Abstract
The structures, electron affinities, and dissociation energies of EuSi n (n = 3–11) and their anions have been examined by means of four hybrid and pure density functional theory (DFT) methods. Basis sets used in this work are of segmented (SEG) Gaussian valence basis sets and relativistic small-core effective core potentials (ECP) with additional diffuse 2pdfg functions, denoted aug-SEG/ECP for Eu atoms and aug-cc-pVTZ for Si atoms. The geometries are fully optimized with each DFT method independently. The ground-state structures for all of these species are found to be substitutional type, which can be regarded as being derived from the ground-state structure of Si n+1 (and/or Si − n+1 ) by replacing a Si atom with a Eu atom. The theoretical adiabatic electron affinities (AEAs) of EuSi n predicted by the four DFT schemes are in excellent agreement with the experimental data, especially the AEAs of TPSSh and B2PLYP. The average absolute deviations from experiment are by 0.10, 0.06, 0.07, and 0.05 eV, and the largest deviations are 0.16, 0.12, 0.18, and 0.10 eV at the B3LYP, TPSSh, PBE, and B2PLYP levels, respectively. The AEA of EuSi n (n = 3–11) is less than that of Si n . With the increase in silicon cluster size, the AEA of EuSi n may be close to that of Si n , but cannot be larger than that of Si n . The Eu atom acts as an electron donor, and the bonding between Eu and silicon clusters is ionic in nature. The bond between Eu and silicon clusters of neutral EuSi n (n = 3–11) is stronger than that of the anions. The total magnetic moments of EuSi n /EuSi − n (n = 3–11) and the magnetic moments on the Eu atom do not quench, and the total magnetic moments are contributed by Eu atom. The dissociation energies of Eu atom from EuSi n and their anions have also been calculated to examine relative stabilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Silicon clusters have been investigated both experimentally and theoretically because they are not only the most important material for the semiconductor industry but also building blocks for the fabrication of new nanostructures with controlled electronic properties, which can be manipulated by changing size, shape, and composition [1–10]. Bare silicon clusters are chemically reactive and unsuitable for building block of self-assembly materials because they much prefer sp 3 hybridization to sp 2. Like carbon atoms, they can appear with sp, sp 2, or sp 3 hybridization in compounds [11, 12]. However, a wide variety of experimental [13–15] and theoretical [16–18] research works elucidated that doping a suitable foreign atom inside silicon clusters can not only enhance the stability, but also influence profoundly the electron properties of these complexes. In particular, the examples of encapsulating a transition metal (TM) atom insider silicon clusters as building blocks of cluster-assembled materials with novel magnetic, electronic, and possibly optical properties are numerous. People hope that by inserting TM atom possessing unpaired d electrons and thus carrying a magnetic moment, the magnetic moment would be retained in a TM@Si n cluster. However, the hybridization between silicon’s sp orbitals and d orbitals of the encapsulated TM atom results in quenching the magnetic moment [19–21]. Instead, the electrons residing in the localized f orbitals of the rare-earth (RE) atom are to a large extent not interacting significantly with the silicon clusters and consequently give rise to often observed magnetic properties of the RE doping silicon clusters [22–24].
There have been some previous studies on silicon clusters. On the experimental aspect, Nakajima et al. [25–27] investigated first the geometric and the electronic structures of LnSi − n (Ln = Tb, Ho, Lu, 6 ≤ n ≤ 20) by means of photoelectron spectroscopy (PES) and a chemical-probe method. Then, Bowen et al. [22, 23] studied the structures and properties of LnSi − n (Ln = Pr, Sm, Eu, Gd, Ho, Yb) by using PES. From the theoretical aspect, the structures and properties such as magnetic moments and stabilities of LnSi n (Ln = La, Sm, Eu, Gd, Ho, Yb, Lu, n < 21) clusters were studied by using density functional theory (DFT) with B3LYP, or GGA-PW91, or ZORA methods and LanL2DZ, or DNP, or TZP basis sets [28–37]. In addition to these, the structural and electronic properties of M@Si6 (M = La, Ce, Pr, Gd, Ho, Yb, Lu) and their anions were reported by Wang et al. [38, 39]. The most stable geometry of Eu@Si20, Sm@Si20, Tm@Si20, and Gd@Si20 − clusters was predicted to be fullerene-like silicon structure and retain significant magnetic moments in their most stable geometry [24, 40].
Even though much effort has been made to research RE atom doping silicon clusters, there are still some problems in the process of determining the ground-state structures. First, the possibility of missing the lowest energy structure exists. This problem may be solved by an extensive search with a global optimization technique. For small sizes, this search can be performed, but as the cluster size increases, it becomes much more difficult because the search for the ground-state structure is dependent on the type of the calculation and on the optimization technique. That is, the search for the ground-state structure needs both accurate potential functions and an efficient optimization method. These conditions cannot be performed for larger size clusters. The second issue is that many isomers sometimes are nearly degenerate in energy resulting from very shallow potential energy surface of some species. Fortunately, the PES is generally sensitive to the structural change; therefore, a more reliable determination of the ground-state structure can be made by comparing the PES to predictions of theory for different isomers. There are two ways of comparison of the experimental PES with predictions of theory. One is the comparison of the first vertical detachment energy (VDE) and/or adiabatic electron affinity (AEA). And another is the comparing of the number of distinct peaks of simulated PES in the low bonding energy and their relative positions. The former is more quantitative than the latter. In this work, we have investigated the ground-state structures, AEAs, dissociation energies, relative stabilities, charge transfer, magnetic moments, and growth pattern of neutral EuSi n (n = 3–11) and their anions with four DFT methods, and with the aim of understanding how their properties differ from that of bare silicon clusters. The predicted AEAs are also compared with those measured previously by PES. The comparison with PES helps to discard wrong structures when the agreement with experiment is poor. Although the theoretical results of the ground-state structures and the properties such as AEAs, population, and magnetic moment have been already reported by Zhao et al. [30], our calculations will provide more accurate results. For instance, the ground-state structures of EuSi n with n = 5, 7, 9, 10, and 11 reported in this paper are different from those reported previously [30].
2 Theoretical methods
The four different density functional forms used here are as follows: Becke’s three-parameter hybrid exchange functional [41] with Lee, Yang, and Parr’s (LYP) [42] correlation functional (B3LYP); the 1996 pure exchange and correlation functional of Perdew, Burke, and Ernzerhof [43, 44] (PBE); the 2003 hybrid functional of Tao, Perdew, Staroverov, and Scuseria [45, 46] (TPSSh); Becke’s exchange and LYP correlation functional with Hartree–Fock exchange and perturbative second-order correlation part [47] (B2PLYP). The basis sets used for silicon are aug-cc-pVTZ [48]. For europium, the segmented (SEG) Gaussian (14s13p10d8f6g)/[10s8p5d4f3g] valence basis sets and relativistic small-core effective core potentials (ECP MWB28) [49] are denoted as SEG/ECP. Since diffuse functions are important for the anions, the Eu-segmented valence basis sets were augmented by 2pdfg diffuse functions with exponents 0.028 and 0.015 (p), 0.032 (d), and 0.05 (f,g) [50] denoted as aug-SEG/ECP.
At the B3LYP, the PBE and the TPSSh levels, harmonic frequency analysis for all EuSi n (n = 3–11) and their anions was performed to guarantee that the optimized structures are local minima. These frequencies are then applied for the zero-point vibration energy (ZPVE) correction at 0 K (the B2PLYP ZPVE adopted that of B3LYP). All of calculations have been performed using the GAUSSIAN 09 program package [51].
To search for the ground-state structures, a large number of isomers need to be studied. Accordingly, in the optimization process of geometries, we considered a great number of isomers which can be classified into the following four types. One is the “substitutional structure,” which can be regarded as being derived from the ground-state structure of Si n+1 (and/or Si − n+1 ) by replacing a Si atom with a Eu atom. The second is the “attaching structure,” in which the Eu atom is attached to different positions on surface or edge or apex of the ground-state structure of Si n (and/or Si − n ). The third type is the “evolving structure,” in which the Si atom is attached to various positions on surface or edge or apex of the lowest energetic structure of EuSi n (and/or EuSi − n ). The remaining geometries were designed by us and are named the “fourth type.” Starting with these structures, we obtained as many of the refined low-lying structures as possible with cc-pVTZ basis set for Si and SEG/ECP basis set for Eu. Then, we refined the energies of the selected low-energy isomers with aug-cc-pVTZ and aug-SEG/ECP basis sets for Si and Eu atom, respectively. In addition, the spin multiplicities of doublet, quartet, sextuplet, octuplet, and decuplet state were taken into account for neutral EuSi n with n ≤ 3 and of singlet, triplet, quintuplet, septet, nonet, and eleven states were taken into account for their anions because the ground state of Si, Si2, and Si3 is triplet. The results show that the ground states of neutral with the exception of EuSi are octuplet (the ground state of EuSi is decuplet), and nonet state for anion excluded EuSi− which is eleven state (see Table 1, the total energies of EuSi1–3 and their anions are listed). Therefore, from n = 4, we only considered octuplet state for neutral and nonet for anion. Although we obtained many isomers for neutral and EuSi n (n = 3–11) and their anions, we reported mainly the ground-state structures in this paper.
3 Results and discussion
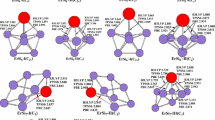
The geometries optimized with all of these methods for EuSi n (n = 3–11) clusters and their anions are shown in Fig. 1.
3.1 Lowest energy structures and isomers of EuSi n and their anions
The lowest energy structure for EuSi3 is predicted to be a planar rhombus with C 2v symmetry and 8A2 ground state, which is the same as the results reported by Zhao et al. [30]. Similar to CaSi3 [52], it can be viewed as being derived from not only the ground-state Si4 structure [1, 2, 4, 5] by replacing a Si atom with a Eu atom but also the ground-state Si3 structure [1, 2, 4] by attaching a Eu atom. For anion EuSi3 −, the lowest energy structure is also a planar rhombus, but 9A2 ground state. The equivalent Eu–Si bond lengths are by 0.11 Å longer than its neutral counterparts.
Zhao et al. [30] reported that the lowest energy structure of EuSi4 is C 2v symmetry. Our results are also C 2v symmetry with 8A2 ground state. It can be viewed as being derived from the trigonal bipyramid of Si5 [1, 2, 4] by replacing a Si atom with a Eu atom. For anion EuSi4 −, the lowest energy structure of 9A2 ground state can also be viewed as being derived from the trigonal bipyramid of Si5 and/or Si5 − [1, 2, 4, 6] by replacing a Si atom with an Eu atom. A pair of equal Eu–Si bond lengths is by 0.11–0.14 Å longer than its neutral counterparts.
The lowest energy structure of EuSi5 (shown in Fig. 1 EuSi5-I) of 8A” ground state belongs to not only “substitutional structure” but also “attaching structure” (for Si6, tetragonal bipyramid, face-capped trigonal bipyramid, and edge-capped trigonal bipyramid compete with each other for the ground-state structure, see Refs. [1, 2, 4–6]). Our result differs from the outcome reported by Zhao et al. [30]. Zhao et al. [30] presented that the lowest energy structure of EuSi5 was C 2v symmetry. The C 2v-symmetry structures (shown in Fig. 1 EuSi5-II) we obtained possess two electronic states. One is 8B1 state, and another is 8A2 state. The 8B1 isomer is less stable than that of EuSi5-I by 0.81, 1.08, and 0.93 eV in energy at the B3LYP, the TPSSh, and the B2PLYP levels, respectively. The 8A2 isomers are a saddle point on the potential surface due to having an imaginary 48i, 49i, and 50i frequency with b 2 mode at the B3LYP, the TPSSh, and the PBE levels, respectively. They undergo Jahn–Teller distortion to give the ground-state structures. For anion EuSi5 −, the lowest energy structure is also C s symmetry, but 9A” ground state.
The lowest energy structure of EuSi6 is predicted to be C 2v symmetry with 8A2 ground state, which are the same as in previous study of Zhao et al. [30]. Similar to CaSi6 [52], it belongs to “substitutional structure.” For anion EuSi6 −, the lowest energy geometry of 9A2 ground state keeps the frame of the corresponding neutral unchanged.
The C 1 symmetry EuSi7-I structure of octuplet state is predicted to be the ground state for neutral EuSi7. It can be viewed as being derived from the distorted bicapped octahedron of Si8 [2, 3, 6, 7] by replacing a Si atom with a Eu atom, analogous to CaSi7 [52]. This result is different from previous study of Zhao et al. [30]. The geometry reported in Ref. [30] is similar to EuSi7-I (see Fig. 1) with C s symmetry and 8A′ state. It can be viewed as attaching a Si atom to the face of the ground state of EuSi6, that is, “evolving structure.” Energetically, it is higher than that of EuSi7-I by 0.57, 0.43, 0.38, and 0.62 eV at the B3LYP, the PBE, the TPSSh, and the B2PLYP levels of theory, respectively. For anion EuSi7 −, the framework of lowest energy structure of nonet ground state is unchanged compared to its neutral. The Eu–Si bond lengths of the anion are longer than its neutral counterparts by 0.08–0.16 Å.
The lowest energy structure of EuSi8 is predicted to be C 2v symmetry with 8A2 ground state, which are the same as previous study of Zhao et al. [30]. It can be viewed as being derived from the bicapped pentagonal bipyramid of Si9 [2, 3, 7] by replacing a Si atom with a Eu atom, analogous to CaSi8 [52]. For anion EuSi8 −, the geometry of 9A2 ground state is unchanged compared to its neutral. The Eu–Si bond lengths of the anion are by 0.10–0.17 Å longer than its neutral counterparts.
Two isomers for neutral EuSi9 are reported. Both EuSi9-I and EuSi9-II can be viewed as being derived from the tetracapped trigonal prism of Si10 [2, 3, 6, 7] by replacing a Si atom located at different position with a Eu atom. Energetically, the EuSi9-I structure of 8A” ground state is more stable than the EuSi9-II isomer of 8A2 state by 0.12, 0.04, 0.08, and 0.14 eV at the B3LYP, the TPSSh, the PBE, and the B2PLYP levels, respectively. Our results are different from previous study [30]. The geometry reported by Zhao et al. [30] is similar to C 3v symmetry EuSi9-III of 8A2 state. Energetically, it is less stable than that of EuSi9-I by 0.22, 0.13, and 0.21 eV at the B3LYP, the TPSSh, and the PBE levels, respectively. For anion EuSi9 −, two isomers are also presented. The C 3v symmetry EuSi9 −-I structure of 9A2 state is predicted to be the ground state. Energetically, it is more stable than the C s symmetry EuSi9 −-II structure of 9A” electronic state by 0.36, 0.46, 0.41, and 0.34 eV at the B3LYP, the TPSSh, the PBE, and the B2PLYP levels, respectively. It is note that although both the ground-state structures of EuSi9 and its anion belong to substitutional type, the substitutional sites are not identical.
The EuSi10-I structure of 8A” ground state can be viewed as being derived from the distorted tricapped tetragonal antiprism of Si11 [3] by replacing a Si atom with a Eu atom. This result is different from previous study of Zhao et al. [30]. The geometry reported by Zhao et al. [30] is similar to EuSi10-II (see Fig. 1) with C s symmetry and 8A” state. It is higher in energy than that of EuSi10-I by 0.81, 0.98, and 1.12 eV at the B3LYP, the PBE, and the TPSSh levels, respectively. Compared to neutral, the anionic geometry of the 9A” ground state is unchanged. The Eu–Si bond lengths of the anion are longer than its neutral counterparts by 0.15–0.20 Å.
The EuSi11-I structure of 8A” ground state can be viewed as being derived from the hexacapped trigonal prism of Si12 [8] by replacing a Si atom with a Eu atom. Our results differ from ones reported previously [30]. The geometry reported previously [30] is similar to EuSi11-II (see Fig. 1). It is higher in energy than that of EuSi11-I by 0.39, 0.41, and 0.43 eV at the B3LYP, the PBE, and the TPSSh levels, respectively. For anion EuSi11 −, the structure of 9A” ground state is unchanged compared to its neutral.
From described above, we can conclude that the lowest energy structure of EuSi n (n = 3–11) can be viewed as being derived from the ground-state structure of Si n+1 (and/or Si − n+1 ) by replacing a Si atom with a Eu atom, that is, “substitutional structure.” This result is similar to that of CaSi n [52], but not for MgSi n [53] and KSi n [54]. The reason may be explained that although the electron configurations ([core]6s 24f 75d 0) of Eu include 4f orbitals, the electrons residing in the f orbitals are to a large extent not responsible for bonding in EuSi n clusters (see below). Consequently, the electron configurations ([core]6s 25d 0) of Eu are similar to those of Ca ([core]4s 23d 0), but not K ([core]4s 13d 0) and Mg ([core]3s 23p 0). In terms of predicting the Eu–Si bond lengths, the B3LYP and B2PLYP bond distances are nearly identical, while the PBE and TPSSh bond lengths are nearly identical. The bond distances of B3LYP and B2PLYP are averagely larger than those of PBE and TPSSh by 0.04 Å. The Eu–Si bond lengths are slightly longer than or nearly close to corresponding Ca–Si bonds of CaSi n . These indicate that the modification of calcium–silicon-based materials via doping Eu atom would be easily implemented. For anion, the lowest energy geometries of EuSi − n (n = 3–11) with the exception of n = 9 are unchanged compared to corresponding neutrals. The Eu–Si bond lengths of the anions are averagely longer than its neutral counterparts by 0.12 Å.
3.2 Electron affinities
The adiabatic electron affinities (AEAs) [defined as the difference of total energies in the manner AEA = E(optimized neutral) − E(optimized anion)] of EuSi n and Si n clusters with n = 3–11 are evaluated. These values and their experimental ones are listed in Table 2. From Table 2, we can see that (1) the theoretical AEAs of EuSi n predicted by the four schemes are in excellent agreement with the experimental values (taken from Ref. [22]), especially the AEAs of TPSSh and B2PLYP. The average absolute deviations from experiment are by 0.10, 0.06, 0.07, and 0.05 eV at the B3LYP, the TPSSh, the PBE, and the B2PLYP levels, respectively. The largest deviations are 0.16 eV (the B3LYP), 0.12 eV (the TPSSh), 0.18 eV (the PBE), and 0.10 eV (the B2PLYP). (2) The theoretical AEAs of Si n are also in excellent agreement with available experimental results. The average absolute deviations from experiment are by 0.07, 0.08, 0.07, and 0.12 eV at the B3LYP, the TPSSh, the PBE, and the B2PLYP levels, respectively. The largest deviations with the exception of Si9 are 0.11, 0.10, 0.10, and 0.19 eV, respectively. Though the largest deviations are that of Si9, which are off by 0.21 eV (the B3LYP), 0.20 eV (the TPSSh), 0.15 eV (the PBE), and 0.25 eV (the B2PLYP), they fall within the experimental error bars of ±0.25 eV [9]. (3) The AEAs of EuSi n are less than those of Si n . The reason can be explained that when a Eu atom is attached to Si n cluster, the charge transfer from Eu atom to silicon cluster (see below) results in the decrease in the AEAs of Si n clusters. With the increase in silicon cluster size, the average charge obtained by each silicon atom would become less and less. As a result, the AEAs of EuSi n can be close to the AEAs of Si n , but cannot be larger than the AEAs of Si n . In light of this point of view, we inferred that the experimental value of 2.8 ± 0.2 eV [22] of EuSi12 may be inaccurate because it is larger than the experimental value of 2.66 ± 0.20 eV [57, 58] of Si12. We hope that this prediction will provide strong motivation for further experimental studies of EuSi12 and its anion.
A very good agreement of AEA with experiment is a necessary condition for predicting the ground-state structure. This means that if the ground-state structure is accurate, then the theoretical AEA will be in good agreement with experimental value. But a good agreement with the experiment is not necessarily to say that the geometry is the ground-state structure. Therefore, even though the geometries reported in Ref. [30] are not the ground-state structures for n = 5 and 7, they also obtained the very good agreement of AEA with experiment.
3.3 Dissociation energies
The dissociation energies (DEs) (defined as the energies required in the reactions EuSi n → Eu + Si n for neutral EuSi n and EuSi − n → Eu + Si − n for anion EuSi − n ) of EuSi n and their anions are calculated and sketched in Figs. 2 and 3, respectively. The stability of bonding a Eu atom to silicon clusters can be found from the DEs. The higher values of the DEs indicate that the cluster bonding of a Eu atom is stable. A better way of comparing the local relative stability of various size clusters is by means of the incremental binding energies. From Figs. 2 and 3, we can see that (1) the DE curves for the four methods are parallel. The orders of DE predicted by the four methods are TPSSh > PBE > B2PLYP > B3LYP. (2) The EuSi n for n = 4, 7, and 10 is less stable than for n = 5 and 8 because the DEs are local minimal values for n = 4, 7, and 10 and local maximal values for n = 5 and 8. This also indicates that Si4, Si7, and Si10 are more stable and Si5 and Si8 less stable for Si n cluster. (3) The EuSi − n anion with n = 4 and 7 is less stable than with n = 2 and 9. (4) The DEs of neutral are larger than those of their anions. The reason will be explained in Sect. 3.4.
3.4 Charge transfer and magnetic moment
To further understand the interaction between the silicon clusters and the Eu atom, natural population analysis (NPA) is performed with the TPSSh method. The NPA valence configurations and charge of Eu atom are listed in Table 3. The magnetic moments of 6s, 4f, 5d, and 6p state for Eu atom, total magnetic moments of Eu atom, and total magnetic moments of the ground-state of EuSi n (n = 3–11) and their anions are listed in Table 4. From Table 3, we can see that (1) the valence configuration is 6s 0.14−0.364f 6.98−6.995d 0.37−0.776p 0.05−0.14 for Eu in EuSi n (n = 3–11) species. Obviously, the 4f shell of Eu in the clusters is nearly unchanged (the configuration of free Eu atom is [core]6s 24f 75d 06p 0), which reproduced the conclusion reported by Zhao et al. [30]. The charge transfer takes place mainly from 6s to 5d orbitals, leading to hybridization between the 6s and 5d orbitals. (2) The calculated charges of the Eu atom in EuSi n (n = 3–11) species are 1.02–1.29 e, which indicates Eu atom acts as an electron donor analogous to the results reported in Ref. [30], and the bonding between Eu atom and silicon clusters is ionic in nature. (3) In the cases of anion EuSi − n (n = 3–11), the majority of the extra electron’s charge was found to be localized on the silicon clusters. Compared with neutral EuSi n (n = 3–11), averaged charges of 0.55 e go back to Eu atom from silicon clusters. As a result, the bonds between Eu and silicon clusters are weakened. So the DEs of Eu atom from the ground-state structure of the anions EuSi − n are smaller than those of their neutral. From Table 3, we can see that the total magnetic moments of EuSi n /EuSi − n (n = 3–11) and the magnetic moments on the Eu atom do not quench and the total magnetic moments are contributed by Eu atom.
4 Conclusions
Carefully selected DFT methods applied with aug-SEG/ECP basis set for lanthanide atoms are capable of reliably predicting the available structures, AEAs, and other properties for the EuSi n clusters. The ground-state structures for all of these species are found to be substitutional structure, which can be regarded as being derived from the ground-state structure of Si n+1 (and/or Si − n+1 ) by replacing a Si atom with a Eu atom. The bond distances predicted by the B3LYP and the B2PLYP are larger than those predicted by PBE and the TPSSh. The theoretical AEAs of EuSi n predicted by the four DFT schemes are in excellent agreement with the experimental data, especially the TPSSh and B2PLYP AEAs. The average absolute deviations from experiment are by 0.10, 0.06, 0.07, and 0.05 eV, and the largest deviations are 0.16, 0.12, 0.18, and 0.10 eV at the B3LYP, the TPSSh, the PBE, and the B2PLYP levels, respectively. The AEA of EuSi n (n = 3–11) is less than that of Si n . With the increase in silicon cluster size, the AEA of EuSi n may be close to that of Si n , but cannot be larger than that of Si n . The EuSi n for n = 4, 7, and 10 is less stable than for n = 5 and 8, and the EuSi − n anion with n = 4 and 7 is less stable than with n = 2 and 9. Eu atom acts as an electron donor, and the bonding between Eu and silicon clusters is ionic in nature. The bond between Eu and silicon clusters of neutral EuSi n (n = 3–11) is stronger than that of their anions. The total magnetic moments of EuSi n /EuSi − n (n = 3–11) and the magnetic moments on the Eu atom do not quench, and the total magnetic moments are contributed by Eu atom.
We hope that our theoretical predictions will provide strong motivation for further experimental and theoretical studies of other lanthanide atom-doped silicon clusters and their anions.
References
Raghavachari K (1986) Theoretical study of small silicon clusters: equilibrium geometries and electronic structures of Sin (n = 2–7,10). J Chem Phys 84:5672–5686
Yang JC, Xu WG, Xiao WS (2005) The small silicon clusters Sin (n = 2–10) and their anions: structures, thermochemistry, and electron affinities. J Mol Struct THEOCHEM 719:89–102
Zhu X, Zeng XC (2003) Structures and stabilities of small silicon clusters: Ab initio molecular-orbital calculations of Si7–Si11. J Chem Phys 118:3558–3570
Honea EC, Ogura A, Murray CA, Raghavachari K, Sprenger WO, Jarrold MF, Brown WL (1993) Raman spectra of size-selected silicon clusters and comparison with calculated structures. Nature (London) 366:42–44
Li S, Zee RJV, Weltner W, Raghavachari K (1995) Si3–Si7 experimental and theoretical infrared spectra. Chem Phys Lett 243:275–280
Raghavachari K, Rohlfing CM (1991) Electronic structures of the negative ions Si2 ––Si10 −: electron affinities of small silicon clusters. J Chem Phys 94:3670–3678
Liu B, Lu ZY, Pan BC, Wang CZ, Ho KM, Shvartsburg AA, Jarrold MF (1998) Ionization of medium-sized silicon clusters and the geometries of the cations. J Chem Phys 109:9401–9409
Zhu XL, Zeng XC, Lei YA, Pan B (2004) Structures and stability of medium silicon clusters. II. Ab initio molecular orbital calculations of Si12–Si20. J Chem Phys 120:8985–8995
Kishi R, Kawamata H, Negishi Y, Iwata S, Nakajima A, Kaya K (1997) Geometric and electronic structures of silicon-sodium binary clusters. II. Photoelectron spectroscopy of Si n Na − m cluster anions. J Chem Phys 107:10029–10043
Kawamata H, Negishi Y, Kishi R, Iwata S, Nakajima A, Kaya K (1996) Photoelectron spectroscopy of silicon–fluorine binary cluster anions (Si n F − m ). J Chem Phys 105:5369–5376
Kutzelnigg W (1984) Chemical bonding in higher main group elements. Angew Chem Int Ed Engl 23:272–295
Pak C, Kiracofe JCR, Schaefer HF (2000) Electron affinities of silicon hydrides: SiHn (n = 0–4) and Si2Hn (n = 0–6). J Phys Chem A 104:11232–11242
Beck SM (1987) Studies of silicon cluster-metal atom compound formation in a supersonic molecular beam. J Chem Phys 87:4233–4234
Koyasu K, Akutsu M, Mitsui M, Nakajima A (2005) Selective formation of MSi16 (M = Sc, Ti, and V). J Am Chem Soc 127:4998–4999
Xu HG, Wu MM, Zhang ZG, Yuan JY, Sun Q, Zheng WJ (2012) Photoelectron spectroscopy and density functional calculations of CuSi − n (n = 4–18) clusters. J Chem Phys 136:104308-1–104308-10
Fan HW, Yang JC, Lu W, Ning HM, Zhang QC (2010) Structures and electronic properties of beryllium atom encapsulated in Si (0,−1) n (n = 2–10) clusters. J Phys Chem A 114:1218–1223
Tam NM, Tai TB, Nguyen MT (2012) Thermochemical parameters and growth mechanism of the boron-doped silicon clusters, Si n Bq with n = 1–10 and q = −1, 0, +1. J Phys Chem C 116:20086–20098
Khanna SN, Rao BK, Jena P, Nayak SK (2003) Stability and magnetic properties of iron atoms encapsulated in Si clusters. Chem Phys Lett 373:433–438
Wang JG, Zhao JJ, Ma L, Wang BL, Wang GH (2007) Structure and magnetic properties of cobalt doped Si n (n = 2–14) clusters. Phys Lett A 367:335–344
Guo LJ, Zhao GF, Gu YZ, Liu X, Zeng Z (2008) Density-functional investigation of metal-silicon cage clusters MSin (M = Sc, Ti, V, Cr, Mn, Fe Co, Ni, Cu, Zn; n = 8–16). Phys Rev B 77:195417-1–195417-8
Li JR, Wang GH, Yao CH, Mu YW, Wan JG, Han M (2009) Structures and magnetic properties of Si n Mn (n = 1–15) clusters. J Chem Phys 130:164514-1–164514-9
Grubisic A, Wang HP, Ko YJ, Bowen KH (2008) Photoelectron spectroscopy of europium-silicon clusters anions, EuSi − n (3 ≤ n≤17). J Chem Phys 129:054302-1–054302-5
Grubisic A, Ko YJ, Wang HP, Bowen KH (2009) Photoelectron spectroscopy of Lanthanide-silicon cluster anions LnSi − n (3 ≤ n≤13; Ln = Ho, Gd, Pr, Sm, Eu, Yb): prospect for magnetic silicon-based clusters. J Am Chem Soc 131:10783–10790
Wang J, Liu Y, Li YC (2010) Magnetic silicon fullerene. Phys Chem Chem Phys 12:11428–11431
Ohara M, Miyajima K, Pramann A, Nakajima A, Kaya K (2002) Geometric and electronic structures of terbium-silicon mixed clusters (TbSi n ; 6 ≤ n ≤ 16). J Phys Chem A 106:3702–3705
Ohara M, Miyajima K, Pramann A, Nakajima A, Kaya K (2007) Geometric and electronic structures of terbium-silicon mixed clusters (TbSi n ; 6 ≤ n ≤ 16). J Phys Chem A 111:10884
Koyasu K, Atobe J, Furuse S, Nakajima A (2008) Anion photoelectron spectroscopy of transition metal- and lanthanide metal-silicon clusters: MSi − n (n = 6–20). J Chem Phys 129:214301-1–214301-7
Cao TT, Feng XJ, Zhao LX, Liang X, Lei YM, Luo YH (2008) Structure and magnetic properties of La-doped Si n (n = 1–12, 24) clusters: a density functional theory investigation. Eur Phys J D 49:343–351
Peng Q, Shen J (2008) Growth behavior of La@Si n (n = 1–21) metal-encapsulated clusters. J Chem Phys 128:084711-1–084711--11
Zhao GF, Sun JM, Gu YZ, Wang YX (2009) Density-functional study of structural, electronic, and magnetic properties of the EuSi n (n = 1–13) clusters. J Chem Phys 131:114312-1–114312-7
Li CG, Pan LJ, Shao P, Ding LP, Feng HT, Luo DB, Liu B (2015) Structures, stabilities, and electronic properties of the neutral and anionic Si n Smλ (n = 1–9, λ = 0, −1) clusters: comparison with pure silicon clusters. Theor Chem Acc 134:34-1–34-11
Liu TG, Zhao GF, Wang YX (2011) Structural, electronic and magnetic properties of GdSi n (n = 1–17) clusters: a density functional study. Phys Lett A 375:1120–1127
Liu TG, Zhang WQ, Li YL (2014) First-principles study on the structure, electronic and magnetic properties of HoSi n (n = 1–12, 20) clusters. Front Phys 9:210–218
Zhao RN, Ren ZY, Guo P, Bai JT, Zhang CH, Han JG (2006) Geometries and electronic properties of the neutral and charged rare earth Yb-doped Si n (n = 1–6) clusters: a relativistic density functional investigation. J Phys Chem A 110:4071–4079
Zhao RN, Han JG, Bai JT, Liu FY, Sheng LS (2010) A relativistic density functional study of Si n (n = 7–13) clusters with rare earth ytterbium impurity. Chem Phys 372:89–95
Zhao RN, Han JG, Bai JT, Liu FY, Sheng LS (2010) The medium-sized charged YbSin ± (n = 7–13) clusters: a relativistic computational investigation. Chem Phys 378:82–87
Cao TT, Zhao LX, Feng XJ, Lei YM, Luo YH (2009) Structural and electronic properties of LuSi n (n = 1–12) clusters: a density functional theory investigation. J Mol Struct THEOCHEM 895:148–155
Wang HQ, Li HF (2014) A combined stochastic search and density functional theory study on the neutral and charged silicon-based clusters MSi6 (M = La, Ce, Yb and Lu). RSC Adv 4:29782–29793
Li HF, Kuang XY, Wang HQ (2011) Probing the structural and electronic properties of lanthanide-metal-doped silicon clusters: M@Si6 (M = Pr. Gd, Ho). Phys Lett A 375:2836–2844
Kumar V, Singh AK, Kawazoe Y (2006) Charged and magnetic fullerenes of silicon by metal encapsulation: Predictions from ab initio calculations. Phys Rev B 74:125411-1–125411-5
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Perdew JP, Burke K, Ernzerhof M (1997) Generalized gradient approximation made simple. Phys Rev Lett 78:1396
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401-1–146401-4
Staroverov VN, Scuseria GE, Tao J, Perdew JP (2003) Comparative assessment of a new nonempirical density functional: molecules and hydrogen-bonded complexes. J Chem Phys 119:12129–12137
Grimme S (2006) Semiempirical hybrid density functional with perturbative second-order correlation. J Chem Phys 124:034108-1–034108-16
Woon DE, Dunning TH (1993) Gaussian basis sets for use in correlated molecular calculations. II. The atoms aluminum through argon. J Chem Phys 98:1358–1371
Cao X, Dolg M (2002) Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J Mol Struct THEOCHEM 581:139–147
Buchachenko AA, Chalasiński G, Szeześniak MM (2007) Diffuse basis functions for small-core relativistic pseudopotential basis sets and static dipole polarizabilities of selected lanthanides La, Sm, Eu, Tm and Yb. Struct Chem 18:769–772
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V et al (2010) Gaussian 09 revision C.01, Gaussian Inc, Wallingford
Ning HM, Fan HW, Yang JC (2011) Probing the electronic structures and properties of neutral and charged CaSi − n (n = 2–10) clusters using Gaussian-3 theory. Comput Theor Chem 967:141–147
Fan HW, Ren ZQ, Yang JC, Hao DS, Zhang QC (2010) Study on structures and electronic properties of neutral and charged MgSi − n (n = 2–10) clusters with a Gaussian-3 theory. J Mol Struct THEOCHEM 958:26–32
Hao DS, Liu JR, Wu WG, Yang JC (2009) Study on structures and electron affinities of small potassium-silicon clusters Si n K (n = 2–8) and their anions with Gaussian-3 theory. Theor Chem Acc 124:431–437
Xu C, Taylor TR, Burton GR, Neumark DM (1998) Vibrationally resolved photoelectron spectroscopy of silicon cluster anions Si − n (n = 3–7). J Chem Phys 108:1395–1406
Nakajima A, Taguwa T, Nakao K, Gomei M, Kishi R, Iwata S, Kaya K (1995) Photoelectron spectroscopy of silicon-carbon cluster anions (Si n C − m ). J Chem Phys 103:2050–2057
Ohara M, Koyasu K, Nakajima A, Kaya K (2003) Geometric and electronic structures of metal (M)-doped silicon clusters (M = Ti, Hf, Mo and W). Chem Phys Lett 371:470–490
Cheshnovsky O, Yang SH, Pettiette CL, Craycraft MJ, Liu Y, Smalley RE (1987) Ultraviolet photoelectron spectroscopy of semiconductor clusters: silicon and germanium. Chem Phys Lett 138:119–124
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21263010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, J., Wang, J. & Hao, Y. Europium-doped silicon clusters EuSi n (n = 3–11) and their anions: structures, thermochemistry, electron affinities, and magnetic moments. Theor Chem Acc 134, 81 (2015). https://doi.org/10.1007/s00214-015-1684-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1684-9