Abstract
This research introduces a novel solid-phase microextraction technology, in which the features of heating of sample, cooling of sorbent, and extraction under vacuum condition have been merged. Heating-, cooling- and vacuum-assisted solid-phase microextraction (HCV-SPME) method was developed as an efficient solution for the direct extraction of volatile and semi-volatiles species in complex solid samples. HCV-SPME was coupled with an in-needle capillary adsorption trap (HCV-INCAT) and applied to the direct extraction of polycyclic aromatic hydrocarbons (PAHs) within soil samples. It consisted of polythiophene/carboxylic acid modified multi-walled carbon nanotube nanocomposite, which was synthesized and wall-coated within a platinized stainless-steel needle via electropolymerization. The influential experimental variables (desorption conditions, sample temperature, adsorption temperature, sampling flow rate, and vacuum level) on the extraction efficiency were optimized. The developed HCV-INCAT technique was used in conjunction with GC-FID and applied for the extraction and determination of PAHs in contaminated soil samples, closely matching with those obtained using a validated ultrasonic-assisted solvent extraction procedure. Under the optimal conditions, linear dynamic ranges, limits of detection, and relative standard deviations were obtained 0.007–5 µg g−1, 8–20 pg g−1, and 7.1–12.1%, respectively, for direct extraction of naphthalene, fluorene, phenanthrene, fluoranthene, and pyrene from solid samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Relying on the unique features of solid-phase microextraction (SPME) as a solvent-less and green analytical method, different configurations have been developed in recent years to improve its efficiency and expand its applications [1]. In this way, fiber-SPME, needle trap device, in-needle capillary adsorption trap (INCAT) [2], in-tube SPME, in‐needle coated fiber, and fiber-packed needle SPME techniques have emerged in this subject area [3,4,5]. INCAT is a robust and high capacity SPME format with remarkable advantages over the other configurations [6]. INCAT is an equilibrium microextraction method, but it can also been employed for exhaustive extractions due to its use of a large capacity sorbent and the possibility of dynamic extraction [7]. During recent years, a variety of INCAT-based methods have been prepared using Tenax, activated carbon, polydimethylsiloxane, Porapak Q, polypyrrole, molecularly imprinted polymers, polyaniline/silica, and polyaniline/multi-walled carbon nanotubes (PANI/MWCNTs) [8]. There is also a steadily expanding range of sorbents for SPME being developed utilizing conducting polymers, such as polypyrrole, polyaniline and polythiophene [9, 10]. Among these promising conducting polymers, polythiophene has attracted considerable interest over the past years in different applications, owing to its high electrical conductivity and environmental stability [11]. On the other hand, MWCNTs, have generated extensive research interest, because of their excellent properties of high electrical conductivity, high surface area, and mesoporosity [12]. In comparison with the commercial SPME sorbents, CNTs exhibited better thermal stability and longer life span. They can be coated as thin film directly onto metal substrates, leads to better chemical and mechanical resistance than fused silica.
Besides, proper functionalization of CNTs can and improve their analyte selectivity.
Different methods have been introduced for preparation of polymer/MWCNT composites, including solution/melt processing and in-situ polymerization [13]. However, the preparation of the polymer/MWCNT composites using electropolymerization of monomers is an economical approach, which has recently gained popularity. This approach can create a thin film of conductive polymer on the substrate surface, which enfolds the other constituents of the composite [14]. The physical characteristics of the coating can be controlled more easily using this procedure.
Broadly speaking, it is difficult to analyze solid samples, such as soil, sediment, and plant due to the strongly adsorbed nature of the analytes, within a solid and complex matrix. For effective extraction, the analytes should be released from the native sites, diffuse to the gaseous phase and concentrate on the extraction phase [15]. A few different solutions have been proposed and few corresponding modifications made in SPME to overcome this problem. One of the most promising approaches is to cool down the extraction phase and heat the sample matrix simultaneously. This can provide a wide temperature gap between the sample matrix and the extraction phase. Heating of the sample improves the release of analytes, while cooling of the sorbent enhances their trapping [16]. Consequently, this enhances sensitivity, lowers detection limits and increases extraction efficiency of analytes, especially for more volatile species. Another approach to promote the extraction efficiency and reduce the equilibrium time is exposing the sample matrix to reduced-pressure conditions [17]. During recent years, few reports have demonstrated the significant positive effect of reduced-pressure on the extraction efficiency of organic compounds in liquid and solid samples [18].
In this research, the synergistic effects of three strategies (i.e., heating of sample matrix, cooling of sorbent, and expose sample matrix to vacuum) were merged into a novel technique named heating-, cooling- and vacuum-assisted solid-phase microextraction (HCV-SPME). To evaluate the reliability and performance of this method, an in-needle capillary adsorption trap (INCAT) was prepared and applied within the new HCV-INCAT format. To prepare the INCAT, a PT/MWCNT-COOH nanocomposite was coated on the interior surface of a platinized stainless-steel needle using a flow-through in-situ electropolymerization method. Naphthalene, fluorene, phenanthrene, fluoranthene, and pyrene (with boiling points located at the start, middle and end of the boiling point range of PAHs) were chosen as the model analytes. The HCV-INCAT setup was coupled to GC-FID and applied for the extraction and measurement of the PAHs in contaminated soil samples. The results demonstrated that HCV-INCAT-GC-FID was a simple, sensitive, and robust extraction method for the determination of the PAHs (with a wide range of the boiling points) in complex solid samples. Low limits of detection, wide linear dynamic ranges, short sampling time, and high extraction capability due to dynamic extraction are some of the advantages of the developed procedure. Also, the results were in agreement with those obtained by a validated ultrasonic-assisted solvent extraction procedure [19].
Experimental
Reagents and Supplies
The PAHs (naphthalene, Nap; fluorene, Flr; phenanthrene, Phe; fluoranthene, Flt; and pyrene, Pyr), with purities higher than 99% were purchased from Sigma-Aldrich (Buchs, Switzerland). All inorganic acids and bases and organic solvents were of analytical reagent-grade and provided by Merck (Darmstadt, Germany). MWCNTs (purity > 90%, 110–170 nm diameter and 5–9 µm length) was purchased from Sigma-Aldrich. Extra-pure thiophene (99.5%) and potassium hexachloroplatinate (K2PtCl6) were purchased from Merck. Standard sand was provided by National Water Research Institute of Canada (Burlington, Canada). A stock solution (1000 μg mL−1) was prepared by dissolving appropriate amounts of the PAHs in methanol. The working standard solutions were prepared by diluting the stock solution weekly. The stock and working standard solutions were kept at 4 °C.
Instrumentation
A Shimadzu GC-2010 Plus/AF gas chromatograph (Kyoto, Japan) was employed for separations and quantification of the analytes of interest. The GC system was equipped with a HP-5 fused silica capillary column (15 m × 0.32 mm × 0.5 µm), a flame ionization detection system (FID-2010 Plus) and a split/splitless injector (SPL-2010 Plus). It was run with GC solution software (version 2.4). The chromatographic separations were conducted using an optimized column temperature program. It started at 100 °C and remained constant for 1 min. Then, temperature was increased to 240 °C with a rate of 20 °C min−1 and held constant for 2 min. Finally, the temperature was raised to 280 °C with a rate of 30 °C min−1 and held constant for 3 min. The injector and detector were set at 280 °C. The GC-FID runs were conducted in split mode with a split ratio of 1/10. High purity nitrogen (99.999%) was used as carrier gas at a flow rate of 1 mL min−1. The flow rates of air, hydrogen, and makeup gases were adjusted at 300, 30, and 30 mL min−1, respectively. Stainless-steel needles (21-Gauge, 12 cm L, 0.7 mm I.D., 0.9 mm O.D) were purchased from Vita Needle Co. (Needham, MA, USA) and used for fabrication of the INCAT device. A side hole was created 3 cm from the tip of the INCAT needle. The carrier gas enters the INCAT via side-hole and carries the thermally desorbed analytes into the GC column. Fourier transform infrared spectra were recorded by an FT-IR 8400 spectrometer (Shimadzu) in transmittance mode and employed to characterize the functional groups of the nanomaterials. The surface morphology of the PT/MWCNT-COOH nanocomposite was studied using a CM120 Vega-Tescan (Brno, Czech Republic) field-emission scanning electron microscope (FE-SEM). The cooling setup was fabricated as described previously [16]. To evacuate the vacuum chamber a DV-42505 vacuum pump (J/B Industries Inc., USA) with 6 mbar ultimate vacuum was used. A Flexiflo Enternal peristaltic pump (Ross Products, Columbus, Ohio, USA) was used for circulating the sample headspace through INCAT.
Synthesis of the Nanocomposite and Fabrication of the Microextraction Device
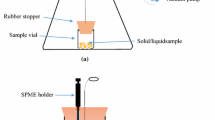
As the first step, the surface of MWCNT was modified by functionalization with carboxylic groups. For this purpose, MWCNTs were treated with concentrated nitric acid and refluxed for 48 h along with constant stirring [20]. The prepared MWCNT functionalized with –COOH groups (MWCNT-COOH) was then rinsed with pure water consecutively until its pH became neutral. Finally, it was dried under vacuum condition for 12 h at 100 °C. According to our previous experiences, the interior surface of the INCAT needle was platinized to create a cohesive, porous, and mechanically/chemically resistant coating's substrate [21]. A two-electrode system coupled to a flow-through system was carried out for electropolymerization of the PT/MWCNT-COOH nanocomposite on the interior surface of INCAT (Fig. 1). For this purpose, 0.1 g thiophene and 0.3 g modified MWCNT was mixed in 30 mL acetonitrile, inside a 40-mL vial with a plastic crimp cap and silicon/Teflon septum. The vial cap was closed, and the mixture dispersed using the sonicator. The tips of the platinized needle and a similar normal needle were passed through the vial’s septum and immersed into the suspension. The ends of two needles were connected to the peristaltic pump. The INCAT needle and normal needle were connected to a DC power supply (2 V), as the working electrode and the counter electrode, respectively. Then, the suspension was circulated through the INCAT for 15 min at a flow rate of 7.5 mL min−1. The suspension was continuously stirred during the electropolymerization process. Afterwards, INCAT was removed and the electrodeposited coating of its outer surface was scraped, and it was washed with water and methanol three times, to remove any possible pollution and remaining monomers. In the final step, the INCAT coated by PT/MWCNT-COOH was conditioned under the nitrogen atmosphere for 1 h at 200 °C.
Characterization of the Synthesized Nanocomposite
The prepared nanocomposite was characterized using both SEM and FT-IR. The SEM images of PT and PT/MWCNT-COOH are shown in Fig. 2a–d. The tubular structures of MWCNT-COOH and their irregular arrangement are clearly depicted in Fig. 2c and d. As shown, MWCNTs are enfolded into the PT layers, as the in-situ electropolymerization is undergoing. The interactions between PT and MWCNTs are mostly non-covalent, of the dipole–dipole type. Indeed, intermolecular forces are caused by interaction between –OH and –COOH groups of MWCNTs and bipolaron system on PT, which is due to delocalization of electrons on sulfur atoms in the thiophene rings [22]. On the other hand, the modified MWCNT carries negative charges, and therefore thiophene can be easily doped into the carbon nanotubes. This makes the nanocomposite more porous and increases its effective surface [23]. This structure is suitably selective for the adsorption of organic polar and semi-polar analytes. The remarkable porosity and high surface area of the nanocomposite guarantee faster mass transfer and high adsorption capacity for trapping of PAHs. These characteristics are enhanced by the platinization of the sorbent substrate, as well its mechanical and chemical stability, more durability and longer lifetime. The functional groups of PT, MWCNT and PT/MWCNT-COOH nanocomposite were investigated by recording their FT-IR spectra in transmission mode (Fig. S1). The vibrational modes of ~ 3470 ∼ 1743 and ∼ 1647 cm−1 in the MWCNT structure indicate the O–H, C=O and C=C bonds, respectively. The aromatic stretching bands in the PT structure are observed at 1649, 1548, 1317, and 1201 cm−1. The strong absorption bands, attributed to the C–C vibration, are seen at 1122 cm−1. A very strong absorption at 786 cm−1 is assigned to the out of the plane vibration of C–H in 2,5-disubstituted thiophene ring (due to α-α′ polymerization). The weak band at 724 cm−1 ascribed to the C–H out of plane vibration of the mono substituted thiophene ring, which indicates a high degree of polymerization. The stretching vibration band attributed to C–S bond appears at 629 cm−1. The peaks seen at 1740, 3397 and 1630 cm−1 are related to the interaction of PT and MWCNT-COOH in the PT/MWCNT-COOH structure. Comparison of PT and PT/MWCNT-COOH spectra demonstrates that the peaks 635, 1247, 1122 and 615 cm−1 are assigned to C=C asymmetric stretching vibration, C–H bending, C–H in-plane deformation, and C–S–C ring deformation, respectively, in the thiophene ring.
The Sampling Procedure
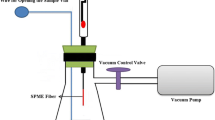
A 250-mL glass chamber with thick walls (suitable for vacuuming) was fabricated as the vacuum chamber (Fig. 3). It was equipped with two glass valves for cleaning (by nitrogen flow) and vacuuming. Solid sample (5 g) was placed inside a 10-mL SPME vial, as the sample container, its cap was closed and fitted at the bottom of the vacuum chamber. Opening of the sample container was done by a special screw lever. Two holes, proper to accommodate GC septum, were embedded in the upper cap of the vial for injection of the INCAT and the normal needle. The cap of the vacuum chamber, the screw lever and the fixing base of the extraction vial were made of Teflon to avoid unwanted adsorption of the analytes. The vacuum chamber was purged with dry nitrogen for 30 min to remove any possible contamination, prior to analysis. The cooling capsule was fixed at the top of the chamber and INCAT was fitted inside it. Then, the chamber was placed into a sand-bath to control extraction temperature. The ends of the INCAT and the normal needle were connected to the peristaltic pump to recirculate the headspace of sample, in the extraction step. To evacuate the chamber the glass valve was opened, and the vacuum pump turned on. When vacuum reached to its maximum level, the glass valve was closed, and the pump turned off. Thereupon, the sample vial’s cap was opened using the screw lever. In this way, the analytes easily released from the solid sample and rapidly dispersed in the chamber. Then, the peristaltic pump was switched on to recirculate the headspace through the INCAT. After completion of the extraction, the peristaltic pump was switched off and the INCAT was removed. It was immediately capped with a septum and injected into the GC injector for the separation and quantification of the analytes.
Analysis of Real Soil Samples Using the Ultrasonic-Assisted Solvent Extraction Method
The developed HCV-INCAT-GC-FID procedure was employed for the sampling and determination of PAHs in contaminated soil samples and the results were compared with a validated ultrasonic-assisted solvent extraction method coupled with FC-FID (UA-SE-GC-FID) method [24]. For this purpose, 5 g of soil sample was mixed with 5 g dry calcium sulfate. Then, 50 mL dichloromethane was added. The mixture was sonicated for 10 min (50 °C) to suspend the contents and release the analytes from the sample matrix. The mixture was then transferred to a conical Teflon tube and centrifuged for 10 min at 4000 rpm. The supernatant solution was decanted and filtered using a 0.45 mm membrane filter. Finally, the solution was made up to volume in a 50-mL volumetric flask and stored at a fridge until the chromatographic analysis.
Results and Discussion
Optimization of the Experimental Variables
Sand contains different amounts of various metal oxides and its composition is like soil and sediment. Consequently, it can be used as a suitable model matrix prior to the analysis of soil samples [25]. Therefore, to optimize the experimental variables standard sand was used as the model matrix and spiked with predetermined volumes of the PAHs standard solutions for the optimization process. For this purpose, the sample was placed inside the extraction vial and its cap was closed. Then, a certain volume of the standard solution (1000 ppm) was injected into the vial. After manual shaking for 1 min, the vial was left in room temperature for 24 h for complete equilibration between the model sample matrix and the analytes.
To achieve the highest possible efficiency, the main parameters influencing the HCV-INCAT performance, including desorption conditions, sample temperature (heated zone), temperature of the INCAT (cooled zone), sampling flow rate, and extraction time were investigated. Desorption conditions have a remarkable influence on sorbent lifetime and carryover effects. Therefore, first the desorption temperature and time were optimized to ensure the complete desorption of analytes from the sorbent, as well as to have access to the highest possible recovery. Desorption temperature was evaluated at 200, 240, 260, and 280 °C. The results indicated that 280 °C led to satisfactory results with RSDs less than 3%. Subsequently, evaluation of desorption time was carried out at 1, 2, 3 and 4 min. The results demonstrated that the complete desorption occurred within 2 min.
Influence of Sample and Sorbent Temperatures
Sample matrix temperature is a key parameter that affects the performance of headspace-based sampling methods. Therefore, the HCV-INCAT efficiency was studied by changing sample temperature in the range of 25–100 °C, while the temperature of the INCAT was kept constant at 0 °C. According to the results (Fig. S2), the extraction recovery increased by raising temperature from 25 to 60 °C and then remained constant. This trend can be explained when simultaneously considering the heating, cooling, and vacuum effects. The INCAT sampling involves equilibration of the analytes between sample matrix/headspace and headspace/INCAT sorbent with the partition coefficients of KS/HS and KHS/INCAT, respectively. Increasing sample temperature enhances KS/HS by improving the release of analytes from sample matrix, while it has a bilateral effect on KHS/INCAT. Rising temperature increases the extraction efficiency of the INCAT sorbent by increasing concentration of analytes in the headspace, while further increase in temperature heats the sorbent and decreases its tendency to adsorb analytes, due to exothermic nature of adsorption. Therefore, in the temperature profile of conventional headspace sampling, there is usually an ascending region, a maximum point and a descending part, respectively [26]. In contrary, the temperature profile of cooling-assisted sampling has a continuously ascending trend due to cooling of the sorbent. In fact, cooling-assisted strategy was developed to overcome this limitation and has made possible the use of high temperatures in headspace sampling [27]. However, in this case, the temperature profile showed a constant region after the increasing zone that seems to be related to the vacuum effect. Applying a vacuum improves the diffusion rates of the analytes and their release of analytes from the matrix, as well as trapping efficiency of the sorbent by removing the air’s interfering molecules. Therefore, it seems that vacuum provides a synergistic effect with both cooling of the sorbent and heating of the sample, and so improves their effectiveness. The temperature of the INCAT was optimized over the range of − 10 to + 10 °C, under the reduced pressure conditions. The experimental results showed that 0 °C resulted in the maximum peak area for all PAHs (Fig. S3). Applying temperatures lower than − 10 °C caused ice particles to appear on the surface of INCAT and reduced the analyte extraction, especially for soil samples with high moisture contents. Thus, 0 and 60 °C were selected as the optimum temperatures of the sample and INCAT for further experiments.
Effect of Sampling Time and Flow Rate
Sampling flow rate is a compromising factor between sampling time and sorption kinetics in dynamic sampling. The effect of sampling flow rate on the extraction efficiency of the HCV-INCAT method was investigated over the range of 1.0–22.3 mL min−1. It was seen that increasing sampling flowrate up to 6.25 mL min−1 raised the extraction efficiencies, while further increase in flowrate caused a slight decrease in the peak areas for most of the analytes (Fig. S4). Therefore, 6.25 mL min−1 was selected as the optimal sampling flow rate. This amount can rationally be considered as the breakthrough volume (BTV) for the HCV-INCAT sampling strategy. To investigate the effect of extraction time, the HCV-INCAT approach was evaluated using different sampling times over the range of 1–30 min. The results revealed that the peak areas increased by increasing of extraction time up to 10 min and then remained constant (Fig. S5). Compared to the similar studies this sampling time is remarkably low for soils samples, which demonstrated a considerable advantage in routine extraction laboratories [7]. It can be deduced from the results that the reduced pressure conditions, along with the heating/cooling processes dramatically improved the extraction kinetics and reduced the equilibration time. Based upon these results, 10 min was chosen as the optimal time for the extraction of PAHs using the HCV-INCAT-GC-FID method.
Comparison of the Developed Method with the Traditional Sampling Procedures
To investigate the credibility and applicability of the HCV-INCAT method, it was compared with conventional INCAT, VA-INCAT, and CA-INCAT procedures for analysis of PAHs in solid samples. The results showed that the extracted amounts of analytes using HCV-INCAT are on average two to three times higher than the other methods, due to the use of three reinforcement factors of cooling of the sorbent, heating of the sample, and extraction under vacuum condition (Fig. 4). This represents a significant improvement in these well-developed individual approaches. As the results show CA-INCAT and VA-INCAT procedures more efficient than conventional INCAT method. The CA-INCAT is basically used for the analysis of volatile compounds in solid samples, while VA-INCAT it more suitable for semi- and non-volatile analytes. Therefore, it can be concluded that HCV-INCAT sampling strategy is efficient for the analysis of organic analytes with a wide range of volatility in complicated solid matrices, as demonstrated herein for the five PAHs (the representative of PAHs with a wide range of boiling points).
Additionally, to survey the extraction ability of the PT/MWCNTs nanocomposite, it was compared with PDMS and C18 (as the most commonly used commercial sorbents), as well as PT. The results (Fig. S6) showed that the proposed nanocomposite sorbent is more efficient than the tested commercial sorbents for the extraction of PAHs using the HCV-INCAT. The analytes of interest, can interact with MWCNTs and functional groups on polythiophene in the sorbent structure through π–π and polar–polar interactions [28]. Additionally, possessing a porous structure provides higher specific surface area and loading capacity. These features make the coating more capable to contribute in π–π and polar–polar interactions.
Method Validation
The performance and practical applicability of the HCV-INCAT-GC-FID method for extraction and analysis of PAHs under the optimized experimental conditions wereinvestigated. To evaluate figures of merit of the proposed method, linear dynamic ranges (LDRs), limits of detection (LODs), limits of quantitation (LOQs), and relative standard deviations (RSDs) were calculated (Table S1). RSDs for six replicate analyses (1 μg g−1 of the PAHs) were calculated 7.1–12.1%. Linear dynamic ranges (LDRs) were obtained over the range of 0.007–5 µg g−1, with determination coefficients (R2) higher than 0.99. The LODs and LOQs were found to be 8–20 pg g−1 and 0.007–0.09 µg g−1, respectively.
Analysis of Contaminated Soil Real Samples
To evaluate the applicability of the HCV-INCAT-GC-FID procedure for the direct extraction and determination of PAHs in solid samples, it was used for the analysis of three real soil samples, collected from the areas of storage stations of oil products in Lorestan Province (West of Iran). The real samples were also analyzed using a validated ultrasonic-assisted solvent extraction method coupled to GC-FID (UA-SE-GC-FID). To more ensure the reliability of the method, each of the samples was also spiked with 0.5 μg g−1 of the analytes and subjected to the HCV-INCAT and UA-SE procedures, three times (Table 1). To certify the agreement between the results of the two methods, statistical t tests were done. The results demonstrated no significant differences between the obtained results at 95% confidence limit (p = 0.05). Generally, matrix effect influences RSDs values in all complicated real samples. However, in this work the matrix effect did not affect the results significantly, except few cases for more volatile analytes. Figure 5 shows a typical GC-FID chromatogram, obtained after HCV-INCAT extraction of the PAHs from a soil sample in spiked and non-spiked conditions.
Comparison of the Proposed Procedure with Published Reports
To assess the reliability of the HCV-INCAT-GC-FID procedure, its analytical performances (including sorbent type, extraction time, sample temperature, LOD, LDR and RSD) were compared with the other important previously published reports [7, 16, 18, 25, 29,30,31,32] for the extraction and determination of PAHs (Table 2). Among the studied methods, HCV-INCAT has wider linearities for the examined PAHs. Lower limits of detection, shorter sampling time, and lower extraction temperature are other features of the developed strategy.
Concluding Remarks
A simple, reliable, and ultrasensitive HCV-INCAT setup and strategy was developed and evaluated for the direct extraction of PAHs in complicated solid samples. To enhance the release of analytes from the sample matrix it was heated, while the sorbent was simultaneously cooled to improve the trapping of analytes. Additionally, the extraction was performed under the reduced-pressure condition to remove the interfering molecules and enhance the release of analytes. The developed method showed 2–3 times higher efficiency compared with CA-INCAT, VA-INCAT and conventional INCAT for the analysis of solid samples. The HCV-INCAT-GC-FID procedure was successfully carried out for analysis of PAHs in contaminated soil samples without any sample pretreatment step and the results were seen to be in a good agreement with those obtained by a validated UA-SE-GC-FID method. LODs, LOQs, RSDs and extraction time of the proposed method were lower or comparable with the similar methods reported in the literature. HCV-INCAT can be coupled to GC–MS and it will assuredly further increase its sensitivity. The results demonstrated that HCV-INCAT sampling strategy can be applied for ultrasensitive and precise analysis of a variety of volatile, semi-volatile and non-volatile analytes in complex solid matrices such as soil, sediment, food, and plants, directly and with minimal sample manipulation. Additionally, it provides an easy, rapid and low-cost method for the analysis of organic pollutants in environmental samples with enough reliability and reproducibility.
References
Jalili V, Barkhordari A, Ghiasvand A (2020) A comprehensive look at solid-phase microextraction technique: a review of reviews. Microchem J 152:104319. https://doi.org/10.1016/j.microc.2019.104319
Bagheri H, Babanezhad E, Khalilian F (2009) An interior needle electropolymerized pyrrole-based coating for headspace solid-phase dynamic extraction. Anal Chim Acta 634:209–214. https://doi.org/10.1016/j.aca.2008.12.047
Heidari N, Ghiasvand A, Abdolhosseini S (2017) Amino-silica/graphene oxide nanocomposite coated cotton as an efficient sorbent for needle trap device. Anal Chim Acta 975:11–19. https://doi.org/10.1016/j.aca.2017.04.031
Kędziora K, Wasiak W (2017) Extraction media used in needle trap devices-progress in development and application. J Chromatogr A 1505:1–17. https://doi.org/10.1016/j.chroma.2017.05.030
Heidari N, Ghiasvand A (2019) A review on magnetic field-assisted solid-phase microextraction techniques. J Liq Chromatogr Relat Technol. https://doi.org/10.1080/10826076.2019.1668804
McComb ME, Oleschuk RD, Giller E, Gesser HD (1997) Microextraction of volatile organic compounds using the inside needle capillary adsorption trap (INCAT) device. Talanta 44:2137–2143. https://doi.org/10.1016/S0039-9140(97)00093-3
Ghiasvand AR, Yazdankhah F (2017) Single-step reinforced microextraction of polycyclic aromatic hydrocarbons from soil samples using an inside needle capillary adsorption trap with electropolymerized aniline/multi-walled carbon nanotube sorbent. J Chromatogr A 1487:47–53. https://doi.org/10.1016/j.chroma.2017.01.056
Jalili V, Barkhordari A, Ghiasvand A (2020) New extraction media in microextraction techniques. A review of reviews. Microchem J 153:104386. https://doi.org/10.1016/j.microc.2019.104386
Jalili V, Barkhordari A, Ghiasvand A (2019) New extraction media in microextraction techniques. A review of reviews. Microchem J. https://doi.org/10.1016/j.microc.2019.104386
Ghiasvand A, Behfar M, Yazdankhah F (2019) Reduced-pressure fiber-in-needle sampling of aldehydes for room temperature assessment of edible oils’ oxidative stability. Chromatographia 82:1405–1414. https://doi.org/10.1007/s10337-019-03752-7
Senthilkumar B, Thenamirtham P, Selvan RK (2011) Structural and electrochemical properties of polythiophene. Appl Surf Sci 257:9063–9067. https://doi.org/10.1016/j.apsusc.2011.05.100
Hinds BJ, Chopra N, Rantell T, Andrews R, Gavalas V, Bachas LG (2004) Aligned multiwalled carbon nanotube membranes. Science 303:62–65. https://doi.org/10.1126/science.1092048
Chang Y-H, Wu M-S, Lin K-F (2014) Graphting polyimide to MWCNT for enhancing dispersion and properties of MWCNT/polyetherimide nanocomposites. J Polym Res 21:419. https://doi.org/10.1007/s10965-014-0419-2
Abdolhosseini S, Ghiasvand A, Heidari N (2017) A high area, porous and resistant platinized stainless steel fiber coated by nanostructured polypyrrole for direct HS-SPME of nicotine in biological samples prior to GC-FID quantification. J Chromatogr B 1061:5–10. https://doi.org/10.1016/j.jchromb.2017.06.042
Ghiasvand AR, Hajipour S, Heidari N (2016) Cooling-assisted microextraction: comparison of techniques and applications. Trends Anal Chem 77:54–65. https://doi.org/10.1016/j.trac.2015.12.008
Ghiasvand AR, Pirdadeh-Beiranvand M (2015) Cooling/heating-assisted headspace solid-phase microextraction of polycyclic aromatic hydrocarbons from contaminated soils. Anal Chim Acta 900:56–66. https://doi.org/10.1016/j.aca.2015.10.016
Brunton N, Cronin D, Monahan F (2001) The effects of temperature and pressure on the performance of Carboxen/PDMS fibres during solid phase microextraction (SPME) of headspace volatiles from cooked and raw turkey breast. Flavour Fragr J 16:294–302. https://doi.org/10.1002/ffj.1000
Psillakis E, Yiantzi E, Kalogerakis N (2013) Downsizing vacuum-assisted headspace solid phase microextraction. J Chromatogr A 1300:119–126. https://doi.org/10.1016/j.chroma.2013.02.009
Banjoo DR, Nelson PK (2005) Improved ultrasonic extraction procedure for the determination of polycyclic aromatic hydrocarbons in sediments. J Chromatogr A 1066:9–18. https://doi.org/10.1016/j.chroma.2005.01.033
Gupta TK, Singh BP, Dhakate SR, Singh VN, Mathur RB (2013) Improved nanoindentation and microwave shielding properties of modified MWCNT reinforced polyurethane composites. J Mater Chem A 1:9138–9149. https://doi.org/10.1039/C3TA11611E
Ghiasvand A, Dowlatshah S, Nouraei N, Heidari N, Yazdankhah F (2015) A solid-phase microextraction platinized stainless steel fiber coated with a multiwalled carbon nanotube-polyaniline nanocomposite film for the extraction of thymol and carvacrol in medicinal plants and honey. J Chromatogr A 1406:87–93. https://doi.org/10.1016/j.chroma.2015.06.052
Zhang H, Hu Z, Li M, Hu L, Jiao S (2014) A high-performance supercapacitor based on a polythiophene/multiwalled carbon nanotube composite by electropolymerization in an ionic liquid microemulsion. J Mater Chem 2:17024–17030. https://doi.org/10.1039/C4TA03369H
Fu C, Zhou H, Liu R, Huang Z, Chen J, Kuang Y (2012) Supercapacitor based on electropolymerized polythiophene and multi-walled carbon nanotubes composites. Mater Chem Phys 132:596–600. https://doi.org/10.1016/j.matchemphys.2011.11.074
Sun F, Littlejohn D, David Gibson M (1998) Ultrasonication extraction and solid phase extraction clean-up for determination of US EPA 16 priority pollutant polycyclic aromatic hydrocarbons in soils by reversed-phase liquid chromatography with ultraviolet absorption detection. Anal Chim Acta 364:1–11. https://doi.org/10.1016/S0003-2670(98)00186-X
Beiranvand M, Ghiasvand A (2017) Simple, low-cost and reliable device for vacuum-assisted headspace solid-phase microextraction of volatile and semivolatile compounds from complex solid samples. Chromatographia 80:1771–1780. https://doi.org/10.1007/s10337-017-3422-z
Zhang Z, Pawliszyn J (1993) Headspace solid-phase microextraction. Anal Chem 65:1843–1852. https://doi.org/10.1021/ac00062a008
Ghiasvand A, Zarghami F, Beiranvand M (2018) Ultrasensitive direct determination of BTEX in polluted soils using a simple and novel pressure-controlled solid-phase microextraction setup. J Iran Chem Soc 15:1051–1059. https://doi.org/10.1007/s13738-018-1302-6
Wang X, Liu Y, Tao S, Xing B (2010) Relative importance of multiple mechanisms in sorption of organic compounds by multiwalled carbon nanotubes. Carbon 48:3721–3728. https://doi.org/10.1016/j.carbon.2010.06.034
Gholivand MB, Abolghasemi MM (2012) Inside needle capillary adsorption trap device for headspace solid-phase dynamic extraction based on polyaniline/hexagonally ordered silica nanocomposite. J Sep Sci 35:695–701. https://doi.org/10.1002/jssc.201100836
Ghiasvand AR, Hosseinzadeh S, Pawliszyn J (2006) New cold-fiber headspace solid-phase microextraction device for quantitative extraction of polycyclic aromatic hydrocarbons in sediment. J Chromatogr A 1124:35–42. https://doi.org/10.1016/j.chroma.2006.04.088
Gholivand MB, Abolghasemi MM, Fattahpour P (2011) Polypyrrole/hexagonally ordered silica nanocomposite as a novel fiber coating for solid-phase microextraction. Anal Chim Acta 704:174–179. https://doi.org/10.1016/j.aca.2011.07.045
Ghiasvand A, Heidari N, Abdolhosseini S, Hamdi A, Haddad P (2018) Evaluation of a cooling/heating-assisted microextraction instrument using a needle trap device packed with aminosilica/graphene oxide nanocomposites, covalently attached to cotton. Analyst 143:2632–2640. https://doi.org/10.1039/C8AN00063H
Acknowledgements
The authors sincerely acknowledge Lorestan University for supporting this research. The authors acknowledge Reza Givehkesh, electronic technician, for his technical support to fabricate some parts of the setup.
Funding
This study was not funded by any grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghiasvand, A., Yazdankhah, F. & Paull, B. Heating-, Cooling- and Vacuum-Assisted Solid-Phase Microextraction (HCV-SPME) for Efficient Sampling of Environmental Pollutants in Complex Matrices. Chromatographia 83, 531–540 (2020). https://doi.org/10.1007/s10337-020-03869-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03869-0