magnetic properties were analyzed by using a vibrating sample magnetometer (Lake Shore 7410 VSM, USA).
MSPE procedure
Ten milligrams of magnetic nanocomposite was dispersed into 100.0 mL of water sample under shaking for 3 min. Then, the NdFeB magnet was held at the bottom of the flask and the sorbent was isolated from the suspension. After about 5 s, the suspension became clear and was decanted. The residual sorbent was eluted with 10.0 mL of acetone/n-hexane (1:1, v/v) to desorb PCBs. Subsequently, the eluted solution was dried under a mild stream of nitrogen at 40 °C. Finally, the residue was reconstituted in 200 μL of n-hexane, and 1.0 μL was used for GC-MS/MS analysis. After PCBs were desorbed from the magnetic sorbent with the NdFeB magnet, the sorbent was recycled by washing with 5.0 mL acetone/n-hexane (1:1, v/v) twice.
GC-MS/MS analysis
Analysis of seven PCB congeners was performed on a 7890A GC interfaced to 7000A triple quadrupole mass spectrometer system (Agilent Technologies, Palo Alto, CA, USA) in the MS/MS mode. The temperatures for the injection port, transfer line, and ion source were set at 270, 270, and 230 °C, respectively, and a solvent delay of 8 min was selected. Helium was used as the carrier gas and quench gas at a constant flow of 1.2 mL min–1 and 2.25 mL min–1, respectively. Nitrogen was used as collision gas at 1.5 mL min–1. A fused silica DB-1701P capillary column (Agilent Technologies, 30 m × 0.25 mm i.d. × 0.25 μm) was used for separation. The column temperature was programmed from 120 °C (held 1 min) at 20 °C min–1 to 220 °C, finally ramped at 8 °C min–1 to 270 °C (held 3 min). The target compounds were unambiguously identified by comparison of their retention times, two ions of the product ion cluster, and the abundance ratio of corresponding product ions to those of the standards. Parameters such as MRM transition (precursor ion→ product ion) and collision energy are presented in Table 1. The data acquisition and analysis were performed by MassHunter WorkStation (Agilent Technologies).
Results and discussion
Morphology and synthesizing mechanism
CS@Fe3O4 is expected to play an important role in the formation of PPD-based nanocomposites, since co-precipitation from aqueous Fe(II) and Fe(III) salt solutions is performed in the presence of CS, then with the addition of sodium hydroxide. These negative charges of Fe3O4 nanoparticles at above 6.5 of pH, due to electrostatic attraction force and coordination bond with positively charge of –NH2 and –OH group in CS, could hold CS on the surface of Fe3O4 nanoparticles. In an aqueous solution containing both m-phenylenediamine and CS@Fe3O4, a complex formation is expected because of the acid-base type and hydrogen bond interactions between the –OH and –NH2 group in CS and –NH2 group in m-phenylenediamine monomers, which could hold these monomers over the surface of CS@Fe3O4. Upon dropwise addition of an APS solution, polymerization only takes place on the surface of the CS@Fe3O4. Therefore, it is expected to form core-shell type self-assembled CS-PPD magnetic nanocomposite, in which CS@Fe3O4 acts as the “core” and PPD acts as the “shell” of the prepared composite.
The SEM images of the Fe3O4@CS (Fig. 2a) and the CS-PPD magnetic nanocomposite (Fig. 2b) show a more porous structure for the latter composite.
The magnetization curves show that CS@Fe3O4 and CS-PPD@Fe3O4 exhibit typical superparamagnetic behavior because of no hysteresis (Fig. 2c). There is no remanence and coercivity, suggesting that such nanocomposite is superparamagnetic. The saturation intensities of magnetization are 58.4 emu g−1 for CS@Fe3O4 and 40.5 emu g−1 for CS-PPD@Fe3O4, which are sufficient for magnetic separation with a conventional magnet. Apparently, the nonmagnetic PPD on the CS@Fe3O4 results in the decrease of the magnetic strength for CS@Fe3O4.
Optimization of extraction conditions
In order to achieve satisfactory extraction efficiency of the proposed MSPE procedure for the PCBs, several parameters that may affect the extraction efficiency were optimized, such as the amount and type of the sorbent, desorption solvent, solution volume, and the extraction time. The influences of all these parameters were evaluated in terms of recovery rate. The optimization experiments were conducted using spiked blank water sample containing 10.0 ng L–1 of each PCB. Each experiment was performed in triplicate.
Effect of the type of sorbent
The morphology and structure of sorbent are key factors in the extraction strategy. In this study, the extraction capabilities of magnetic nanocomposite coated with CS and CS-PPD were examined by extracting PCBs, as model compounds, from aquatic media. According to the obtained results from Fig. 3, the recoveries of PCBs had a significant increase in the presence of PPD, which indicated that PPD had a vital role in the extraction process.
Effect of solution pH
The pH of sample solution could influence the extraction performance of the analytes by changing both the existing forms of the target compounds and the species and density of charges on the adsorbent surface. The pH values of the sample solutions were adjusted with 1 mol L–1 HCl and 1 mol L–1 NaOH aqueous solutions. As shown in Fig. 4A, the extraction recoveries of the seven PCBs were acceptable in the entire pH range of 3.0–11.0, demonstrating that the sorbent could be applied to extract PCBs in a wider pH range. Since the pH of water sample was generally in the range 6.0–8.0, there was no need to adjust the pH of the sample solution before extraction in the following experiments.
Effect of the sorbent amount and extraction time
To appraise the effect of sorbent quantity on the extraction efficiency, different amounts of sorbent within the range of 2.0–20 mg were added to the solution. The results, as illustrated in Fig. 4B, showed that the best extraction efficiency of PCBs could be obtained using 10 mg of the sorbent. Compared with the ordinary sorbents, nano-sized sorbents have higher surface areas, therefore, satisfactory results can be obtained by lower amounts of nano-sized sorbents.
To reveal the effect of extraction time on extraction efficiency, extraction time was varied in the range of 0.5–5 min. It was found that extending the extraction time more than 3 min had no effect on peak area of PCBs, so 3 min was selected as the extraction time. Such a fast adsorption rate could be attributed to the absence of an internal diffusion resistance, since the adsorption of the PCBs occurred only on the surface of the sorbent.
Effect of the salt content of the sample
To investigate the effect of salinity on the extraction efficiency of the target compounds, the concentration of NaCl in sample solution was varied in the range of 0–15 % (w/v). As shown in Fig. 5A, NaCl addition had a significant negative effect on the MSPE efficiency. It was due to the fact that the aqueous solution viscosity would increase with the addition of salt, which resulted in difficult mass transfer and low extraction efficiency. Moreover, it was believed that the addition of salt might change the physical properties of the Nernst diffusion film, reduced the diffusion rates of solutes from water to the adsorbent surface, and ultimately affected the extraction efficiency [28]. Therefore, no salt was added in subsequent experiments.
Desorption conditions
The desorption solvent is crucial for obtaining a satisfactory desorption efficiency for the analytes. Several organic solvents, including acetone, ethyl acetate, cyclohexane, and n-hexane and acetone/n-hexane (1:1, v/v) were used to elute the PCBs from the magnetic sorbent. As shown in Fig. 5B, acetone/n-hexane (1:1, v/v) gained the highest desorption efficiency. Thus, acetone/n-hexane (1:1, v/v) was selected as the desorption solvent. Furthermore, the influence of the elution volume of acetone/n-hexane (1:1, v/v) from 2 to 12 mL on desorption efficiency was also studied. According to the experiments, all the analytes could be completely desorbed from the sorbent by rinsing with 10 mL of acetone/n-hexane (1:1, v/v). Desorption time was evaluated within the range of 0.5–2 min. The results showed that the time of 1 min appeared to be the optimum value for the elution of analytes.
Effect of solution volume
In environmental water samples, the concentrations of targets are usually much lower than the detection limit of analytical instruments. It is generally necessary to enrich target analytes from large volumes of water samples to get a high preconcentration factor. Fortunately, the MSPE technique avoids the time-consuming step of column loading or pressure filtration and exhibits great potential in pretreatment of large volumes of water samples. To investigate the effect of sample volume on PCBs recoveries, the volume of water sample was changed from 20 mL to 100 mL. The recoveries of all the analytes remained constant as sample volume increased from 20 mL to 100 mL. The high extraction ability of the sorbents to PCBs was caused by their large surface areas and strong adsorption ability of m-phenylenediamine coat under the large volume sample.
Reusability of the sorbents
In order to investigate the recycling of the magnetic sorbents, the sorbent was rinsed with 5 mL of acetone/n-hexane (1:1, v/v) twice before application in the next time. After 10 times of recycling, there was no obvious decrease or increase for the recoveries of analytes. The results indicated that the sorbent was reusable with no analyte carryover during MSPE procedure, showing good reusability.
Method evaluation
The linear range of the method was established using blank water samples spiked with the target compounds at six levels from 0.5 or 1.0 to 200 ng L–1 for the PCBs, each injected in triplicate. The correlation coefficients were between 0.9954 and 0.9993. Limits of detection (LOD) and of quantification (LOQ) were calculated by extrapolation of the concentrations giving a signal-to-noise ratio (S/N) of 3 and 10, respectively. The LODs ranged from 0.11 to 0.32 ng L−1, whereas the LOQs ranged from 0.5 to 1.0 ng L−1 (Table 1). The developed method was used to determine PCBs in local aquaculture water, livestock breeding water, and sewage water samples. The analytical results are summarized in Table 2. The recoveries of spiked 10, 20, and 50 ng L−1 PCBs samples ranged from 94 % to 108 %, and the repeatability of the method expressed as relative standard deviations (RSDs) for six replicates ranged from 3 % to 6 %, which demonstrating that the proposed method was suitable, reliable, and reproducible. The representative extracted chromatogram of the PCBs obtained from water samples by the proposed method is shown in Fig. 6, illustrating symmetrical peak shapes and high resolution.
Comparison to previously reported methods
To highlight the application of the magnetic nanocomposite for the determination of PCBs, the proposed method was compared to several published methods for the determination of PCBs from water samples, such as SPE [9], SPME [11], and LPME [17]. According to Table 3, the proposed method considerably accelerated the sample preparation procedure and chromatographic separation time because only 20 min was required to the sample preparation and 15.25 min was required to separate PCBs with high resolution. Moreover, the magnetic adsorbent could be easily and quickly isolated within 5 s and recycled from water samples with an external magnetic field. Additionally, the recoveries and RSDs of the proposed method were better than those obtained with other methods.
Determination of PCBs in environmental water samples
In order to evaluate the applicability of the proposed method, a survey on PCBs in real environmental water samples including aquaculture water, livestock breeding water, sewage water collected in breeding base, and sewage treatment plant was performed. The results indicated that PCB 138 and PCB 153 were found in two sewage water samples with their concentrations ranging from 15.7 ng L–1 to 37.6 ng L–1, and other samples were not contaminated by PCBs.
Conclusion
In the present work, CS-PPD@Fe3O4 core-shell magnetic nanocomposite was used as the sorbent for the MSPE of PCBs at trace levels in water samples. Combined with GC-MS/MS, the developed method offered excellent sensitivity, wide linear range, and ease of operational as well as satisfactory recovery and repeatability under the optimized conditions. The method was successfully used to analyze real environmental water samples with less than 20 min of sample preparation time, which was an advantage relative to other methods. The results demonstrated that the magnetic nanocomposite was a promising material for the MSPE of trace analyte from environmental water samples.
References
Ahmed FE (2003) Analysis of polychlorinated biphenyls in food products. Trends Anal Chem 22:170–185
Brouwer A, Ahlborg UG, van Leeuwen FXR, Feeley MM (1998) Report of the who working group on the assessment of health risks for human infants from exposure to PCDDS, PCDFS, and PCBS. Chemosphere 37:1627–1643
Guéguen F, Stille P, Millet M (2013) Optimization and application of accelerated solvent extraction and flash chromatography for quantification of PCBs in tree barks and XAD-2 passive samplers using GC-ECD with dual columns. Talanta 111:140–146
Liu SS, Liu Y, Yin DQ, Wang XD, Wang LS (2006) Prediction of chromatographic relative retention time of polychlorinated biphenyls from the molecular electronegativity distance vector. J Sep Sci 29:296–301
Zaater M, Tahboub Y, Qasrawy S (2005) Monitoring of polychlorinated biphenyls in surface water using liquid extraction, GC/MS, and GC/ECD. Anal Lett 38:2231–2245
Westbom R, Thörneby L, Zorita S, Mathiasson L, Björklund E (2004) Development of a solid-phase extraction method for the determination of polychlorinated biphenyls in water. J Chromatogr A 1033:1–8
Zhou Q, Wu W, Xiao J (2013) Solid phase extraction with silicon dioxide microspheres for the analysis of polychlorinated biphenyls in environmental water samples prior to gas chromatography with electron capture detector. Int J Environ Anal Chem 93:894–905
Xing H, Chen X, Wang X, Wang M, Zhao R (2014) Feasibility of hydrofluoric acid etched sand particles for enrichment and determination of polychlorinated biphenyls at trace levels in environmental water samples. Anal Bioanal Chem 406:3787–3793
Yang F, Jin S, Meng D, Xu Y (2012) Solid phase extraction with pyrenebutyric acid-bonded silica for analysis of polychlorinated biphenyls in sewage water by gas chromatography-mass spectrometry. Chemosphere 81:1000–1005
Wu Y-Y, Yang C-X, Yan X-P (2014) Fabrication of metal-organic framework MIL-88B films on stainless steel fibers for solid-phase microextraction of polychlorinated biphenyls. J Chromatogr A 1334:1–8
Wang Y, Li Y, Zhang J, Xu S, Yang S, Sun C (2009) A novel fluorinated polyaniline-based solid-phase microextraction coupled with gas chromatography for quantitative determination of polychlorinated biphenyls in water samples. Anal Chim Acta 646:78–84
Hawthone SB, Grabanski CB, Miller DJ (2009) Solid-phase-microextraction measurement of 62 polychlorinated biphenyl congeners in milliliter sediment pore water samples and determination of K DOC values. Anal Chem 81:6936–6943
Derouiche A, Driss MR, Morizur JP, Taphane MH (2007) Simultaneous analysis of polychlorinated biphenyls and organochlorine pesticides in water by headspace solid-phase microextraction with gas chromatography-tandem mass spectrometry. J Chromatogr A 1138:231–243
Rezaei F, Bidari A, Birjandi AP, Hosseini MRM, Assadi Y (2008) Development of a dispersive liquid–liquid microextraction method for the determination of polychlorinated biphenyls in water. J Hazard Mater 158:621–627
Dai L, Cheng J, Matsadiq G, Liu L, Li J (2010) Dispersive liquid–liquid microextraction based on the solidification of floating organic droplet for the determination of polychlorinated biphenyls in aqueous samples. Anal Chim Acta 674:201–205
Ozcan S, Tor A, Aydin ME (2009) Determination of selected polychlorinated biphenyls in water samples by ultrasound-assisted emulsification-microextraction and gas chromatography-mass-selective detection. Anal Chim Acta 647:182–188
Li G, Zhang L, Zhang Z (2008) Determination of polychlorinated biphenyls in water using dynamic hollow fiber liquid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr A 1204:119–122
Yan H, Gao M, Yang C, Qiu M (2014) Ionic liquid-modified magnetic polymeric microspheres as dispersive solid phase extraction adsorbent: a separation strategy applied to the screening of sulfamonomethoxine and sulfachloropyrazine from urine. Anal Bioanal Chem 406:2669–2677
Moliner-Martinez Y, Vitta Y, Prima-Garcia H, González-Fuenzalida RA, Ribera A, Campíns-Falcó P, Coronado E (2014) Silica supported Fe3O4 magnetic nanoparticles for magnetic solid-phase extraction and magnetic in-tube solid-phase microextraction: application to organophosphorous compounds. Anal Bioanal Chem 406:2211–2215
Ding J, Gao Q, Luo D, Shi ZG, Feng YQ (2010) n-Octadecylphosphonic acid grafted mesoporous magnetic nanoparticle: preparation, characterization, and application in magnetic solid-phase extraction. J Chromatogr A 1217:7351–7358
Cao X, Chen J, Ye X, Zhang F, Shen L, Mo W (2013) Ultrasound-assisted magnetic SPE based on Fe3O4-grafted graphene for the determination of polychlorinated biphenyls in water samples. J Sep Sci 36:3579–3585
Karamani AA, Douvalis AP, Stalikas CD (2013) Zero-valent iron/iron oxide-oxyhydroxide/graphene as a magnetic sorbent for the enrichment of polychlorinated biphenyls, polyaromatic hydrocarbons, and phthalates prior to gas chromatography-mass spectrometry. J Chromatogr A 1271:1–9
Chen X, Ding N, Zang H, Yeung H, Zhao R-S, Cheng C, Liu J, Chan T-WD (2013) Fe3O4@MOF core-shell magnetic microspheres for magnetic solid-phase extraction of polychlorinated biphenyls from environmental water samples. J Chromatogr A 1304:241–245
Lakouraj MM, Zare EN, Moghadam PN (2014) Synthesis of novel conductive poly(p-phenylenediamine)/Fe3O4 nanocomposite via emulsion polymerization and investigation of antioxidant activity. Adv Polym Technol 33:21385
Baghayeri M, Nazarzadeh ZE, Mansour LM (2014) A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly(p-phenylenediamine)@Fe3O4 nanocomposite. Biosens Bioelectron 55:259–265
Yang S, Liu D, Liao F, Guo T, Wu Z, Zhang T (2012) Synthesis, characterization, morphology control of poly (p-phenylenediamine)-Fe3O4 magnetic micro-composite, and their application for the removal of Cr2O7 2− from water. Synth Met 162:2329–2336
Zhou Q, Huang Y, Xie G (2012) Investigation of the applicability of highly ordered TiO2 nanotube array for enrichment and determination of polychlorinated biphenyls at trace level in environmental water samples. J Chromatogr A 1237:24–29
Tahmasebi E, Yamini Y, Seidi S, Rezazadeh M (2013) Extraction of three nitrophenols using polypyrrole-coated magnetic nanoparticles based on anion exchange process. J Chromatogr A 1314:15–23
Acknowledgments
This work was supported by an innovation fund of Jiangxi Academy of Agricultural Sciences (no. 2013CJJ003) and National quality and safety risk assessment of livestock and poultry products in 2014 (no. GJFP2014007). The authors are grateful to Dr. Jian Ling (Yunnan University, China) for providing the SEM apparatus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, Q.G., Wang, D.G. & Luo, L.G. Chitosan-poly(m-phenylenediamine)@Fe3O4 nanocomposite for magnetic solid-phase extraction of polychlorinated biphenyls from water samples. Anal Bioanal Chem 406, 7571–7579 (2014). https://doi.org/10.1007/s00216-014-8215-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8215-8