Abstract
A solid-phase microextraction was performed using Fe3O4 nanoparticle coated with the chitosan, as a green and biodegradable polymer, for the pre-concentration of La (III) in aqueous solutions. The Fe3O4 magnetic nanoparticles were initially synthesized and properly coated with chitosan as adsorbent, and desorption step was successfully conducted using nitric acid. After desorption, analyte was determined with ICP-OES. The effects of different parameters, including pH, nanoparticle amount, concentration and volume of desorption acid, adsorption and desorption time, stirring rate, temperature and salt effect on the microextraction process, were investigated. The calibration curve was plotted, and a linear response range of 0.008–0.1 ng L−1 with a detection limit of 0.008 ng L−1 was obtained. This method was successfully implemented on two samples of river water, and favorable results were observed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lanthanum is one of the most abundant elements on the earth’s crust and soils. Lanthanum is amount of 18.3 g/metric ton in the Earth’s crust [1]. The element is usually extracted from some sources, such as Monaxit, Bastnasite, Allanit and Cerite [2, 3]. Lanthanides have a valuable potential for use as an alloy due to their proprietary properties. The elements of lanthanides and their compounds are highly paramagnetic. This has important results because the measurement of the magnetic sensitivity of lanthanide compounds can give a precise estimate of the electrical charge of these compounds [4]. Lanthanides have many uses, including their applications in catalysts, fuel cells and wireless technologies, as well as in nuclear, construction and spatial industries, medical and dental areas and lasers, fertilizers, superconductors and optical fibers [5].
Lanthanides are generally non-toxic because they cannot pass through cell membranes. Therefore, they are not absorbed through the mouth. However, if they are injected intravenously, they can be poisonous because they can pass through cells through calcium channels.
Lanthanide complexes play an essential role in treating cancer, which is commonly used in MRI imaging of tumors [6]. Lanthanide radioisotopes have also been studied for both photography and treatment. This unique feature allows the creation of probes based on lanthanides for biological studies, such as investigating the interactions of metals with proteins or the detection of calcium in biological environments [7].
Chitosan is a natural polysaccharide with many useful features such as hydrophilicity, biocompatibility, biodegradability, antibacterial properties and remarkable affinity for many bio-macromolecules. Chitosan and its derivatives have great potential applications in the areas of biotechnology, bio-medicine, food ingredients and cosmetics [8,9,10]. Noteworthily, chitosan is also capable of adsorbing a number of metal ions because its amino groups can serve as chelation sites [11,12,13]. Chitosan is a suitable biopolymer, holds rich amino and hydroxyl groups and is regarded as a useful starting support for adsorption purposes [14, 15]. Thus, the binding of chitosan onto Fe3O4 nanoparticles will probably yield another novel magnetic nanoadsorbent for the efficient removal of heavy metal ions. The preparations of chitosan-conjugated Fe3O4 particles by a co-precipitation method, cross-linking method and covalent binding method using coupling agents have been reported [16,17,18].
So far, both instrumental and electrochemical methods have been used to measure lanthanum and its compounds. Instrumental methods such as inductively coupled plasma with emission spectrometer (ICP-AES) [19, 20], ICP-MS [21], gravimetry [22], luminescence spectrometry [23] and X-ray florescence spectrometry [24] have been used for the analysis of the elements. One of the electrochemical methods used for this purpose so far is adsorptive stripping voltammetry (AdSV) [25]. Several techniques have been applied for the uptake of lanthanides from aqueous medium, such as solid-phase extraction [26] and adsorption process [27, 28].
The goal of this research is to measure lanthanum in environmental and industrial samples.
Due to low concentrations of rare elements in natural waters, rapid, simple, low-cost and green sample preparation methods have been used to pre-concentration, refining and increase in the selectivity and sensitivity target analyte from the sample [29]. Solid-phase microextraction (SPME) and liquid-phase microextraction (LPME) are new miniaturized and inexpensive sample preparation among the preparation methods. SPME is especially favorable since it is solvent free and environment friendly and has hence found a lot of uses.
The development of nanomaterials for pre-concentration and determination of cations because of their remarkable properties are of interest to scientists [30]. Recently, the nanocomposites such as nano-Fe3O4 coated with various materials have been applied in many researches [31,32,33].
The magnetic sorbents effortlessly easily absorbed by an external magnet and removed from the solution [34]. In this paper, SPME procedure based on application of Fe3O4@chitisan has been evaluated for pre-concentration through the determination of La (III) by ICP-OES.
2 Experimental
2.1 Materials
La (NO3)3.6H2O salts of other cations were obtained from Sigma-Aldrich. Medium molecular weight chitosan and high-purity glutaraldehyde and other solvents were purchased from Sigma-Aldrich. All solutions were prepared using distilled or deionized water. Stock solutions of cations were prepared by solving 0.01 g of their salts in 100 mL of distilled water. All working solutions were daily prepared from stock solution.
2.2 Instruments
The following instruments were applied:
A Varian/Bruker tensor FT-IR spectroscope and a Genesis 76004555 SPECTRO ARCOS System ICP-OES instrument (RF power supply: 1400 W; Ar carrier gas flow rate: 0.6 L min−1; Ar auxiliary gas flow: 1.0 L min−1; Ar coolant gas flow rate: 14.0 L min−1; viewing height: 15 mm; wavelength of element: La: 408.672 nm) was used for the determination of metal ions.
A 780 Metrohm pH meter and a Telo magnetic stirrer, a Sartorius GC 7603 P Digital balance with an precision of 0.0001 g were used FESEM HITACHI S4160 (Japan) scanning electron microscopy (SEM) was applied for morphologic investigation and transmission electron microscopy (TEM) images were taken by TEM PHILIPS CM30 (Netherland).
2.3 Synthesis of Nanomaterials
2.3.1 Synthesis of Magnetic Fe3O4 Nanoparticles
The Fe3O4 nanoparticles were synthesis as expressed by Liu [32]: Briefly, 0.8 g of sodium hydroxide, 1.4 g of trisodium citrate and 17.04 g of sodium nitrate were dissolved in 90 mL of H2O and heated to 100 °C until a clear solution was obtained. Next, 20 mL of a 1.0 M solution of FeSO4∙7H2O was added to the reaction solution and the resulting solution was stored at 100 °C. After 1 h, the solution was cooled to room temperature, and the resulting black was isolated using a magnet, repeatedly washed with deionized water and dried in an oven at 60 °C for 6 h.
2.3.2 Synthesis of the Fe3O4@Chitosan Nanocomposite
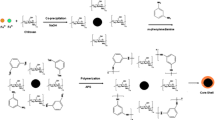
At this stage, 0.5 g of chitosan and 50 mL of acetic acid 2% were added to the beaker and vigorously stirred using a magnet stirrer, until a clear jell polymer was obtained. 0.1 g of Fe3O4 nanoparticles was then dispersed in 10 ml of the chitosan solution for 4 h using a vortex apparatus to obtain a viscose chitosan solution containing uniformly dispersed nanoparticles.
Next, NaOH solution 0.1 M was drop-wise added to the stirring solution, over a period of 15 min. Then, using a magnet, the nanoparticles were collected and the supernatant was disposed. The collected nanoparticles were repeatedly washed with acetate buffer solution (pH 4).
The nanoparticles were next dispersed in 10 ml of deionized water and mixed with 2 ml of glutaraldehyde (25% C5H8O2), as a reagent that establishes transverse bonds in the nanocomposite structure.
The solution was stirred by vortex for 12 h, before the nanoparticles were collected by placing a magnet under the reaction vessel and discarding the supernatant. The nanoparticles were washed with distilled water 3–4 times and then transferred into a desiccator [31].
2.4 Analytical Procedure
A 2000 µg/mL solution of La (III) nitrate hexahydrate was prepared as the stock solution, and the standard solutions were freshly prepared by diluting appropriate amounts of this stock solution with distilled water. After preparing dilute solutions from the stock solution, 10 ml was transferred a 10-ml vial. Then, 0.1 g of magnetic nanocomposites was added to the vial, and after adjusting the pH, the contents of the vial were subjected to ultrasonic waves. After a suitable time, the supernatant was removed from the magnetic particles using a magnet. Next, 0.1 ml of the desorption solvent was added to the Fe3O4 nanoparticles and was subjected to ultrasonic waves. The magnetic nanoparticles were collected using a magnet again, and the supernatant was analyzed using the ICP-OES instrument. Figure 1 shows a typical procedure used in this study.
3 Results and Discussion
3.1 Identification of Synthetic Fe3O4@Chitosan Nanocomposite
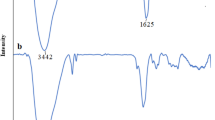
Figure 2 illustrates the IR spectra of nano-Fe3O4 (a) and Fe3O4@Chitosan nanocomposites (b). As shown in Fig. 2a, the absorption peak at 582.33 cm−1 was attributed to the bending vibrations of the Fe–O bond. The absorption peak at 1066 cm−1 can reflect the stretching vibrations of the O–H bond. The bands at 3409 and 1637 cm−1 are attributed to the vibrational, stretching and bending modes of H–O–H of the water molecules which have been adsorbed onto the sample.
The FT-IR spectrum of synthesized nanocomposite is shown in Fig. 2 b. IR bonds related to functional groups chitosan are also illustrated, and the absorption peak at 3417.5 cm−1 can be attributed to the stretching vibrations of the –OH bond and the –NH2 group. The absorption peak in the region of 1601.11 cm−1 is related to the vibrations of the N–H bond in –NH2. The absorption peak in the 1424 cm−1 region is related to the stretching vibrations of the C–N bond. In addition, 1383.57 cm−1 is related to the deformation vibrations of N–H in –NH2 group. The 1086.54 cm−1 peak corresponds to the stretching vibration of CO in C–OH [35]. As it can be seen, all of the main functional groups are found in chitosan confirmed the electrostatic reaction between Fe3O4 nanoparticles and chitosan; also the absorption bond in 598.18 cm−1 refers to the stretching vibrations of the Fe–O bond in the Fe3O4 nanoparticles and nanocomposite. Figure 3 (SEM and TEM) indicates that the Fe3O4@chitosan nanoparticles are approximately uniform and small. The layered structure and spherical numerous Fe3O4@chitosan nanocomposites could be clearly detected in Fig. 3a, b. These nanoparticles were formed with porous surface which constitute for adsorption and desorption processes. The average size was estimated about 20 nm by TEM. The formation of chitosan layer around the magnetic core is viewed in Fig. 3c, d.
3.2 The optimization Process
Various parameters can affect the extraction efficiency, and that of La (III) through the SPME method is no exception. These factors include pH, stirring rate at the extraction phase, temperature of the aqueous phase, concentration of desorption solvent, amount of the nanocomposite, extraction time, volume of the analyte solution, absorption and desorption times and salt effect. To obtain the highest La (III) extraction efficiency, it would be necessary to consider and optimize these, and hence after ensuring from the success of the method, optimization experiments were performed through the one variable at a time (OVAT) method. The results are shown in Table 1.
3.3 Effect of pH
Optimal pH values not only improve the emission efficiency, in the solid-phase microextraction process, but it also reduces the interference of the sample matrix. In addition, the pH of the sample plays a key role in determining the mechanical stability of adsorbents with little resistance against acids and strong bases [36].
In this study, the effect of pH on lanthanum extraction efficiency was investigated within the range of 1.0–11.0. The pH was adjusted using 0.1 mol/L HCl or NaOH. As shown in Fig. S1 (Supplementary information data), the extraction efficiency of La (III) cation is better at acidic pH and the maximum emission intensity was observed at pH 3.0. From pH 1.0–3.0, the emission intensity increased and then decreased.
Extraction efficiency enhancement can be due to the adsorbent structure and the presence of amino groups in chitosan. Given that the adsorbent is protonated in acidic solutions, more suitable cationic cites are formed for the adsorption of the analyte.
3.4 Effect of the Stirring Rate
Stirring of the solution increases the analyte transfer rate and also the loading of the analyte on the nanoparticle and further improves the repeatability of the results. Moreover, the time required for reaching thermodynamic equilibrium reduces. Hence, it seems that vigorous stirring should increase the extraction efficiency and facilitate mass transfer. The optimum stirring rate, which led to the maximum intensity, was 500 rpm (Figure S2). Below this rate, transferring of the analyte in the aqueous phase to the surface of the nanoparticles does not reach its highest level, reducing the adsorption efficiency.
3.5 Temperature of the Aqueous Phase
Clearly, choice of the temperature is important in the progress of reactions and interactions and the appropriate temperature should hence be determined for various processes. To optimize the temperature of the aqueous phase at the extraction stage, the extraction process was carried out at several temperatures (Figure S3). At 35 °C, the extraction efficiency decreased, but over this temperature, the transfer rate of the analyte molecules to the nanoparticles increased with increasing temperature, leading to an increase in the extraction efficiency. It was concluded that the optimum response could be observed at about 25 °C.
3.6 The effect of Concentration of Desorption Solvent
The choice of the organic solvent is one of the most important factors in the extraction process, and accurate selection of the solvent leads to good selectivity of the sample. The extraction capacity for a sample depends on its chemical structure, and as a result, the choice of solvents is also made based on its chemical structure.
Optimization of desorption solvent was performed using 0.1 ml of different concentrations of nitric acid (0.1, 0.01, 0.001, 0.0001 mol L−1). The results (Figure S4) show that the most suitable solvent was the 0.001 mol L−1 solution of nitric acid.
3.7 Amount of the Fe3O4@Chitosan Nanocomposite
The extraction efficiency often depends on the absorbent volume, which in the case of SPME is equivalent to the amount of the adsorbent or the thickness of the absorbent coating layer. The amount of the analyte absorbed on the absorbent coating depends on the amount of absorbent [37]. It should also be noted that increasing the amount of the nanoparticles may cause particles to stick together and prevent analyte adsorption on the surface of the nanoparticle.
To study the effect of the amount of the nanoparticle, various amounts in the range of 0.01–0.3 g were tested and 0.1 g of the nanoparticle was chosen as the optimal amount, as shown in Figure S5. Below 0.1 g, the extraction efficiency was low. The results indicated that the absorption increases by increasing the amount of the nanoparticles. The reason for this is the increase in the available sites for the adsorption of the adsorption. The amounts of more than 0.1 g do not have an effect on increasing the extraction efficiency.
3.8 Extraction Time
Optimization of the time is required to complete the reaction and pre-concentration of the sample. For a complete extraction, enough time is a naturally necessary. On the other hand, SPME process is relative to equilibrium activity. The time is very important for percentage recovery of La(III) between two phases.
Long-term equilibrium times are not effective on the extraction parameters. In quantitative measurements, reaching equilibrium is not a necessary condition, but it is sufficient to give perfectly identical and repetitive times for the transmission of a specific mass, because computations are performed relative in comparison with standard solutions. In this stage, different times (i.e., 10, 20, 30, 40, 50 and 60 min) were investigated and, as shown in Figure S6, it was concluded that the highest intensity was achieved after 30 min.
3.9 Desorption Time
One of the parameters that influences on the equilibrium of analyte distribution between magnetic nanoparticles and solution is the stirring rate of the solution during extraction. Here, the most efficient method of analyte extraction, i.e., the use of ultrasonic waves, which results in a very short time equilibrium, was used. In this technique, it is often possible to determine the theoretical limit for stirring. Accordingly, in this research, ultrasonic waves were used to desorb the analyte using changing in time 5, 10, 15 and 20 min were applied, and it was found that the enrichment factor gradually increased up to 15 min (Figure S7). After 15 min, the changes were negligible and constant, and the balance was established so 15 min was chosen as the optimal value. The optimum condition for procedure is shown in Table 1.
3.10 Figures of Merit
To plot the calibration curve, the optimal extraction process was performed on standard aqueous solutions of lanthanum with different concentrations. The calibration graph was plotted for the emission signal intensity of standard solutions after extraction versus concentration, and the linear calibration range for the analyte was determined. Under optimal conditions, according to Fig. 4, the calibration curve was plotted in the concentration range of 0.0001–0.14 µg/ml.
The analytical figures of merit were evaluated, the values for linear range (LR), detection limit (DL), limit of quantification (LOQ), pre-concentration factor (PF) and relative standard deviation (RSD) were calculated, and the results are shown in Table 2. Theoretically, the enrichment factor is the ratio of the final concentration of the desired species in the receptor phase to its initial concentration in the donor solution, which indicates the amount of enrichment of the target species during the pre-concentration process. The pre-concentration factor is obtained from the analyte concentration ratio after extraction to analyte concentration before extraction multiplied by dilution factor (see Table 2).
3.11 Study of Interferences Effect
This step was performed in order to consider the interference of some cations that are commonly present in water samples. The disturbance can be due to the competition of other metal cations with La (III) to form complexes and hence being extracted.
Different amounts of interfering ions, such as alkali metals, alkaline earth metals and intermediates, with equal concentrations of 10 and 100 times that of the analyte (0.01 and 0.1 μg/mL), were added in optimal conditions.
The results of emission samples are presented in Table 3. As can be seen, acceptable recovery percentages were obtained in all samples. The interference of the desired ions did not have an effect on the recovery of the analyte, which indicates the good performance of the method for quantitative measurement of La(III) in sample solution.
3.12 Validation of the Methods
Table 4 compares the proposed method and methods which used pre-concentration for La (III) determination. The good capabilities of the Fe3O4@chitosan in the pre-concentration SPME of La(III) and combination with ICP-OES method showed a low detection limit. The reported LOD in the proposed method is 0.008 ng L−1. This might be due to high microextraction and leads to higher extraction efficiency.
To evaluate applicability of the proposed method in real samples, different water samples were extracted using Fe3O4@chitosan in SPME with ICP-OES determination.
The microextraction method using nano-magnetite was applied successfully for studying the technique accuracy and evaluating the matrix effect in water samples of Zoshk and the Karun River. Extraction operations under optimal conditions were performed on a real sample without spiking and with spiking a solution containing lanthanum, and relative recovery (RR) was obtained for desired samples. RRs% were obtained 97.6–104.8 (see Table 5).
4 Conclusion
SPME was used for the pre-concentration and determination of La (III). The method has advantages such as simplicity, low cost, low solvent consumption and the ability to achieve a favorable enrichment factor. The use of Fe3O4 nanoparticles with chitosan coating, as an adsorbent, increased the sensitivity and efficiency of the method.
Inductively coupled plasma spectrometry (ICP-OES), as one of the best methods for determining the nature and concentration of various elements, with low detection limits and less spectral and chemical interference than other methods of emission, was used for the analysis of the samples. Combining SPME and ICP-OES led to enhanced sensitivity and very low detection limit, high precision, efficient sample preparation, simultaneous determination of La (III) without interferences ions. The method was used for the determination of La (III) in river water as real samples, and the obtained recoveries were acceptable.
References
De Boer, M.; Lammertsma, K.: Scarcity of rare earth elements. Chemsuschem 6(11), 2045–2055 (2013)
Harkins, W.D.; Davies, E.C.; Clark, G.L.: The orientation of molecules in the surfaces of liquids, the energy relations at surfaces, solubility, adsorption, emulsification, molecular association, and the effect of acids and bases on interfacial tension. 1 (surface energy vi.). J. Am. Chem. Soc. 39(4), 541–596 (1917)
Taylor, S.; McLennan, S.: The Continental Crust: Its Evolution and Composition. Blackwell, London (1985)
Moeller, T.: The Chemistry of the Lanthanides: Pergamon Texts in Inorganic Chemistry. Elsevier, Amsterdam (2013)
Singh, A.K.; Saxena, P.; Mehtab, S.; Gupta, B.: A selective membrane electrode for lanthanum(III) ion based on a hexaaza macrocycle derivative as ionophore. Anal. Sci. 22, 1339–1334 (2006)
Haghniaz, R.; Bhayani, K.R.; Umrani, R.D.; Paknikar, K.M.: Dextran stabilized lanthanum strontium manganese oxide nanoparticles for magnetic resonance imaging. RSC Adv. 3(40), 18489–18497 (2013)
Martin, B.; Richardson, F.S.: Lanthanides as probes for calcium in biological systems. Q. Rev. Biophys. 12(2), 181–209 (1979)
Chen, X.-G.; Lee, C.M.; Park, H.-J.: O/W emulsification for the self-aggregation and nanoparticle formation of linoleic acid modified chitosan in the aqueous system. J. Agric. Food Chem. 51(10), 3135–3139 (2003)
Alonso, M.J.: Nanomedicines for overcoming biological barriers. Biomed. Pharmacother. 58(3), 168–172 (2004)
Bozkir, A.; Saka, O.M.: Chitosan nanoparticles for plasmid DNA delivery: effect of chitosan molecular structure on formulation and release characteristics. Drug Deliv. 11(2), 107–112 (2004)
Juang, R.-S.; Shao, H.-J.: A simplified equilibrium model for sorption of heavy metal ions from aqueous solutions on chitosan. Water Res. 36(12), 2999–3008 (2002)
Ng, J.; Cheung, W.; McKay, G.: Equilibrium studies of the sorption of Cu (II) ions onto chitosan. J. Colloid Interface Sci. 255(1), 64–74 (2002)
Karthikeyan, G.; Anbalagan, K.; Andal, N.M.: Adsorption dynamics and equilibrium studies of Zn (II) onto chitosan. J. Chem. Sci. 116(2), 119–127 (2004)
Suginta, W.; Khunkaewla, P.; Schulte, A.: Electrochemical biosensor applications of polysaccharides chitin and chitosan. Chem. Rev. 113(7), 5458–5479 (2013)
Tong, J.; Chen, L.: Preparation and application of magnetic chitosan derivatives in separation processes. Anal. Lett. 46(17), 2635–2656 (2013)
Honda, H.; Kawabe, A.; Shinkai, M.; Kobayashi, T.: Development of chitosan-conjugated magnetite for magnetic cell separation. J. Ferment. Bioeng. 86(2), 191–196 (1998)
Atar, N.; Eren, T.; Yola, M.L.; Karimi-Maleh, H.; Demirdögen, B.: Magnetic iron oxide and iron oxide@ gold nanoparticle anchored nitrogen and sulfur-functionalized reduced graphene oxide electrocatalyst for methanol oxidation. RSC Adv. 5(33), 26402–26409 (2015)
Gnanasekaran, L.; Hemamalini, R.; Rajendran, S.; Qin, J.; Yola, M.L.; Atar, N.; Gracia, F.: Nanosized Fe3O4 incorporated on a TiO2 surface for the enhanced photocatalytic degradation of organic pollutants. J. Mol. Liq. 287, 110967 (2019)
Vicente, O.; Masi, A.; Martinez, L.; Olsina, R.; Marchevsky, E.: On-line preconcentration system for lanthanum determination in urine using FI-ICP-AES. Anal. Chim. Acta 366(1–3), 201–207 (1998)
Agrawal, Y.K.; Shrivastav, P.: Solvent extraction, spectrophotometric and inductively coupled plasma atomic emission spectroscopic (ICP-AES) determination of lanthanum (III) with crown hydroxamic acid. Talanta 44(7), 1307–1312 (1997)
Fujimori, E.; Hayashi, T.; Inagaki, K.; Haraguchi, H.: Determination of lanthanum and rare earth elements in bovine whole blood reference material by ICP-MS after coprecipitation preconcentration with heme-iron as coprecipitant. Fresenius J. Anal. Chem. 363(3), 277–282 (1999)
Kapoor, H.; Agrawal, Y.; Verma, P.: Separation and gravimetric determination of cerium and lanthanum with N-m-tolyl-m-nitrobenzohydroxamic acid. Talanta 22(2), 193–196 (1975)
Sita, N.M.; Prasada, T.; Iyer, C.; Damodaran, A.: Ultratrace determination of europium in high-purity lanthanum, praseodymium and dysprosium oxides by luminescence spectrometry. Talanta 44(3), 423–426 (1997)
Wenli, L.; Curini, R.; Gasparini, G.; Casarci, M.; Mattia, B.; Traverso, D.; Bellisario, F.: Study of on-line analysis using energy dispersive X-ray fluorescence spectrometry for controlling lanthanum and neodymium extraction. Anal. Chim. Acta 362(2–3), 253–260 (1998)
Wang, J.; Farias, P.A.; Mahmoud, J.S.: Trace determination of lanthanum, cerium, and praseodymium based on adsorptive stripping voltammetry. Anal. Chim. Acta 171, 215–223 (1985)
Hosseini, M.; Ganjali, M.R.; Abkenar, S.D.; Veismohammadi, B.; Riahl, S.; Norouzi, P.; Salavati-Niasari, M.: Highly selective ratiometric fluorescent sensor for La (III) ion based on a new Schiff’s base. Anal. Lett. 42(7), 1029–1040 (2009)
Ali, T.A.; Mohamed, G.G.: Potentiometric determination of La (III) in polluted water samples using modified screen-printed electrode by self-assembled mercapto compound on silver nanoparticles. Sens. Actuator B Chem 216, 542–550 (2015)
Du, P.-Y.; Li, H.; Fu, X.; Gu, W.; Liu, X.: A 1D anionic lanthanide coordination polymer as an adsorbent material for the selective uptake of cationic dyes from aqueous solutions. Dalton Trans. 44(30), 13752–13759 (2015)
Fisher, A.; Kara, D.: Determination of rare earth elements in natural water samples—a review of sample separation, preconcentration and direct methodologies. Anal. Chim. Acta 935, 1–29 (2016)
Khan, A.A.; Paquiza, L.; Khan, A.: An advanced nano-composite cation-exchanger polypyrrole zirconium titanium phosphate as a Th(IV)-selective potentiometric sensor: preparation, characterization and its analytical application. J. Mater. Sci. 45(13), 3610–3625 (2010)
Kaushik, A.; Khan, R.; Solanki, P.R.; Pandey, P.; Alam, J.; Ahmad, S.; Malhotra, B.: Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosens. Bioelectron. 24(4), 676–683 (2008)
Karimi-Maleh, H.; Fakude, C.T.; Mabuba, N.; Peleyeju, G.M.; Arotiba, O.A.: The determination of 2-phenylphenol in the presence of 4-chlorophenol using nano-Fe3O4/ionic liquid paste electrode as an electrochemical sensor. J. Colloid Interface Sci. 554, 603–610 (2019)
Zhang, M.; Qin, J.; Rajendran, S.; Zhang, X.; Liu, R.: Heterostructured d-Ti3C2/TiO2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic hydrogen production activity. Chemsuschem 11(24), 4226–4236 (2018)
Suleiman, J.S.; Hu, B.; Peng, H.; Huang, C.: Separation/preconcentration of trace amounts of Cr, Cu and Pb in environmental samples by magnetic solid-phase extraction with Bismuthiol-II-immobilized magnetic nanoparticles and their determination by ICP-OES. Talanta 77(5), 1579–1583 (2009)
Kumar, A.S.K.; Jiang, S.-J.: Chitosan-functionalized graphene oxide: a novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 4(2), 1698–1713 (2016)
Mester, Z.; Sturgeon, R.; Pawliszyn, J.: Solid phase microextraction as a tool for trace element speciation. Spectrochim. Acta B 56(3), 233–260 (2001)
Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namieśnik, J.: Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 39(11), 4524–4537 (2010)
Liang, P.; Liu, Y.; Guo, L.: Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim. Acta B 60(1), 125–129 (2005)
Agrawal, Y.: Poly (β-styryl)-(1, 2-methanofullerene-C60)-61-formo hydroxamic acid for the solid phase extraction, separation and preconcentration of rare earth elements. Fuller. Nanotube Carbon Nanostruct. 15(5), 353–365 (2007)
Berijani, S.; Ganjali, M.R.; Sereshti, H.; Tabatabaei, S.H.; Norouzi, P.: Application of a new modified magnetic nanoparticle as a selective sorbent for preconcentration and extraction of europium in environmental water samples prior to ICP-OES determination. J. Iran. Chem. Soc. 12(4), 737–742 (2015)
Su, S.; Chen, B.; He, M.; Hu, B.; Xiao, Z.: Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@ SiO2@ polyaniline–graphene oxide composite. Talanta 119, 458–466 (2014)
Zhang, Y.; Zhong, C.; Zhang, Q.; Chen, B.; He, M.; Hua, B.: Graphene oxide–TiO2 composite as a novel adsorbent for the preconcentration of heavy metals and rare earth elements in environmental samples followed by on-line inductively coupled plasma optical emission spectrometry detection. RSC Adv. 5(8), 5996–6005 (2015)
Li, D.; Chang, X.; Hu, Z.; Wang, Q.; Li, R.; Chai, X.: Samarium (III) adsorption on bentonite modified with N-(2-hydroxyethyl) ethylenediamine. Talanta 83(5), 1742–1747 (2011)
Murty, D.; Chakrapani, G.: Preconcentration of rare earth elements on activated carbon and its application to groundwater and sea-water analysis. J. Anal. Atom Spectrom. 11(9), 815–820 (1996)
Wang, Z.-H.; Yan, X.P.; Wang, Z.P.; Zhang, Z.P.; Liu, L.W.: Flow injection on-line solid phase extraction coupled with inductively coupled plasma mass spectrometry for determination of (ultra) trace rare earth elements in environmental materials using maleic acid grafted polytetrafluoroethylene fibers as sorbent. J. Am. Soc. Mass Spectrom. 17(9), 1258–1264 (2006)
Acknowledgements
The authors thank Islamic Azad University Branch of Mashhad for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rajabi, N., Masrournia, M. & Abedi, M. Measuring and Pre-concentration of Lanthanum Using Fe3O4@Chitosan Nanocomposite with Solid-phase Microextraction for ICP-OES Determination. Arab J Sci Eng 45, 121–129 (2020). https://doi.org/10.1007/s13369-019-04237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-019-04237-3